Back to Journals » Cancer Management and Research » Volume 11
Silencing of Peroxiredoxin 1 Inhibits the Proliferation of Esophageal Cancer Cells and Promotes Apoptosis by Inhibiting the Activity of the PI3K/AKT Pathway
Authors Song Y, Liu H, Cui C, Peng X, Wang C, Tian X, Li W
Received 19 October 2019
Accepted for publication 12 December 2019
Published 30 December 2019 Volume 2019:11 Pages 10883—10890
DOI https://doi.org/10.2147/CMAR.S235317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Yingjian Song,1,* Huimin Liu,2,* Chunling Cui,3 Xiaonu Peng,1 Chaoyang Wang,1 Xudong Tian,4 Wenjun Li1
1Department of Thoracic Surgery, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai 264000, Shandong, People’s Republic of China; 2Department of Gastroenterology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai 264000, Shandong, People’s Republic of China; 3Library, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai 264000, Shandong, People’s Republic of China; 4Department of Thoracic Surgery, Liaocheng People’s Hospital, Liaocheng 252000, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xudong Tian
Department of Thoracic Surgery, Liaocheng People’s Hospital, No. 67, Changxi Road, Liaocheng, Shandong 252000, People’s Republic of China
Tel +86-15653112705
Email [email protected]
Wenjun Li
Department of Thoracic Surgery, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, No. 20 Yuhungding East Road, Zhifu District, Yantai 264000, Shandong, People’s Republic of China
Tel +86-18653525277
Email [email protected]
Objective: To study the effect of peroxiredoxin 1 (PRDX1) on esophageal squamous carcinoma cells and determine whether it plays a role in regulating the PI3K/AKT signaling pathway.
Methods: Three esophageal squamous cell carcinoma cell lines (Eca-109, EC9706, and KYSE150) and one normal cell line (human esophageal epithelial cells) were selected. The protein expression of peroxiredoxin 1 (PRDX1) and the activity of the PI3K/AKT pathway were detected via Western blotting. The proliferation ability of cells was detected through the MTT assay and cell clone formation. Apoptosis was detected using flow cytometry. Subsequently, cells were treated with a PI3K/AKT pathway inhibitor and activator, alone or in combination with silencing of PRDX1, and the above indicators were re-tested.
Results: The expression of PRDX1 and activity of PI3K/AKT pathway-associated proteins were higher in esophageal cancer cells than in normal esophageal epithelial cells. Compared with normal human esophageal epithelial cells, the proliferation of the three types of esophageal cancer cells was increased, whereas their level of apoptosis was decreased (p<0.05). In Eca-109 cells (cell line with silenced expression of PRDX1), the expression of PRDX1 was significantly decreased. In contrast to the control group, the proliferation and clonality of cells in the silencing PRDX1 group was decreased, the proportion of apoptotic cells was increased, and the phosphorylation levels of PI3K and AKT were decreased (p<0.05). Compared with the control group, treatment with the inhibitor LY294002 alone significantly inhibited cell proliferation and promoted apoptosis (p<0.05); this effect was similar to that observed in the silencing PRDX1 group.
Conclusion: PRDX1 was highly expressed in esophageal cancer cells. Silencing of PRDX1 can inhibit the proliferation of esophageal cancer cells and promote apoptosis. The mechanism involved in this process may be related to the inhibition of the PI3K/AKT signaling pathway.
Keywords: peroxiredoxin 1, esophageal squamous cell carcinoma, PI3K/AKT signaling pathway, proliferation, apoptosis
Introduction
Esophageal cancer (EC) is one of the most mortality malignancies. Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma are two major histological subtypes of EC, accounting for approximately 90% of all cases of EC. Esophageal adenocarcinoma is more common in Western countries; however, ESCC is the main subtype encountered in the Middle East and Asia.1 Most patients are diagnosed at an advanced stage and often have metastasis to the lymph node region.2,3 The accumulation of multiple genetic/epigenetic changes is often associated with the development of ESCC, including the stimulation of oncogenes or inactivation of tumor suppressor genes. ESCC is a fatal disease, and there are numerous factors that are responsible for its development, such as drinking, smoking, lack of appropriate nutrition, excessive intake of food rich in nitrosamines or mold contamination.4–6 The current main treatment for ESCC is surgical resection; nevertheless, tumor recurrence and metastasis after surgical resection occur in most cases due to the high invasiveness of ESCC. This leads to poor prognosis, short median survival, poor postoperative quality of life, and low postoperative survival.7,8 In addition to surgical resection, chemotherapy is generally used in combination with chemotherapy for the treatment of ESCC. However, owing to the resistance of tumor tissues to drugs, the therapeutic efficacy of chemotherapeutic drugs is greatly reduced.9,10 Therefore, there is an urgent need to clarify the underlying mechanisms of ESCC, identify relevant biomarkers, and develop novel and effective treatments.
Peroxiredoxin 1 (PRDX1) protein is a key antioxidant enzyme and a member of the peroxidase family, playing an effective role in scavenging oxidants.11 It has been reported that the expression of PRDX1 is increased in ESCC. In addition, PRDX1 is a tumor suppressor that can be used as an effective prognostic indicator for EC cell carcinoma.12,13 The relationship between PRDX1 and the P13K/AKT signaling pathway was rarely investigated in previous studies.
Materials and Methods
Cell Culture
The human ESCC cell lines Eca-109 (BNCC337687; North Natron Biotechnology Research Institute, Beijing, China), KYSE150 (HYC3413; Heyuan Biotechnology Co., Ltd., Shanghai, China), EC9706 (BNCC339892; North Natron Biotechnology Research Institute), and normal human esophageal epithelial cells (HEEC) (BNCC337729; North Natron Biotechnology Research Institute) were maintained in RPMI 1640 (Gibco, Rockville, MD, USA) medium supplemented with 10% fetal bovine serum (Sigma–Aldrich, St. Louis, MO, USA). Cells were cultured at 37°C in 5% CO2, and those in the logarithmic growth phase were selected for experiments.
Cell Processing and Grouping
The experiment was divided into the following five groups: blank control group (control), negative control group (lv-NC), silencing PRDX1 group (lv-PRDX1), PI3K/AKT pathway inhibitor group (LY294002), and PRDX1 silencing plus activator group (lv-PRDX1+740 Y-P).
The cells in the control group were not treated. In the lv-NC group, the negative control lentiviral vector was used. In the lv-PRDX group, cells were treated with the silencing PRDX1 gene lentiviral vector. The cells in the LY294002 group were treated with 10 µmol/L of LY294002 (HY-10108; MCE, USA). The cells in the Lv-PRDX1+740 Y-P group were transfected with PRDX1 siRNA sequence and treated with 20 μM 740 Y-P (ApexBio, Houston, TX, USA).14
The ESCC cell line with the most differential expression of PRDX1 was selected for the transfection of PRDX1 with siRNA. The siRNA against PRDX1 and lentiviral vector were purchased from Shanghai GenePharm Pharmaceutical Technology Co., Ltd. (Shanghai, China). The recombinant lentiviral vector carrying siRNA was transfected into the ESCC cell line using the liposome LipofectamineTM 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA). The transfection efficiency of the cells was detected through quantitative reverse transcription-polymerase chain reaction (quantitative reverse transcription-PCR) 72 h after infection.
The PRDX1 siRNA sequences were as follows:
Sense:5ʹ-CUGGAAACCUGGCAGUGAUTT-3ʹ
Anti-sense: 5ʹ-AUCACUGCCAGGUUUCCAGTT-3ʹ
Negative control siRNA:
Sense:5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ
Anti-sense: 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ
MTT Assay
The cells in each group were seeded into 96-well plates at a concentration of 1×104 cells/mL. After incubation for 24, 48, 72, and 96 h, MTT (20 μL; 5 mg/mL) was added, and the cells were cultured in an incubator at 37°C with 5% CO2. After 4 h, the supernatant solution was discarded, and dimethyl sulfoxide (200 μL) was added (Sigma–Aldrich). Subsequently, it was blown evenly, and the absorption value (optical density value) in each well was measured at the wavelength of 490 nm using Bio-Rad Laboratories (Hercules, CA, USA).
Plate Cloning Experiment
Each group of cells in the logarithmic growth phase was trypsinized to prepare a single cell suspension, and the concentration of cells was adjusted. Each cell suspension (2 mL) was seeded into a flat-bottom six-well plate (500 cells per well), and three replicate wells were prepared for each group. The cells were evenly dispersed by shaking the plate, and incubated at 37°C, 5% CO2, and saturated humidity. Following the appearance of macroscopic clones in the culture plates, the culture was terminated, the medium was discarded, and the cells were carefully washed twice with phosphate-buffered saline (PBS). The cells were fixed with methanol for 15 min at room temperature. Subsequently, the fixative was discarded and the cells were stained with Giemsa staining solution for 10–30 min. The stain was gently washed off with running water, the cell cultures were allowed to dry at room temperature, and the number of formed clones was counted.
Flow Cytometry
The cells were trypsinized, centrifuged (1000 rpm, 5 min), and the collected cells were washed twice with pre-cooled sterile PBS at 4°C to obtain a total of approximately 1×106 cells/mL. The cells were resuspended in 250 μL of 1× binding buffer. Fluorescein isothiocyanate-labeled Annexin-V (5 μL) was added to 195 μL of the cell suspension. The cell suspension was gently mixed and maintained at room temperature for 3 min. The cells were incubated for 10 min at room temperature in the dark. Subsequently, 1× binding buffer (400 μL) was added, and the cells were gently mixed and placed in a flow cytometer (Gallios; Beckman Coulter, Inc., Brea, CA, USA). for detection. The results were analyzed using the Cell Quest software (BD Biosciences, San Diego, CA, USA).
Western Blotting
Western blotting was used to detect the expression levels of PRDX1, p-PI3K, PI3K, AKT, and p-AKT in each cell line. Each group of cells was collected, and the total protein in the cells was extracted according to the instructions provided by the manufacturer. The concentration of protein in the samples was measured using a bicinchoninic acid protein quantification kit (23225, PierceTM BCA Protein Assay Kit; Thermo Fisher Scientific, Waltham, MA, USA). Each protein sample (40 μg) was loaded on gels, separated through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Mini-Protean-3; Bio-Rad Laboratories), and transferred onto a polyvinylidene difluoride membrane (Millipore, MA, USA). The membrane was blocked with 5% skim milk powder for 1 h, and 5% bovine serum albumin was added to dilute the primary antibody against each protein. The primary antibodies were rabbit anti-p-PI3K (1:500, orb338965; Biorbyt, Cambridge, UK), anti-PI3K (1:500, orb137259; Biorbyt), anti-AKT (1:2000, ab235958; Abcam, Cambridge, UK), anti-p-AKT (1:1000, ab8932; Abcam), anti-PRDX1 (1:500, orb335510; Biorbyt), and polyclonal β-actin (1:1000, ab8227; Abcam). The samples were incubated with goat anti-rabbit Ig G (1:2000, ab6721; Abcam) for 1 h. The enhanced chemiluminescence method was used for detection, and the gray scale scanning and quantification were performed using the ImageJ software. Protein expression levels were normalized to those of β-actin.
Real-Time PCR
Total RNA was extracted from each cell line using the total RNA extraction kit (Invitrogen). After transcription into cDNA, cDNA template (2 μL) was added, and the real-time PCR reaction was performed under the following conditions: 95°C for 10 min, 95°C for 15 s, 57°C for 10 s, amplification for 40 cycles. The glyceraldehyde-3-phosphate dehydrogenase gene (MQP-0202; Guangzhou Ruibo Biotechnology Co., Ltd., Guangzhou, China) was used as the internal reference, and the relative expression of PRDX1 was calculated via the 2−△△Ct method.
Statistical Analysis
The SPSS version 19.0 statistical software was used for the statistical analysis of data. The results were expressed as the mean ± standard deviation. The t-test was used for data analysis between two groups. The data of multiple groups were analyzed through analysis of variance, and the least significant difference test was used for subsequent analysis. A p<0.05 indicated statistically significant difference.
Results
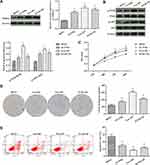
Detection of the Proliferation and Apoptosis of Human ESCC Cells and Normal HEEC
As shown in Figure 1, the results of the Western blotting analysis showed that the expression of PRDX1 in three ESCC cells was significantly higher than that observed in normal HEEC; the difference was statistically significant in Eca-109 cells (p<0.05). The phosphorylation levels of PI3K and AKT were significantly elevated in the three ESCCs compared with HEEC (p<0.05).
The results of the cell biological behavior test showed that the viability and proliferation of EC cells were significantly higher than those of normal HEEC, whereas the rate of apoptosis level was markedly lower; the difference was statistically significant in Eca-109 cells (p<0.05).
Silencing of PRDX1 Inhibits the Proliferation of Eca-109 Cells and Promotes Apoptosis
Eca-109 cells were used for the experiment, as shown in Figure 2. After transfection of Eca-109 cells with PRDX1 siRNA, the mRNA expression levels of PRDX1 were clearly downregulated (p<0.05). In comparison with the control group, the proliferation and colony formation abilities of cells in the lv-PRDX1 group were significantly decreased, whereas the rate of apoptosis was markedly increased (p<0.05).
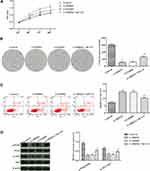
Silencing of PRDX1 Reduces the Activity of the PI3K/AKT Pathway
Western blotting was used to detect changes in the activity of the PI3K/AKT pathway after silencing PRDX1. As shown in Figure 3, the phosphorylation levels of PI3K and AKT in the lv-PRDX1 group were significantly reduced (p<0.05), while the total protein content was not visibly changed compared with the control group.
 |
Figure 3 WB assay for the activity of the PI3K/AKT pathway in esophageal squamous cell carcinoma cells. *p<0.05, compared with the control group; #p<0.05, compared with the lv-NC group. |
Silencing of PRDX1 Inhibits the Proliferation of Eca-109 Cells and Promotes Apoptosis by Decreasing the Activity of the PI3K/AKT Pathway
We further investigated whether the effect of PRDX1 on the proliferation and apoptosis of ESCC involves the PI3K/AKT signaling pathway. Cells were treated with the inhibitor LY294002 to inhibit the signaling pathway, and in combination with the activator 740 Y-P on the basis of silencing PRDX1. The effects of this treatment on Eca-109 cells were re-examined. The experimental results are shown in Figure 4. The proliferation and clonality of cells treated with LY294002 were significantly decreased, the proportion of apoptotic was increased, and the protein expression levels of p-AKT and p-PI3K were decreased (p<0.05) compared with those of the control group. The effect was similar to that observed in the lv-PRDX1 group. In comparison with the lv-PRDX1 group, the cells were treated with the activator 740 Y-P on the basis of silencing PRDX1. The proliferation ability of the lv-PRDX1 +740 Y-P group was significantly enhanced, the number of apoptotic cells was decreased, and the phosphorylation levels of p-AKT and p-P13k were increased (p<0.05).
Discussion
PRDX1 plays an important role in hydrogen peroxide-mediated cell signaling. It is a peroxide scavenger in mammalian cells, and associated with poor prognosis in several types of human cancer. The overexpression of PRDX1 in osteosarcoma and fibrosarcoma tumors can inhibit the invasion and migration of these cancerous cells, and retard tumor progression.15 During myocardial ischemia/reperfusion damage, PRDX1 can also reduce myocardial apoptosis through the reactive oxygen species-activated mitogen-activated protein kinase pathway.16 According to autophagy studies, PRDX1 can negatively regulate the signaling and autophagy functions of toll-like receptor 4 activated by nuclear factor-κB by inhibiting the activity of tumor necrosis factor receptor-associated factor 6 ubiquitin-ligase. Moreover, it can enhance bactericidal activity and inhibit the invasion and migration of tumor cells.17 Among the proteins identified by proteomics for ESCC, PRDX1 is considered a tumor-associated antigen for EC cells. In the present study, PRDX1 was shown to be a tumor suppressor gene that is highly expressed in three ESCC cell lines. Further experiments showed that the proliferation and clonality of the three ESCC cells were significantly higher than those reported for HEEC, whereas the number of apoptotic cells was significantly lower.
It has been shown that the P13K/AKT pathway is related to the proliferation, migration and invasion of ESCC.18 In the P13K/AKT pathway, following the activation of PI3K, 3,4,5-phosphatidylinositol trisphosphate phosphorylation is catalyzed and the protein kinase AKT is activated to promote the growth and proliferation of cells.19 Our investigation of the specific mechanism of PRDX in EC cells showed that the effects of silencing PRDX1 alone and adding the P13K/AKT pathway inhibitor alone were similar. Both interventions inhibited the proliferation and clonality of ESCC cells and increased the rate of apoptosis.
However, in combination with an activator of the P13K/AKT pathway on the basis of silencing PRDX1, the inhibitory effect on tumor development is offset. The results of the present study showed that silencing of PRDX1 inhibited the activity of the P13K/AKT pathway, thereby inhibiting the proliferation of ESCC cells and promoting apoptosis. However, the specific mechanism involved in this process remains unclear. In this study, we focused on the effects of PRDX1 on the proliferation, clonality, and apoptosis of ESCC cells. However, the role of PRDX as a clinical marker for clinical diagnosis and prognosis has not been examined thus far. After confirming the role of PRDX1 in subsequent experiments, we will focus our research on its usefulness in tumor identification. The aim of these future studies would be to utilize PRDX1 for the prompt detection of the occurrence of tumors and early initiation of appropriate treatment to improve the survival of patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Meng W, Smith JS, Wei WQ. Tissue protein biomarker candidates to predict progression of esophageal squamous cell carcinoma and precancerous lesions. Ann N Y Acad Sci. 2018;1434(1):59–69.
2. Lian Y, Niu X, Cai H, et al. Clinicopathological significance of c-MYC in esophageal squamous cell carcinoma. Tumour Biol. 2017;39(7):. doi:10.1177/1010428317715804
3. Lu Y-F, Yu J-R, Yang Z, et al. Promoter hypomethylation mediated upregulation of MicroRNA-10b-3p targets FOXO3 to promote the progression of esophageal squamous cell carcinoma (ESCC). J Exp Clin Cancer Res. 2018;37(1):301. doi:10.1186/s13046-018-0966-1
4. Wu Y, Yang Y, Xian Y-S. HCRP1 inhibits cell proliferation and invasion and promotes chemosensitivity in esophageal squamous cell carcinoma. Chem Biol Interact. 2019;308:357–363. doi:10.1016/j.cbi.2019.05.032
5. Maehara Y. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus. Int J Clin Oncol. 2010;15(2):135–144. doi:10.1007/s10147-010-0055-8
6. Morita M, Kumashiro R, Kubo N, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15(2):126–134. doi:10.1007/s10147-010-0056-7
7. Goseki N, Koike M, Yoshida M. Histopathologic characteristics of early stage esophageal carcinoma. A comparative study with gastric carcinoma. Cancer. 2010;70(11):2743.
8. Tian W, Jiang C, Huang Z, Xu D, Zheng S. Comprehensive analysis of dysregulated lncRNAs, miRNAs and mRNAs with associated ceRNA network in esophageal squamous cell carcinoma. Gene. 2019;696:206–218.
9. Kawakami T, Tsushima T, Omae K, et al. Risk factors for esophageal fistula in thoracic esophageal squamous cell carcinoma invading adjacent organs treated with definitive chemoradiotherapy: a monocentric case-control study. BMC Cancer. 2018;18(1):573. doi:10.1186/s12885-018-4486-3
10. Li R, Leng AM, Liu XM, et al. Overexpressed PTOV1 associates with tumorigenesis and progression of esophageal squamous cell carcinoma. Tumour Biol. 2017;39(6):. doi:10.1177/1010428317705013
11. Conway JP, Michael K. Dual role of peroxiredoxin I in macrophage-derived foam cells. J Biol Chem. 2006;281(38):27991. doi:10.1074/jbc.M605026200
12. Ren P, Ye H, Dai L, et al. Peroxiredoxin 1 is a tumor-associated antigen in esophageal squamous cell carcinoma. Oncol Rep. 2013;30(5):2297–2303. doi:10.3892/or.2013.2714
13. Isamu H, Hisahiro M, Yasunori A, et al. Tumor suppressor Prdx1 is a prognostic factor in esophageal squamous cell carcinoma patients. Oncol Rep. 2007;18(4):867–871.
14. An G, Liang S, Sheng C, Liu Y, Yao W. Upregulation of microRNA-205 suppresses vascular endothelial growth factor expression-mediated PI3K/Akt signaling transduction in human keloid fibroblasts. Exp Biol Med. 2017;242(3):. doi:10.1177/1535370216669839.
15. Wang Y, Liu M, Yang P, Peng H. Peroxiredoxin 1 (PRDX1) suppresses progressions and metastasis of osteosarcoma and fibrosarcoma of bone. Med Sci Monit. 2018;24:4113–4120. doi:10.12659/MSM.908736
16. Guo W, Liu X, Li J, et al. Prdx1 alleviates cardiomyocyte apoptosis through ROS-activated MAPK pathway during myocardial ischemia/reperfusion injury. Int J Biol Macromol. 2018;112:608–615. doi:10.1016/j.ijbiomac.2018.02.009
17. Min Y, Kim M-J, Lee S, Chun E, Lee K-Y. Inhibition of TRAF6 ubiquitin-ligase activity by PRDX1 leads to inhibition of NFKB activation and autophagy activation. Autophagy. 2018;14(8):1347–1358. doi:10.1080/15548627.2018.1474995
18. Pan S, Sun Y, Sui D, et al. Lobaplatin promotes radiosensitivity, induces apoptosis, attenuates cancer stemness and inhibits proliferation through PI3K/AKT pathway in esophageal squamous cell carcinoma. Biomed Pharmacother. 2018;102:567–574. doi:10.1016/j.biopha.2018.03.109
19. Liu GL, Yang HJ, Liu B, Liu T. Effects of microRNA-19b on the proliferation, apoptosis and migration of wilm’s tumor cells via the PTEN/PI3K/AKT signaling pathway. J Cell Biochem. 2017;118(10):3424–3434. doi:10.1002/jcb.25999
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.