Back to Journals » OncoTargets and Therapy » Volume 13
Silencing of Long Non-Coding RNA-HCG18 Inhibits the Tumorigenesis of Gastric Cancer Through Blocking PI3K/Akt Pathway
Received 3 December 2019
Accepted for publication 25 February 2020
Published 18 March 2020 Volume 2020:13 Pages 2225—2234
DOI https://doi.org/10.2147/OTT.S240965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
This paper has been retracted.
Fengzhen Ma, 1 Kexiang An, 2 Yuqin Li 3
1Department of Gastroenterology, Affiliated Hospital of Binzhou Medical University, Binzhou City, Shandong Province 256603, People’s Republic of China; 2Department of Gastrointestinal Surgery, Rizhao Central Hospital, Rizhao City, Shandong Province 276800, People’s Republic of China; 3Department of Internal Medicine V, Linyi Cancer Hospital, Linyi City, Shandong Province 276001, People’s Republic of China
Correspondence: Yuqin Li
Department of Internal Medicine V, Linyi Cancer Hospital, No. 6, Lingyuan East Street, Lanshan District, Linyi City, Shandong Province 276001, People’s Republic of China
Tel +86-13563058562
Email [email protected]
Purpose: Long non-coding RNAs (lncRNAs) play critical regulatory roles in the tumorigenesis of GC. This study aimed to investigate the regulatory effect and mechanism of lncRNA-HCG18 on GC.
Methods: The expression of lncRNA-HCG18 was detected in GC tissues and cell lines by qRT-PCR. LncRNA-HCG18 was silenced in AGS and MGC803 cells by the transfection of lncRNA-HCG18 siRNA (si-HCG18). MTT, transwell and Annexin V-PI double staining assay were performed to assess the proliferation, migration, invasion and apoptosis of GC cells. The expression of PI3K/Akt pathway-, apoptosis-, and migration-related proteins were detected by Western blot. An activator of PI3K/Akt pathway 740 Y-P was used to activate the PI3K/Akt pathway in AGS cells. A human tumor xenograft model was established in mice to evaluate the effects of si-HCG18 in vivo.
Results: LncRNA-HCG18 was overexpressed in GC tissues and cells. Up-regulation of lncRNA-HCG18 was positively correlated with the stage of tumor node metastasis and invasion depth. Silencing of lncRNA-HCG18 suppressed the proliferation, migration and invasion, and induced the apoptosis of GC cells. Silencing of lncRNA-HCG18 blocked the PI3K/Akt pathway. The intervention of 740Y-P reversed the anti-tumor effect of lncRNA-HCG18 on GC cells. In addition, silencing of lncRNA-HCG18 suppressed the growth of GC xenografts in mice.
Conclusion: Silencing of lncRNA-HCG18 inhibited the tumorigenesis of GC through blocking the PI3K/Akt pathway, suggesting a novel therapeutic target for GC.
Keywords: gastric cancer, lncRNA-HCG18, PI3K/Akt pathway, proliferation, migration
Introduction
Gastric cancer (GC) is a common type of malignancy, which is the second leading cause of cancer-related death worldwide.1,2 The high metastatic potential of GC leads to the poor prognosis of patients, with a 5-year survival rate of less than 20%.3 Although the surgery and adjuvant chemotherapy have made great progress in the treatment of GC, the prognosis of GC patients is still poor since more than 80% of patients are diagnosed in the advanced stage.4–6 Therefore, identifying novel targets for the diagnosis and treatment of GC are urgently needed.
Long non-coding RNAs (lncRNAs) are linear RNA transcripts of the mammalian genome without protein-coding function.7 Recently, various cancer-related lncRNAs have been identified, and their biological functions in tumorigenesis have also been confirmed, such as lncRNA-MALAT1 in prostate cancer,8 and lncRNA-HOTAIR9 and lncRNA-ANRIL10 in cervical cancer. It is worth mentioning that lncRNAs exert critical regulatory roles in the development of GC. Zhao et al11 have shown that overexpression of lncRNA-HULC promotes the proliferation and invasion, and inhibits the apoptosis of SGC7901 cells. Li et al12 have indicated that overexpression of lncRNA-CASC2 inhibits the growth of GC cells via blocking the MAPK pathway. Wu et al have proved that silencing of lncRNA-FEZF1-AS1 represses the tumorigenesis of GC by activating the Wnt/β-catenin pathway.13 LncRNA-human leucocyte antigen complex group 18 (HCG18) is a 2430-bp lncRNA located on chromosome 6p22.1. Xi et al14 have found that lncRNA-HCG18 represses the growth of nucleus pulposus (NP) cells and accelerates the development of intervertebral disc degeneration (IDD). Si-Yu et al15 have determined a tumor-promoting role of lncRNA-HCG18 on liver cancer. However, the biological function of lncRNA-HCG18 on GC remains unclear.
Phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway is indispensable in the progression of cancers, which can modulate the tumorigenesis, metastasis, and cell proliferation and apoptosis.16 Recently, accumulating researches have suggested that the regulatory effects of lncRNAs on GC are closely related to the PI3K/Akt pathway.17 For examples, lncRNA AK023391 accelerates the proliferation and invasion of GC cells via activating the PI3K/Akt pathway.18 LncRNA-UCA1 accelerates the tumorigenesis of GC via modulating the proteins and downstream mediators involving the PI3K/Akt pathway.19 LncRNA CRNDE plays an important role in promoting GC progression by activating the PI3K/Akt pathway.20 Thus, we attempted to determine whether the regulatory role of lncRNA-HCG18 on GC is associated with the PI3K/Akt pathway.
In this study, we investigated the regulatory effect of lncRNA-HCG18 on GC and the underlying mechanism involving the PI3K/Akt pathway. The expression of lncRNA-HCG18 was detected in GC tissues and cell lines. Functional experiments were performed to determine the role of lncRNA-HCG18 on the tumorigenesis of GC in vitro and in vivo. Our findings may reveal a novel therapeutic target for GC, and provide a new insight into the underlying mechanism for the treatment of GC.
Materials and Methods
Tissue Samples
Forty-five patients with GC (29 males and 16 females; average age 59.12 ± 6.79 years) were screened from April 2017 to May 2018 in our hospital. Paired tumor tissues and adjacent normal tissues (ANT) were obtained from patients underwent surgical resection. Patients had not received preoperative adjuvant chemotherapy, radiotherapy, targeted therapy or immunotherapy before surgical resection. Pathological diagnosis was performed in accordance with the WHO classification criteria of digestive system tumors (2010 edition). This study was approved by the Local ethics committee, and informed consents were obtained from all patients.
Cell Culture
Normal human gastric epithelial cell line (GES-1) and GC cell lines (MKN45, MGC803, AGS) were obtained from Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, logan, Utah, USA) containing 10% fetal bovine serum (FBS; Hyclone) and cultured in an incubator (MCO-15AC, SANYO, Japan) at 37°C. When reaching 90% confluence, cells were passaged at a ratio of 1:3. Logarithmic growth phase cells were used for subsequent experiments.
Cell Transfection
LncRNA-HCG18 siRNAs (si-HGC18-1 and si-HGC18-2) and siRNA negative control (si-NC) were purchased from Sangon Biotech Co., Ltd (Shanghai, China). AGS and MGC803 cells were transfected with si-HCG18 or si-NC using Lipofectamine 2000 reagent (Invitrogen, Waltham, MA, USA). Cells without transfection were considered as the Blank control. In addition, the transfected AGS cells were further treated with an activator of PI3K/Akt pathway 740 Y-P (50 μg/mL, TocrisBioscience, Ellisville, MO, USA).
qRT-PCR
Total RNA was extracted from GC tissues and cells using TRIzolTMPlus RNA Isolation Reagents (Invitrogen, Waltham, MA, USA). A Reverse Transcription Kit (Takara, Otsu, Japan) was used for reverse transcription. qRT-PCR was performed on an ABI 7500HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) under the following conditions: 95°C for 10 min, 40 cycles at 95°C for 10 s, 60°C for 20 s and 72°C for 34 s. GAPDH was used as the internal control. The mRNA expression level was calculated using the 2−ΔΔCt method. The primer sequences were HCG18, forward: 5′-ATCCTGCCAATAGATGCTGCTCAC-3, reverse: 5′-AGCCACCTTGGTCTCCAGTCTC-3′; GAPDH, forward: 5′-TGACGTGCCGCCTGGAGAAAC-3, reverse: 5′-CCGGCATCGAAGGTGGAAGAG-3′.
Western Blot
Total proteins were isolated from cells using RIPA Lysis Buffer (Elabscience, Wuhan, China), and then quantified using a BCA Protein Assay Kit (ThermoFisher, SanJose, CA, USA). The protein samples were separated by 10% SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane. The membrane was then blocked in 5.0% non-fat milk for 45 min and incubated with primary antibody at 4°C overnight. The primary antibodies included anti-GAPDH (1:1000, ab9485, Abcam, Cambridge, England), -PI3K (1:1000, 4292, CST, Danvers, MA, USA), -p-PI3K (1:1000, 17,366, CST), -Akt (1:1000, 9272, CST), -p-Akt (1:1000, 4060, CST), -MMP-2 (1:1000, ab97779, Abcam), -MMP-9 (1:1000, ab38898, Abcam,), -Bcl-2 (1:1000, ab32124, Abcam), and -Bax (1:1000, ab32503, Abcam). Subsequently, the membrane was incubated with HRP-conjugated goat anti-rabbit IgG (1:10,000, G-21234, Invitrogen) for 1 h at 25°C. Protein bands were visualized using a Chemiluminescent Substrate Kit (Bio-Rad, Hercules, CA, USA). GAPDH was used as the internal control.
MTT Assay
Cells were seeded into 96-well plates (6 × 103 cells/well, 200 μL/well) and cultured in an incubator at 37°C for 0, 24, 48, and 72 h, respectively. MTT (5 mg/mL, 20 μL/well, Sigma, San Antonio, TX, USA) was then added into each well. After 4 h of incubation, DMSO (150 μL/well) was added to terminate the reaction. The optical density at 495 nm (OD495) was measured by a Microplate Reader (Applied Biosystems).
Annexin V-Propidium Iodide (PI) Double Staining
Cell apoptosis was detected using an Annexin V-fluorescein isothiocyanate (FITC)/PI Kit (Invitrogen). Briefly, cells were seeded into 6-well plates (1 × 105 cells/well, 500 µL/well) and then stained with 5 µL Annexin V‐FITC and 5 mL PI for 10 min at 25°C in the dark. The cell apoptosis was assessed by a MUSETM flow cytometer (MerckMillipore, Billerica, MA, USA).
Wound Healing Assay
Cells were seeded into 6-well plates (1 × 106 cells/well), and cultured until 90% confluence. A scratch was then made using a sterile pipette tip. After 48 h of culturing, the wound area was photographed and measured under an optical microscope (Olympus Ckx53, Tokyo, Japan) using Image J Software.
Transwell Invasion Assay
Cell invasion was detected using a Transwell Chamber (BD Biosciences, Franklin Lakes, NJ, USA). Briefly, cells were seeded in the upper chamber pre-coated with matrigel. The medium containing 10% FBS was added to the lower chamber. After 48 h of culturing, cells on the upper chamber were removed. Cells in the lower chamber were stained with 0.1% crystal violet for 30 min, and counted under a microscope (Olympus Ckx53) at five randomly selected fields.
Establishment of Xenograft Tumor Model in Mice
Twelve Male nude mice (BALB/c, 4 weeks old) were obtained from Shanghai experimental animal center, Chinese academy of sciences (Shanghai, China). Mice were randomly divided into three groups: Mock, si-NC and si-HCG18 group (n = 4 each group). AGS cells were subcutaneously injected into the left axilla (1 × 107 cells/mice, 0.2mL/mice). The longest diameter (L) and the shortest diameter (W) of the tumor xenograft were measured with a vernier caliper every 7 days after injection. The tumor volume was calculated using the following formula: V = L ×W2/2. At the end of the 4th-week post-injection, mice were anesthetized with pentobarbital sodium (60 mg/kg), and killed by cervical dislocation. The tumor xenografts were dissected completely and then weighed. Animal experiments were performed in accordance with the “Guidelines for the Care and Use of Laboratory Animals” published by the National Institutes of Health and were approved by Linyi Cancer Hospital’s ethics committee.
Statistical Analysis
All experiments were performed in triplicates and repeated at least three times. Data were presented as means ± standard deviation (SD) and analyzed by SPSS 22.0 statistical software (SPSS Inc., Chicago, IL). Student’s t-test was used to analyze the differences between the two groups. One-way ANOVA followed by Tukey’s post hoc test was used to analyze the differences among multi-groups. Differences were considered statistically significant at P < 0.05.
Results
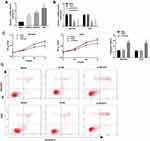
LncRNA-HCG18 Was Overexpressed in GC Tissues
The expression of lncRNA-HCG18 in tumor tissues (GC) and adjacent normal tissues (ANT) were detected by qRT-PCR. As shown in Figure 1A, the expression of lncRNA-HCG18 was significantly higher in the GC group than that in the ANT group (P < 0.0001). In addition, the expression of lncRNA-HCG18 was significantly higher in tumors at I/II stage than that in tumors at III/IV stage (P < 0.0001), and was significantly higher in tumors at T1-2 stage than that in tumors at T3-4 stage (P < 0.0001, Figure 1B and C). The above results indicated that lncRNA-HCG18 was overexpressed in GC tissues, and overexpression of lncRNA-HCG18 was positively correlated with the stage of tumor node metastasis and invasion depth.
Silencing of lncRNA-HCG18 Repressed the Proliferation and Induced the Apoptosis of GC Cells
The expression of lncRNA-HCG18 was detected in three GC cell lines (MKN45, MGC803, AGS) and normal human gastric epithelial cells (GES-1). qRT-PCR showed that the expression of lncRNA-HCG18 expression in GC cell lines (MKN45, MGC803, AGS) was significantly higher than that in GES-1 cells (P < 0.001, Figure 2A). MGC803 and AGS cells with relatively high lncRNA-HCG18 expression were used for the following experiments. LncRNA-HCG18 was then silenced in MGC803 and AGS cells by the transfection of si-HCG18-1/2. qRT-PCR showed that the expression of lncRNA-HCG18 in the si-HCG18-1/2 group was significantly lower than that in the Blank group (P < 0.01, Figure 2B). si-HCG18-1 with relatively high silence efficiency was used for the subsequent experiments. MTT assay showed that the OD495 values at 48 and 72 h post-culturing were significantly decreased in the si-HCG18 group compared with Blank group (P < 0.05, Figure 2C). In contrast to cell proliferation, si-HCG18 promoted the apoptosis of MGC803 and AGS cells (P < 0.01, Figure 2D).
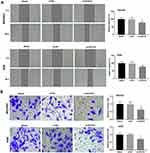
Silencing of lncRNA-HCG18 Inhibited the Migration and Invasion of GC Cells
Wound healing and transwell assay were performed to detect the migration and invasion of MGC803 and AGS cells, respectively. Both the migration and invasion ability of cells in si-HCG18 group were significantly decreased compared with those in the Blank group (P < 0.01, Figure 3A and B).
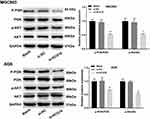
Silencing of lncRNA-HCG18 Blocked the PI3K/Akt Pathway
In order to explore the regulatory mechanism of lncRNA-HCG18 involving the PI3K/Akt pathway, the expression of PI3K/Akt pathway-related proteins was detected by Western blot. The protein expression levels of p-AKT/AKT and p-PI3K/PI3K in MGC803 and AGS cells were significantly decreased in the si-HCG18 group compared with the Blank group (P < 0.01, Figure 4).
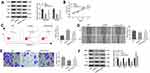
Activation of the PI3K/Akt Pathway Reversed the Anti-Tumor Effect of LncRNA-HCG18 Silencing on GC Cells
In order to investigate whether the effects of lncRNA-HCG18 on GC cells were associated with the PI3K/Akt pathway, an activator of PI3K/Akt pathway, 740 Y-P was used to activate the PI3K/Akt pathway in AGS cells. As shown in Figure 5A, 740 Y-P reversed the inhibiting effect of si-HCG18 on the PI3K/Akt pathway (P < 0.05). In addition, 740 Y-P reversed the inhibiting effects of si-HCG18 on the proliferation, migration, invasion of AGS cells, and reversed the promoting effect of si-HCG18 on the apoptosis of AGS cells (P < 0.05, Figure 5B–E). The expression of apoptosis- and migration-related proteins was further detected by Western blot. As shown in Figure 5F, the protein expression of MMP-2, MMP-9 and Bcl-2 was significantly decreased, and the protein expression of Bax was significantly increased in si-HCG18 group compared with those in si-NC group (P < 0.01). The intervention of 740 Y-P significantly reversed the expression of the above proteins in AGS cells (P < 0.05, Figure 5F).
Silencing of lncRNA-HCG18 Inhibited the Growth of GC Xenografts in Mice
A human tumor xenograft model in mice was established to evaluate the anti-tumor effect of lncRNA-HCG18 silencing on GC in vivo. As shown in Figure 6A–C, the tumor volume and weight were significantly decreased in the si-HCG18 group compared with the Mock group (P < 0.01). These results demonstrated that the silencing of lncRNA-HCG18 repressed the growth of GC xenografts in mice.
Discussion
The development of GC is a complicated biological process involving multiple factors and signaling pathways. Previous studies have proved that lncRNAs are dysregulated in GC, such as the up-regulation of lncRNA-HOAIR and -ANRIL, and the down-regulation of lncRNA-MEG3 and -GAS5.21–24 Huang et al25 have shown that the expression of lncRNA-LINC00673 is up-regulated in GC and positively related to the poor prognosis of GC patients. Du et al26 have found that the expression of lncRNA-CRNDE is increased in GC tissues and cell lines, and is positively associated with the depth of invasion, TNM stage and lymph node metastasis. Sun et al23 have revealed that lncRNA-GAS5 acts as a tumor-inhibitor in GC and a negative prognostic factor in GC patients. However, the specific role of lncRNA-HCG18 in GC remains unclear. In this study, the expression of lncRNA-HCG18 was significantly up-regulated in GC tumor tissues and was positively associated with the stage of tumor node metastasis and invasion depth. Similar with the previous researches, our findings indicate that lncRNA-HCG18 is a tumor promoter in GC.
LncRNA-HCG18 plays a promoting role in the development of IDD, which is involved in the proliferation and apoptosis of NP cells.14 However, researches on the regulatory effects of lncRNA-HCG18 on GC cells are limited. Xu et al27 have demonstrated that NOTCH1 represses the progression of bladder cancer via cooperating with lncRNA-HCG18. Si-Yu et al15 have found that the up-regulation of lncRNA-HCG18 promotes the proliferation and invasion, inhibits the apoptosis of hepatocarcinoma (HCC) cells. Qu et al28 have illustrated that lncRNA-HCG18 promotes the proliferation and migration of non-small cell lung cancer (NSCLC) cells. Consistent with the function of lncRNA-HCG18 in HCC and NSCLC, 为lncRNA-HCG18 promoted the proliferation, migration, invasion, and inhibited apoptosis of GC cells. Our results indicate that lncRNA-HCG18 is an oncogenic factor in GC. Furthermore, a tumor xenograft model in mice was established to evaluate the role of lncRNA-HCG18 on GC in vivo. In consistent with in vitro results, we found that silencing of lncRNA-HCG18 inhibited the growth of GC xenografts in mice.
PI3K/Akt pathway exerts critical effects in modulating the biological processes of cell growth, survival, proliferation, invasion and apoptosis.29 PI3K/Akt pathway is involved in many types of human cancers including GC.30–32 The aberrant activation of the PI3K/Akt pathway is closely related to the progression of GC.16 Li et al33 have shown that LEMD1 promotes the proliferation of GC cells through activating the PI3K/Akt pathway. Huang et al18 have revealed that lncRNA-AK023391 promotes the tumorigenesis of GC through activating the PI3K/Akt pathway. Du et al20 have proved that lncRNA-CRNDE promotes the proliferation, migration and invasion of GC cells through activating the PI3K/Akt pathway.25 In this study, we found that silencing of si-HCG18 blocked the PI3K/Akt pathway in GC cells. In order to further identify whether the effects of lncRNA-HCG18 on GC cells were associated with the PI3K/Akt pathway, 740 Y-P (an activator of PI3K/Akt pathway) was used to treat GC cells. The results showed that 740 Y-P significantly reversed the anti-tumor effect of si-HCG18 on AGS cells. We speculate that silencing of lncRNA-HCG18 may promote the proliferation, migration and invasion, and inhibit the apoptosis of GC cells by blocking the PI3K/Akt pathway.
Conclusions
In summary, lncRNA-HCG18 was up-regulated in GC tissues and cell lines. Silencing of lncRNA-HCG18 promoted the proliferation, migration and invasion, and inhibited the apoptosis of GC cells by blocking the PI3K/Akt pathway. Silencing of lncRNA-HCG18 could also inhibit the growth of GC xenografts in mice. LncRNA-HCG18 is an oncogene in GC, which may be used as a novel therapeutic target in clinical practice.
Ethics Approval and Consent to Participate
This study was conducted after obtaining approval of Linyi Cancer Hospital’s ethical committee and written informed consent from the patients.
Animal experiments were performed in accordance with the “Guidelines for the Care and Use of Laboratory Animals” published by the National Institutes of Health and were approved by Linyi Cancer Hospital’s ethics committee.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Pisan P, Parkin D, Pisani P, Jemal A. Global cancer statistics. CA: Cancer J Clinicians. 2011;49(1):33.
2. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomark Prev. 2014;23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
3. Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107(3):230–236. doi:10.1002/jso.23262
4. Rona KA, Schwameis K, Zehetner J, et al. Gastric cancer in the young: an advanced disease with poor prognostic features. J Surg Oncol. 2017;115(4):371–375. doi:10.1002/jso.v115.4
5. Li R, Liu B, Gao J. The application of nanoparticles in diagnosis and theranostics of gastric cancer. Cancer Lett. 2016;386:123. doi:10.1016/j.canlet.2016.10.032
6. Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer. 2015;136(8):1921–1930. doi:10.1002/ijc.29227
7. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521
8. Chang J, Xu W, Du X, Hou J. MALAT1 silencing suppresses prostate cancer progression by upregulating miR-1 and downregulating KRAS. Onco Targets Ther. 2018;11:3461–3473. doi:10.2147/OTT.S164131
9. Liu M, Jia J, Wang X, Liu Y, Wang C. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol Ther. 2018;19(5). doi:10.1080/15384047.2018.1423921
10. Zhang -J-J, Wang -D-D, Du C-X, Wang Y. Long noncoding RNA ANRIL promotes cervical cancer development by acting as a sponge of miR-186. Oncol Res. 2018;26(3):345–352. doi:10.3727/096504017X14953948675449
11. Zhao Y, Guo Q, Chen J, Hu J, Wang S, Sun Y. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigation. Oncol Rep. 2014;31(1):358–364. doi:10.3892/or.2013.2850
12. Li P, Xue W-J, Feng Y, Mao Q-S. Long non-coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8(8):3522–3529.
13. Wu X, Zhang P, Zhu H, Li S, Chen X, Shi L. Long noncoding RNA FEZF1-AS1 indicates a poor prognosis of gastric cancer and promotes tumorigenesis via activation of Wnt signaling pathway. Biomed Pharmacother. 2017;96:1103–1108. doi:10.1016/j.biopha.2017.11.113
14. Xi Y, Jiang T, Wang W, et al. Long non-coding HCG18 promotes intervertebral disc degeneration by sponging miR-146a-5p and regulating TRAF6 expression. Sci Rep. 2017;7(1):13234. doi:10.1038/s41598-017-13364-6
15. Si-Yu C, Jian-an C, Li-wen L, et al. Effects of long noncoding RNA HCG18 on biological activity of hepatoma cells. Henan Med Res. 2019.
16. Wei Z, Shaoqing J, Rongrong J, Ming C. Long non-coding RNA-mediated regulation of signaling pathways in gastric cancer. Clin Chem Lab Med. 2018;56(11):1828–1837.
17. Ooi CH, Ivanova T, Wu J, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5(10):e1000676. doi:10.1371/journal.pgen.1000676
18. Huang Y, Zhang J, Hou L, et al. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36(1):194. doi:10.1186/s13046-017-0666-2
19. Li C, Liang G, Yang S, et al. Dysregulated lncRNA-UCA1 contributes to the progression of gastric cancer through regulation of the PI3K-Akt-mTOR signaling pathway. Oncotarget. 2017;8(55):93476–93491. doi:10.18632/oncotarget.19281
20. Du D-X, Lian D-B, Amin B-H, Yan W. Long non-coding RNA CRNDE is a novel tumor promoter by modulating PI3K/AKT signal pathways in human gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(23):5392–5398. doi:10.26355/eurrev_201712_13925
21. X-h L, Sun M, F-q N, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014. 13(1):92.
22. Shenghong Z, Shuling C, Guang Y, et al. Long noncoding RNA HOTAIR as an independent prognostic marker in cancer: a meta-analysis. PLoS One. 2014. 9(8):e105538.
23. Sun M, Jin F-Y, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14(1):319. doi:10.1186/1471-2407-14-319
24. Sun M, Xia R, Jin F, et al. Downregulated long noncoding RNAMEG3is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumor Biol. 2014;35(2):1065–1073. doi:10.1007/s13277-013-1142-z
25. Huang M, Hou J, Wang Y, et al. Long noncoding RNA LINC00673 is activated by SP1 and exerts oncogenic properties by interacting with LSD1 and EZH2 in gastric cancer. Mol Ther. 1014-1026;25(4).
26. Du D-X, Lian D-B, Amin B-H, Yan W. Long non-coding RNA CRNDE is a novel tumor promoter by modulating PI3K/AKT signal pathways in human gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(23):5392–5398.
27. Xu Z, Huang B, Zhang Q, He X, Wei H, Zhang D. NOTCH1 regulates the proliferation and migration of bladder cancer cells by cooperating with long non-coding RNA HCG18 and microRNA-34c-5p. J Cell Biochem. 2019;120(4):6596–6604. doi:10.1002/jcb.v120.4
28. Qu bao liang MHB, Qiang GJ. LncRNA HCG18 regulates the proliferation and metastasis of non-small cell lung cancer cells through targeting miR-17-5p/HMGA2 axis. Chin J Cancer Biother. 2019;26(4):409–416.
29. Bartholomeusz C, Gonzalez-angulo AM. Targeting the PI3K signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):121–130. doi:10.1517/14728222.2011.644788
30. Davis WJ, Lehmann PZ, Li W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front Cell Develop Biol. 2015;3:24.
31. Brown KK, Toker A. The phosphoinositide 3-kinase pathway and therapy resistance in cancer. F1000Prime Rep. 2015;7.
32. Zheng D, Zhu G, Liao S, et al. Dysregulation of the PI3K/Akt signaling pathway affects cell cycle and apoptosis of side population cells in nasopharyngeal carcinoma. Oncol Lett. 2015;10(1):182–188.
33. Li Q, Ge Y, Chen X, et al. LEM domain containing 1 promotes proliferation via activating the PI3K/Akt signaling pathway in gastric cancer. J Cell Biochem. 2019;120(9):15190–15201.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.