Back to Journals » Cancer Management and Research » Volume 12
Silencing of lncRNA LINC00346 Inhibits the Proliferation and Promotes the Apoptosis of Colorectal Cancer Cells Through Inhibiting JAK1/STAT3 Signaling
Received 12 February 2020
Accepted for publication 26 May 2020
Published 16 June 2020 Volume 2020:12 Pages 4605—4614
DOI https://doi.org/10.2147/CMAR.S249491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
This paper has been retracted.
Dan Li, 1 Shuang Wen 2
1Department of Pathology, Tianjin Baodi Hospital, Baodi Clinical College of Tianjin Medical University, Tianjin City 301800, People’s Republic of China; 2Department of Pathology, The Friendship Hospital of Dalian, Dalian City, Liaoning Province 116000, People’s Republic of China
Correspondence: Shuang Wen
Department of Pathology, The Friendship Hospital of Dalian, No. 8, Sanba Square, Zhongshan District, Dalian City, Liaoning Province 116000, People’s Republic of China
Tel +86-0411-82718822
Email [email protected]
Purpose: The study was aimed to investigate the effect and mechanism of lncRNA LINC00346 on cell proliferation and apoptosis of colorectal cancer (CRC).
Methods: The expression of lncRNA LINC00346 in CRC tissues and cells was detected by qRT-PCR. LINC00346 was overexpressed and silenced in HT29 and LoVo cells by the transfection of pcDNA-LINC00346 and si-LINC00346. The proliferation of CRC cells was detected by CCK-8 and colony-formation assay. The apoptosis was detected by flow cytometry assay. The expression of apoptosis-associated proteins (Caspase-3, Bcl-2, Bax) and JAK1/STAT3 signaling-associated proteins (JAK1, STAT3, p-JAK1, p-STAT3) was detected by Western blot. The tumor growth was detected in mice subcutaneous injected with transfected HT29 cells.
Results: LINC00346 was significantly upregulated in CRC tissues and cells. Overexpression of LINC00346 significantly increased the OD 450 values, number of colonies, decreased the apoptosis rate, upregulated Bcl-2, and downregulated Caspase-3 and Bax in HT29 and LoVo cells. Knockdown of LINC00346 exerted opposite results of proliferation and apoptosis on HT29 and LoVo cells. The expression levels of JAK1/JAK1 and p-STAT3/STAT3 were upregulated by LINC00346 overexpression. Tofacitinib (JAK1 inhibitor) reversed the tumor-promoting effect of LINC00346 overexpression on CRC cells. In vivo experiments further validated that LINC00346 overexpression promoted the growth of CRC xenograft tumors.
Conclusion: LncRNA LINC00346 promoted the proliferation and inhibited the apoptosis of CRC cells through activating JAK1/STAT3 signaling.
Keywords: LncRNA LINC00346, colorectal cancer, proliferation, apoptosis, JAK1/STAT3
Introduction
Colorectal cancer (CRC) is a frequent malignancy globally and the leading cause of death in patients.1 Most CRC patients have reportedly died from distant metastases, particularly liver metastases.2 Surgery and chemotherapy are currently common treatments, but traditional chemotherapy for CRC has many limitations, including using highly toxic drugs caused adverse side effects.3 Thus, it is necessary to investigate the mechanisms and targets associated with the treatment of CRC.
Long non-coding RNAs (lncRNAs) are a class of important non-coding RNA with limited or no protein-coding capacity.4 LncRNAs have been proved to be a major regulator of gene expression, and they can play key roles in all kinds of biological functions and cancers processes.5 Many lncRNAs including MALAT1,6 UCA1,7 and TUG1,8 are upregulated in CRC, and play important roles in promoting CRC development and metastasis. LncRNA LINC00346, belongs to the intergenic lncRNA, has been found to be involved in many cancers. LINC00346 is upregulated in bladder cancer tissues, knockdown of LINC00346 inhibits the proliferation and migration of T24 and SW780 cells, and induces cell cycle arrest and apoptosis.9 Shi et al,10 have reported that LINC00346 overexpression remarkably enhances the proliferation and tumorigenesis of pancreatic cancer cells. However, the regulatory effects of LINC00346 in CRC are unclear.
JAK/STAT3 signaling pathway participates in various physiological processes, such as differentiation, cell growth, hematopoiesis and immune function.11 More and more evidences indicate that abnormalities in the JAK1/STAT3 signaling are crucial in tumorigenesis. For example, Xiong et al,12 have demonstrated that STAT3, JAK1 and JAK2 are involved in CRC cell growth, invasion, survival and migration. JAK2, STAT1, STAT3 and STAT6 are related with colon cancer and STAT3, STAT4 and STAT6 are related with rectal cancer.13 In addition, the expression of LINC00346 is increased in non-small cell lung cancer (NSCLC) cells and tissues, and LINC00346 promotes the proliferation and inhibits the apoptosis of NSCLC cells through regulating the JAK/STAT3 signaling pathway.14 However, the relationship between JAK1/STAT3 and LINC00346 in CRC is still unclear.
In this study, LINC00346 expression was detected in CRC tissues and cells. The regulatory effects of LINC00346 on the proliferation and apoptosis of CRC cells were analyzed. Then the mechanism of LINC00346 involving JAK1/STAT3 signaling was evaluated. Our findings may reveal a potential therapeutic target for CRC.
Materials and Methods
Tissue Samples
Tumor tissues and adjacent normal tissues were obtained from 52 CRC patients (22 males and 30 females, aged 35–53 years) from January 2017 to December 2018. This study was permitted by our hospital ethics committee, and informed consents were obtained from all patients.
Cell Culture
Human colon cancer cell lines (HT29 and LoVo) and normal human colon epithelial cell line FHC were obtained from American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in DMEM (GIBCO, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS) (GIBCO), 100 IU/mL penicillin and 100 mg/mL streptomycin (GIBCO). Cells were maintained at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Cell Transfection
SiRNA LINC00346 (si-LINC00346) and siRNA negative control (si-NC) were purchased by Invitrogen (Invitrogen, Carlsbad, CA, USA). Full length fragments of LINC00346 and negative control coding sequences were amplified by PCR and constructed into pcDNA3.1 vector (Invitrogen) to generate pcDNA-LINC00346 and pcDNA-NC. HT29 and LoVo cells were plated in 24-well plates (1 × 105 cells/well) and incubated at 37°C for 24 h. Then the above plasmids and si-RNAs were transfected into HT29 and LoVo cells using Lipofectamine 3000 (Invitrogen, USA). After 48 h of transfection, cells were used for further assays. In addition, transfected cells were treated with JAK1 inhibitor Tofacitinib (#14,703, Cell Signaling Technology, MA, USA) for 30 min before transfection.
qRT-PCR
Total RNA was extracted from tissues and cells using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). RNA was reverse transcribed to cDNA using Takara PrimeScript RT reagent kit gDNA Eraser. PCR was performed with the following conditions: an initial of 10 min at 95°C, followed by 40 cycles of 95°C for 10 s, 60°C for 30 s, 72°C for 34 s. GAPDH was used as an internal control. The relative expression level was calculated using the 2−ΔΔCT method. The primer sequences are shown in Table 1.
 |
Table 1 Primer Sequences Used in qRT-PCR |
Western Blot
Cells were lysed by ice-cold lysis buffer. The concentration of protein was measured using BCA kit (Invitrogen, Carlsbad, CA, USA). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene fluoride (PVDF) membranes. Then the membrane was incubated with diluted primary antibody overnight at 4°C. Primary antibodies are shown as follows: anti-phospho-JAK1 (1:1000, #74,129), anti-JAK1 (1:1000, #3344), anti-phospho-STAT3 (1:1000, #9145), anti-STAT3 (1:1000, #12,640), anti-GAPDH (1:1000, #5174), anti-Bax (1:1000, 14796S) (Cell Signaling Technology); anti-Caspase-3 (1:1000, ab197202), and anti-Bcl-2 (1:1000, ab32124) (Abcam, UK). Followed by three times of washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (anti-rabbit, #7074, Cell Signaling Technology) for 1 h at 37°C. The protein bands were visualized by ECL exposure solution, and quantified by a gel imaging system.
Cell Viability Assay
Cells were seeded in 96-well plates (2 × 104), and cultured for 24, 48, 72 and 96 h. Then 10 μL CCK-8 solution (BD Biosciences, USA) was added to each well, and incubated for 2 h at 37°C. The optical density 450 nm (OD450) was measured using a microplate reader.
Colony-Formation Assay
Cells were seeded in 6-well plates, and cultured for 14 days. After washed twice with PBS, the colonies were fixed with methanol for 15 min, and stained with crystal violet for 15 min. Positive stained colonies (more than 30 cells) were observed under an inverted microscope (Olympus Ckx53, Japan), and the number was counted randomly using Image J (1.48V).
Flow Cytometry Assay
Cells were seeded in 96-well plates, and cultured for 24 h. After washed 3 times with PBS, cells were resuspended and adjusted to 1 × 106 cells/mL. Then 500 μL cells were stained with 5 μL V-FITC and 10 μL of PI for 20 min in the dark. The apoptosis rate was detected by flow cytometry.
Establishment of Tumor Model in Mice
A total of 24 male nude mice (BALB/c, 4 weeks old) were purchased from Huafukang Biotechnology Co, Ltd. (Beijing, China). Mice were fed in an SPF environment (temperature 25–27°C, humidity 45–50%) with free access to food and water. Mice were randomly divided into 4 groups, including pcDNA-LINC00346 group, pcDNA-NC group, pcDNA-LINC00346 + Tofacitinib, and BLANK group (6 mice in each group). Approximately 1 × 106 prepared transfected HT29 cells were subcutaneous injected into the left armpit of mice. The volume of HT29 xenografts in mice was measured weekly until the 4th week according to the following formula: volume = 1/2 (length × width2). The mice were killed by neck dislocation after 4 weeks of injection. The tumor was removed and weighed. All animal experimental procedures were permitted by the institutional animal care and ethics committee of the Friendship Hospital of Dalian. Animal testing procedures were performed on the basis of the “Guidelines for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Statistical Analysis
Each assay was performed at least three times. Data statistical analysis was performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviations. Comparison between two groups was determined by t-test, and comparison among more than two groups was determined by One-Way ANOVA, followed LSD test. Survival analysis was performed using Kaplan-Meier curve and analyzed using the Log rank test. A P value less than 0.05 was considered to be significant.
Results
LINC00346 Is Overexpressed in CRC Tissues and Cell Lines
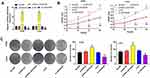
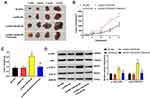
LINC00346 expression was detected in 52 cases of CRC tissues and normal tissues by using qRT-PCR. LINC00346 expression was significantly higher in CRC tissues compared with normal tissues (P < 0.001) (Figure 1A). Simultaneously, LINC00346 expression was detected in CRC cell lines HT29 and LoVo, as well as normal human colon epithelial cell line FHC. The results showed that the expression of LINC00346 was upregulated in CRC cell lines (P < 0.01) (Figure 1B). Kaplan-Meier survival analysis showed that the overall survival rate of CRC patients with high expression of lncRNA LINC00346 was significantly shortened compared with those with low expression (P = 0.0046) (Figure 1C). Then the correlation between the expression of LINC00346 and clinicopathological features of CRC patients was further analyzed. As presented in Table 2, LINC00346 expression was positively related to TNM stage and lymphatic metastasis (P < 0.05), but not correlated with age, gender, or differentiation (P > 0.05).
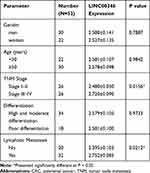 |
Table 2 Correlation Between the Expression of LINC00346 and Clinicopathological Features of CRC |
Silencing of LINC00346 Inhibits CRC Cell Proliferation
To further confirm the effect of LINC00346 on the proliferation of CRC cells, lncRNA LINC00346 was overexpressed by the transfection of pcDNA-LINC00346, and silenced by the transfection of si-LINC00346 (P < 0.001) (Figure 2A). CCK-8 assay showed that silencing of LINC00346 significantly decreased the OD450 values of HT29 and LoVo cells at 24, 48, 72, and 96 h post-culturing (P < 0.05). On the contrary, overexpression of LINC00346 significantly increased the OD450 values of HT29 and LoVo cells (P < 0.05) (Figure 2B). In addition, colony-formation assay showed that silencing of LINC00346 remarkably decreased the number of colonies of HT29 and LoVo cells, and overexpression of LINC00346 significantly increased the number of colonies (P < 0.05) (Figure 2C).
Silencing of LINC00346 Promotes CRC Cell Apoptosis
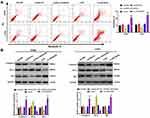
To research the function of lncRNA LINC00346 on CRC cell apoptosis, the apoptosis rate was detected by flow cytometry assay. As shown in Figure 3A, the apoptosis rate of si-LINC00346 group was significantly increased in HT29 and LoVo cells (P < 0.01), and the apoptosis rate of pcDNA-LINC00346 group was significantly decreased (P < 0.01). In addition, the expression of apoptosis-related proteins, such as Caspase-3, Bax, and Bcl-2 was detected by Western blot. We found that the expression of Bcl-2 was elevated in pcDNA-LINC00346 group, but reduced in si-LINC00346 group. Furthermore, the expression of Caspase-3 and Bax were remarkably reduced in pcDNA-LINC00346 group, but elevated in si-LINC00346 group (Figure 3B).
Silencing of lncRNA LINC00346 Blocks JAK1/STAT3 Signaling in CRC Cells
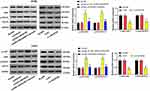
To further verify the effect of lncRNA LINC00346 on JAK1/STAT3 signaling, the expression of JAK1/STAT3 signaling-related proteins was detected in CRC cells using Western blot (Figure 4). We found that overexpression of LINC00346 promoted p-JAK1/JAK1 and p-STAT3/STAT3 expression, and LINC00346 knockdown inhibited p-JAK1/JAK1 and p-STAT3/STAT3 expression (P < 0.001). Additionally, JAK1/STAT3 signaling inhibitor Tofacitinib could reverse the promotion role of LINC00346 on p-JAK1/JAK1 and p-STAT3/STAT3 expression (P < 0.001).
LINC00346 Promotes CRC Cell Proliferation and Inhibits Apoptosis by Activating JAK1/STAT3 Signaling
We further verified whether the regulatory effects of LINC00346 on CRC cells are associated with JAK1/STAT3 signaling. CCK-8 and colony-formation assay showed that overexpression of LINC00346 remarkably increased the OD450 values and number of colonies of HT29 cells (P < 0.05). Note worthily, Tofacitinib significantly reversed the promoting effect of pcDNA-LINC00346 on the proliferation of HT29 cells (P < 0.05) (Figure 5A and B). In addition, flow cytometry assay showed that up-regulated LINC00346 remarkably decreased the apoptosis rate of HT29 cells (P < 0.001), while Tofacitinib significantly reversed the inhibiting effect of LINC00346 overexpression (P < 0.01) (Figure 5C). Tofacitinib also significantly reversed the reducing effect of LINC00346 overexpression on the expression of Caspase-3 and Bax, and the promoting effect on Bcl-2 expression (P < 0.01) (Figure 5D).
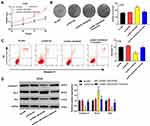
LINC00346 Promotes Tumor Growth of CRC in vivo
To further confirm the function of LINC00346 in CRC in vivo, HT29 cells were subcutaneously injected into nude mice. As shown in Figure 6A and B, the tumor volume in mice was increased with injection times. Overexpression of LINC00346 increased the tumor volume (P < 0.001) and Tofacitinib significantly reversed the promoting effect of LINC00346 on tumor volume (P < 0.001). Meanwhile, the tumor weight was remarkably increased in the pcDNA-LINC00346 group after 4 weeks, while reversed by Tofacitinib (P < 0.001) (Figure 6C). As expected, p-JAK1/JAK1 and p-STAT3/STAT3 expression was significantly decreased with Tofacitinib in mice injected with pcDNA-LINC00346-transfected HT29 cells (P < 0.001) (Figure 6D).
Discussion
LncRNAs are a class of non-coding RNAs involved in gene expression regulation and cancer pathogenesis. Emerging evidence has proved that some lncRNAs are upregulated in CRC, such as PANDAR,15 MALAT1,6 and ZFAS1.16 LINC00346 has been found to be up-regulated in CRC and increased the WBSCR22 expression via inhibiting miR-509-5p.17 LINC00346 is recurrently amplified and high-expressed in gastric cancer, and its expression is positively correlated with poor prognosis.18 Also, LINC00346 is upregulated in pancreatic tumor specimens and contributes to pancreatic cancer progression.19 Here, we found that expression of LINC00346 was significantly elevated in CRC cells and tissues. Our research is in accordance with previous research, and indicates that LINC00346 may act as tumor-promoting factor in CRC. Guo et al,20 have found that lncRNA FTX is significantly upregulated in CRC and significantly associated with differentiation grade, clinical stage, lymph vascular invasion, and poor survival. LncRNA ZFAS1 expression is upregulated in CRC, and upregulated ZFAS1 is correlated with advanced TNM stage, poor overall survival, and lymph nodes metastasis of CRC patients.16 Likewise, we found that LINC00346 overexpression was closely correlated with a decreased survival rate in CRC patients, and was positive associated with TNM stage and lymphatic metastasis. These results suggest that LINC00346 may be a potential prognostic factor for CRC.
Recent studies have suggested that LINC00346 plays vital role in cancer growth and apoptosis. LINC00346 silencing significantly suppresses cell viability, colony formation ability and DNA replication, and also downregulates the expression of cyclin D1, CDK 4 and CDK 6 in bladder cancer.9 LINC00346 silencing promotes the apoptosis and inhibits the proliferation of NSCLC cells.14 Furthermore, LINC00346 overexpression enhances the colony formation and proliferation of pancreatic cancer cells.10 Here, knockdown of LINC00346 remarkably reduced the OD450 value, number of colonies, increased the apoptosis rate, downregulated Bcl-2, and upregulated Caspase-3 and Bax in HT29 and LoVo cells. These results are consistent with previous studies, and indicate that silencing of LINC00346 inhibits the proliferation and promotes the apoptosis of CRC cells in vitro. To further research the role of LINC00346 in vivo, HT29 cells were injected into mice. We found that LINC00346 overexpression significantly increased the tumor volume and weight. The above phenomena illustrate that LINC00346 plays a tumor-promoting role in CRC, and LINC00346 silencing may be used as a potential therapeutic target for CRC.
Recently, more and more evidence indicates that the JAK/STAT3 signaling is involved in the progression of CRC.21 Blocking JAK/STAT3 signaling can not only inhibit CRC cell proliferation but also promote cell apoptosis.12 RPTS significantly induces cell apoptosis in SW480 CRC cells through inhibiting the IL-6/JAK-STAT3 signaling.22 Triptolide inhibits the colony formation, proliferation, and migration of colon cancer cells, also reduces the levels of JAK1 and phosphorylated STAT3.23 LncRNA AB073614 induces epithelial-mesenchymal transition of CRC cells by activation of the JAK/STAT3 pathway.24 In our study, we found that LINC00346 overexpression increased the expression of p-JAK1/JAK1 and p-STAT3/STAT3 in HT29 and LoVo cells and Tofacitinib (JAK/STAT3 signaling inhibitor) reversed the effect of up-regulated LINC00346 on the promoting of proliferation and the inhibiting of apoptosis of CRC cells. To sum up, LINC00346 may promote the proliferation and inhibit the apoptosis of CRC cells through activating JAK1/STAT3 signaling.
Conclusions
In conclusion, LINC00346 was upregulated in CRC tissues and cells. Silencing of LINC00346 inhibited the proliferation, and promoted the apoptosis of CRC cells through blocking JAK1/STATS3 singling. In addition, overexpression of LINC00346 promoted the tumor growth in mice. LINC00346 may be used as a therapeutic target for CRC.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of The Friendship Hospital of Dalian. Written informed consent was obtained from all subjects.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
None.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi:10.3322/caac.21220
2. Landreau P, Drouillard A, Launoy G, et al. Incidence and survival in late liver metastases of colorectal cancer. J Gastroenterol Hepatol. 2015;30(1):82–85. doi:10.1111/jgh.12685
3. Eng C. Toxic effects and their management: daily clinical challenges in the treatment of colorectal cancer. Nat Rev Clin Oncol. 2009;6(4):207–218. doi:10.1038/nrclinonc.2009.16
4. Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. doi:10.1038/nrg.2015.10
5. Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. doi:10.1038/onc.2017.184
6. Ji Q, Zhang L, Liu X, et al. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer. 2014;111(4):736. doi:10.1038/bjc.2014.383
7. Yu H, Ying-Nan Y, Heng-Heng Y, et al. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology. 2014;46(5):396–401. doi:10.1097/PAT.0000000000000125
8. Sun J, Ding C, Yang Z, et al. The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial-mesenchymal transition. J Transl Med. 2016;14(1):42. doi:10.1186/s12967-016-0786-z
9. Ye T, Ding W, Wang N, Huang H, Pan Y, Wei A. Long noncoding RNA linc00346 promotes the malignant phenotypes of bladder cancer. Biochem Biophys Res Commun. 2017;491(1):79–84. doi:10.1016/j.bbrc.2017.07.045
10. Shi W, Zhang C, Ning Z, et al. Long non-coding RNA LINC00346 promotes pancreatic cancer growth and gemcitabine resistance by sponging miR-188-3p to derepress BRD4 expression. J Exp Clin Cancer Res. 2019;38(1):60. doi:10.1186/s13046-019-1055-9
11. Niwa Y, Kanda H, Shikauchi Y, et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24(42):6406–6417. doi:10.1038/sj.onc.1208788
12. Xiong H, Zhang ZG, Tian XQ, et al. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10(3):287–297. doi:10.1593/neo.07971
13. Slattery ML, Lundgreen A, Kadlubar SA, Bondurant KL, Wolff RK. JAK/STAT/SOCS-signaling pathway and colon and rectal cancer. Mol Carcinog. 2013;52(2):155–166. doi:10.1002/mc.21841
14. Wang F, Chen JG, Wang LL, Yan ZZ, Chen SP, Wang XG. Up-regulation of LINC00346 inhibits proliferation of non-small cell lung cancer cells through mediating JAK-STAT3 signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(22):5135–5142. doi:10.26355/eurrev_201711_13830
15. Lu M, Liu Z, Li B, Wang G, Li D, Zhu Y. The high expression of long non-coding RNA PANDAR indicates a poor prognosis for colorectal cancer and promotes metastasis by EMT pathway. J Cancer Res Clin Oncol. 2017;143(1):1–11. doi:10.1007/s00432-016-2252-y
16. Xie S, Ge Q, Wang X, Sun X, Kang Y. Long non-coding RNA ZFAS1 sponges miR-484 to promote cell proliferation and invasion in colorectal cancer. Cell Cycle. 2017;17(5):1–21. doi:10.1080/15384101.2017.1386514
17. Zhao H, Su W, Sun Y, Wu Z. WBSCR22 competes with long non-coding RNA Linc00346 for miR-509-5p binding site to regulate cancer stem cell phenotypes of colorectal cancer. Biochem Genet. 2020;58:384–398. doi:10.1007/s10528-020-09949-y
18. Xu TP, Ma P, Wang WY, et al. KLF5 and MYC modulated LINC00346 contributes to gastric cancer progression through acting as a competing endogeous RNA and indicates poor outcome. Cell Death Differ. 2019;26(11):2179–2193. doi:10.1038/s41418-018-0236-y
19. Peng WX, He RZ, Zhang Z, Yang L, Mo YY. LINC00346 promotes pancreatic cancer progression through the CTCF-mediated Myc transcription. Oncogene. 2019;38(41):6770–6780. doi:10.1038/s41388-019-0918-z
20. Guo XB, Hua Z, Li C, et al. Biological significance of long non-coding RNA FTX expression in human colorectal cancer. Int J Clin Exp Med. 2015;8(9):15591.
21. Shu-Wei W, Yue-Ming S. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review). Int J Oncol. 2014;44(4):1032–1040. doi:10.3892/ijo.2014.2259
22. T W-J, Chen P, Z F-Y, et al. Effect of Rhizoma paridis total saponins on apoptosis of colorectal cancer cells and imbalance of the JAK/STAT3 molecular pathway induced by IL-6 suppression. Genet Mol Res. 2015;14(2):5793–5803. doi:10.4238/2015.May.29.11
23. Zhipeng W, Haifeng J, Ruodan X, Qibing M, Daiming F. Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression. Exp Mol Med. 2009;41(10):717. doi:10.3858/emm.2009.41.10.078
24. Xue J, Liao L, Yin F, Kuang H, Zhou X, Wang Y. LncRNA AB073614 induces epithelial- mesenchymal transition of colorectal cancer cells via regulating the JAK/STAT3 pathway. Cancer Biomark. 2018;21(4):849–858. doi:10.3233/CBM-170780
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.