Back to Journals » Cancer Management and Research » Volume 12
Silenced Myeloblastosis Protein Suppresses Oral Tongue Squamous Cell Carcinoma via the microRNA-130a/Cylindromatosis Axis
Authors Yang R, Shui Y, Hu S, Zhang K, Wang Y, Peng Y
Received 3 March 2020
Accepted for publication 29 June 2020
Published 5 August 2020 Volume 2020:12 Pages 6935—6946
DOI https://doi.org/10.2147/CMAR.S252340
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
This paper has been retracted.
Ran Yang, 1 Yusen Shui, 2 Shoushan Hu, 2 Kun Zhang, 2 Yuru Wang, 2 Yiran Peng 1
1State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Chengdu 610041, Sichuan, People’s Republic of China; 2Department of Pediatric Dentistry, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan, People’s Republic of China
Correspondence: Yiran Peng
Department of Pediatric Dentistry, West China Hospital of Stomatology, Sichuan University, 14 Renmin South Road Third Section, Chengdu 610041, Sichuan, People’s Republic of China
Tel/ Fax +86-28-85503527
Email [email protected]
Background: Oral tongue squamous cell carcinoma (OTSCC) represents oral epithelial cell damage. Myeloblastosis (MYB) is involved in OTSCC. This study tried to probe roles of MYB in OSCC with potential axis.
Methods: Expression of MYB and miR-130a in OTSCC was detected. Western blot analysis was utilized to determine epithelial–mesenchymal transition-related protein levels. Dual-luciferase reporter gene assay certified the target relation between miR-130a and CYLD. Moreover, xenograft tumors in nude mice were applied to confirm the in vitro experiments.
Results: Both MYB and miR-130a were highly expressed in OTSCC, which promoted cell growth. Meanwhile, silenced miR-130a discouraged cell development enhanced by overexpressed MYB. CYLD was poorly expressed in OTSCC and targeted by miR-130a. Additionally, MYB knockdown activated CYLD to suppress OTSCC by downregulating miR-130a.
Conclusion: Our experiment supported that silenced MYB suppressed OTSCC malignancy by inhibiting miR-130a and activating CYLD. This investigation may provide novel insights for OTSCC treatment.
Keywords: oral tongue squamous cell carcinoma, MYB, microRNA-130a, CYLD, epithelial–mesenchymal transition, proliferation
Introduction
The most common oral cancer is squamous cell carcinoma (SCC) and its highest occurrence is in the tongue.1 Oral squamous cell carcinoma (OSCC) represents a life-threatening cancer originating from oral mucosal epithelium and may exacerbate by tumor invasion, lymph node metastasis, oral-facial destruction and blood-borne dissemination.2 Oral tongue squamous cell carcinoma (OTSCC) is an aggressive tumor, with high rates of regional lymph node metastasis and local recurrence.3 Pathogenesis of OSCC is significantly involved in epithelial–mesenchymal transition (EMT).4 Cigarette smoking, betel-quid chewing and excessive alcohol drinking remain risk factors for OSCC.5 As the most prevalent oral cancer (OC), OSCC occupies 90% oral malignancy, with a relatively high survival rate of 80% at early stage but only 20–30% if diagnosed at the advanced stage.6 Despite the existing therapies for OSCC, such as chemoradiotherapy, epidermal growth factor receptor inhibitors, photodynamic therapy, cyclooxygenase-2 and surgery, all of them are potentially associated with a serious problem of non-specific cell death.3 In this context, biomarkers at the early stage and novel therapeutic targets for OSCC are in urgent need.
As a class of fundamental transcription factors, myeloblastosis (MYB) gene family is increasingly reported in the pathology and tumorigenesis in diverse human cancers, including brain cancer, breast cancer and leukemia.7 Recent researches have documented that c-MYB expression is elevated in laryngeal SCC (LSCC).8 Alterations of b-MYB expression mediate esophageal SCC (ESCC) cell growth, migration and invasion.9 Furthermore, MYB modulates OSCC cell survival, proliferation and invasion by binding to microRNA (miR)-1258 promoter.10 However, given the inadequate research, the solid mechanism of MYB in OSCC is not yet to know, let alone the interaction between MYB and its downstream genes. miRs are found to be indispensable biomarkers in cancer treatment as they can vary from oncogenes to tumor suppressors in different cancer progression.11 What’s more, dysregulated miRs also play a master role in cutaneous SCC, making them noticeable biomarkers in similar malignancies.12 Eichelmann et al have suggested that miR-130a-3p upregulation alters ESCC cell migration, adherence and apoptosis.13 miR-130a-3p expression is higher in aggressive OSCC tumors than that in the non-aggressive ones.14 Furthermore, growing evidence has supported the detrimental effects of the network of MYB and miR-130a on diverse human diseases. As a well-known oncogene in salivary adenoid cystic carcinoma, MYB positively correlates with miR-130a to make the malignancy more aggressive.15 In addition, the interaction between c-MYB and miR-130a accelerates gastric cancer progression by encouraging angiogenesis and cancer cell development.16 Moreover, CYLD was a target gene of miR-130a, and miR-130a mimic reduced the level of CYLD in hypertrophic scars and the derived fibroblasts.1 As a deubiquitinated enzyme which served as a tumor suppressor in OSCC, CYLD secured a favored prognosis and improved OSCC cell sensitivity to chemotherapeutic approaches.17 Collectively, according to the evidence listed above, it is reasonable to hypothesize that MYB might play a key role in OSCC development with its downstream pathways.
Materials and Methods
Ethics Statement
This study was approved and supervised by the ethics committee of West China Hospital of Stomatology, and conformed to Declaration of Helsinki. All the subjects signed the informed consent. The protocol was also approved by the Institutional Animal Care and Use Committee of West China Hospital of Stomatology. Significant efforts were made to reduce the animal number and suffering.
Sample Collection
Thirty OTSCC patients treated in West China Hospital of Stomatology were enlisted in this study for collection of OTSCC tissues and paracancerous tissues. The resected tissues were preserved at −80°C. Patients received preoperative chemotherapy, radiotherapy and immunotherapy would be excluded.
Cell Culture
Human oral keratinocytes (HOK, GD-C10819440, Shanghai Guandao Bioengineering Co., Ltd., Shanghai, China) and OTSCC cell lines CAL-27 (CC0701, Guangzhou Cellcook Biotech Co., Ltd., Guangzhou, China), SCC-4 (CC0705, Guangzhou Cellcook Biotech), SCC-9 (CC0704, Guangzhou Cellcook Biotech) and SCC-25 (ZY-H272, Shanghai Zeye Biotechnology Co., Ltd., Shanghai, China), and HEK293T cells (KCB200744YJ, Kunming Cell Bank of Chinese Academy of Sciences, Kunming, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Company, Grand Island, NY, USA) consisting 10% fetal bovine serum, 100 U/mL penicillin and 100 mg/mL streptomycin at 37°C with 5% CO2.
Cell Grouping and Transfection
MYB overexpressing plasmids (MYB) were constructed using pIRES2-CMV, which were designed by Genepharma Co., Ltd. (Shanghai, China), with a control scrambled sequence served as the control. MYB small interfere (si) RNAs (si-MYB-1 and si-MYB-2) were designed and synthesized by Genepharma to silence MYB expression. miR-130a mimic, miR-130a inhibitor and their negative control (NC) were also designed and synthesized by Genepharma. According to the instructions of the Lipofectamine 2000 (Invitrogen, Carlsbad, USA), CAL-27 cells were transfected with si-MYB-1, si-MYB-2 or miR-130a mimic, and SCC-4 cells were transfected with MYB or miR-130a inhibitor. Subsequently, CAL-27 cells were transfected with combination of si-MYB-2 and miR-130a mimic, while SCC-4 cells were transfected with combination of MYB and miR-130a inhibitor for 48 h before they were analyzed.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
TRIzol reagent (Invitrogen) was utilized to extract the total RNA of cells and the extracted RNA was reverse transcribed into cDNA using One Step PrimeScript miRNA cDNA Synthesis kit or PrimeScript RT Master Mix (both from Takara Biotechnology Ltd., Dalian, China). RT-qPCR was conducted with SYBR Premix Ex Taq II (Takara) on RT-qPCR device (7500, ABI, Inc., Foster City, CA, USA). The primer sequences are exhibited in Table 1. U6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were employed as the internal references. The 2−ΔΔCT method was applied to calculate the expression of mRNA.
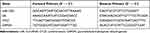 |
Table 1 Primers Sequence for RT-qPCR |
Dual-Luciferase Reporter Gene Assay
The targeted binding site between miR-130a and CYLD 3ʹ UTR was predicted using TargetScan (http://www.targetscan.org/vert_72/). The binding sequence and mutant (MUT) sequence between miR-130a and CYLD were amplified and cloned to pmiR-GLO luciferase vector (Promega, Madison, WI, USA) to construct CYLD-wild type (WT) plasmids and CYLD-MUT plasmids. These plasmids were co-transfected with miR-NC mimic or miR-130a mimic into HEK293T cells in line with the instructions of LipofectamineTM 2000 (Invitrogen). After 48 h, the activity of firefly and Renilla luciferase was detected in strict accordance with the instructions of the dual-luciferase reporter assay system (Promega, Madison, WI, USA), and the relative luciferase activity was calculated. This experiment was conducted for 3 times.
RNA Pull-Down Assay
CAL-27 and SCC-4 cells were treated with biotin-labeled WT-miR-130a and MUT-miR-130a for 48 h. After that, cells were harvested and incubated with lysis buffer (5 mM MgCl2, 100 mM KCl, 20 mM Tris 0.3%, NP-40, 50 U of RNase OUT and complete protease suppressor cocktail) (Ambion, Austin, TX, USA) for 10 min. Then, the cell lysates were cultured with M-280 streptavidin magnetic beads (S3762, Millipore Sigma, Billerica, MA, USA), which were precoated with bovine serum albumin without yeast tRNA and RNase. The magnetic beads were then incubated at 4°C for 3 h, followed by twice washes in lysis solution, 3 washes in low-salt buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and once in high-salt buffer (Solarbio). TRIzol kit (Invitrogen) was used to separate RNA adhered on beads. RT-qPCR was performed to assess CYLD mRNA expression. All the operations above were conducted as previously described.18
Cell Counting Kit-8 (CCK-8) Method
CCK-8 kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) was employed for cell proliferation assessment according to its instructions. Cells were seeded into 96-well plates at 1 × 104 cells/well and observed at 450 nm at 24 h, 48 h and 72 h, respectively. This experiment was repeated 3 times.
Colony Formation Assay
Afterwards, 1× 103 cells were seeded into 6-well plates in triplicate in a 37°C incubator with 5% CO2 for 2 weeks. After the incubation, the colonies were fixed and stained with 0.5% crystal violet for observation and counting under an optical microscope (Olympus Optical Co., Ltd, Tokyo, Japan).
Transwell Assays
To verify cell migration, CAL-27 and SCC-4 cells from different groups were collected and filled with DMEM basic medium for cell resuspension. Cells were put into the Transwell chambers (24-well insert, 8 μm pore size, Corning Incorporated, Corning, NY, USA) when cell concentration was adjusted into 1 × 103 cells/well. Next, basolateral chambers were added with 500 μL DMEM complete medium. After 24-h incubation at 37ºC with 5% CO2, the chambers were taken out with medium removed. The basement membranes were stained with 1% crystal violet. Five fields of views were randomly selected, and counted and photographed under the optical microscope.
Before the cell invasion experiment, Transwell chambers were enveloped by Matrigel (3.9 μg/μL, BD Biosciences Inc., San Jose, CA, USA) gel. The remaining operations were identical as the cell migration experiment. Finally, the cells in the basement membranes were counted under the microscope.
Western Blot Analysis
Total protein of CAL-27 and SCC-4 cells was extracted using radio-Immunoprecipitation assay cell lysis buffer and the concentration and purity were tested using a bicinchoninic acid kit (Jiancheng). The extracted proteins (30 μg) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred into polyvinylidene fluoride membranes using semi-dry method. Membranes were sealed with 5% skim milk powder for 2 h, and then incubated with the primary antibodies (all from Abcam Inc., Cambridge, MA, USA): CYLD (1:1000; ab137524), Vimentin (1:1000; ab92547), Snail (1:500; ab82846), E-cadherin (1:10,000; ab40772), N-cadherin (1:500; ab18203) and GAPDH (1:10,000; ab181602) at 4°C overnight. The following day, the membranes were washed with tris-buffered saline-tween buffer for 3 times with primary antibodies discarded, and incubated with horseradish peroxidase labeled goat anti-rabbit immunoglobulin G antibody (1:5000; ab205718) for 1 h. Subsequently, the membranes were developed using enhanced chemiluminescence reagent (Invitrogen), and visualized using Odyssey infrared imaging system (Li-Cor Bioscience, Lincoln, NE, USA). Image Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA) was utilized to conduct semi-quantitative analysis on the bands.
Xenograft Tumors in Nude Mice
Ten BALB/c nude mice (18 ~ 20 g) (Shandong Scobess Biotechnology Co., Ltd., Shandong, China, SCXK (Shandong) 2016–0001) were raised in specific pathogen-free environment for 1 week. All the treatment and welfare of laboratory animals were performed based on Guidelines for Animal Experimentation released by Ministry of Science and Technology in China.
After subcutaneous injection of 200 μL Cal-27 cell suspension (1 × 107 cells/mL) to BALB/c nude mice to build the solid tumors, the nude mice were randomly allocated to two groups (n = 5 in each group). Then, 10 μg of si-MYB-2 or si-NC was injected subcutaneously to the tumor site every 2 days. Tumor volume was measured every 7 days according to the formula V = 0.5 × L × W2, in which L represented the long diameter of the tumor and W represented for the short diameter. Four weeks after the injection of CAL-2 cells transfected with si-MYB-2, tumors were weighed and immediately preserved with liquid nitrogen freezing for RT-qPCR and Western blot analysis.
Statistical Analysis
SPSS 21.0 (IBM Corp. Armonk, NY, USA) was employed for data analysis. Kolmogorov–Smirnov test indicated whether the data were in normal distribution. The data were shown in mean ± standard deviation. The t-test was used for analyzing comparisons between two groups, one-way analysis of variance (ANOVA) or two-way ANOVA for comparing different groups, and Tukey’s multiple comparisons test for pairwise comparisons after ANOVA. Chi-square test was used to analyze counting data. The p value was attained using a two-tailed test and p < 0.05 or p < 0.01indicated significant difference.
Results
Silencing MYB Reduces Cell Proliferation, Migration and Invasion
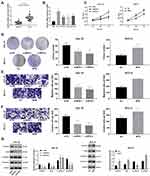
MYB was highly expressed in OTSCC tissues compared with that in paracancerous tissues (p < 0.01) (Figure 1A). Then, we classified the patients into high expression group and low expression group with the mean value of MYB expression as the critical threshold. We analyzed the correlation between the expression of MYB and the clinical parameters of patients, and found that the expression of MYB was not related to the gender and age of patients (p > 0.05), but related to the clinical stage and lymph node metastasis (p < 0.01, Table 2). Besides, CAL-27 cells had the highest MYB expression while SCC-4 cells had relatively poor MYB expression (all p < 0.01) (Figure 1B), so there two kinds of cell lines were selected for further experiments. CAL-27 cell migration was reduced when MYB expression in CAL-27 was silenced by MYB siRNA; on the contrary, SCC-4 cell proliferation was promoted when MYB was overexpressed (all p < 0.01) (Figure 1C and D). Transwell assays exhibited that migration and invasion rates of CAL-27 cells with declined MYB expression were decreased whereas those in SCC-4 cells with overexpressed MYB were increased (all p < 0.01) (Figure 1E and F). Detection on levels of EMT-related proteins, including Vimentin, Snail, N-cadherin and E-cadherin found that when MYB was silenced in CAL-27 cells, the levels of the first three proteins were reduced, but E-cadherin level was increased. Meanwhile, SCC-4 cells with overexpressed MYB had opposite trends (all p < 0.01) (Figure 1G).
 |
Table 2 Correlation Between MYB Expression and Clinical Parameters of Patients |
miR-130a Expression is Upregulated by MYB and Induces Cell Proliferation, Migration and Invasion
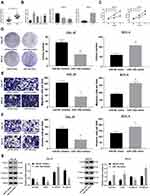
miR-130a was highly expressed in OTSCC tissues compared with that in paracancerous tissues (p < 0.01) (Figure 2A). The mean value of miR-130a expression in cancer tissue was used as the critical threshold to classify the patients into high expression group and low expression group. The correlation between the expression of miR-130a and the clinical parameters of the patients was analyzed. It was found that the expression of miR-130a was not related to the gender and age of the patients (p > 0.05), but related to the clinical stage and lymph node metastasis (p < 0.01) (Table 3). In different OTSCC cell lines, the level of miR-130a was significantly higher than that in HOK cells. CAL-27 cells with silencing MYB evidently displayed miR-130a expression, while SCC-4 cells with overexpressing MYB showed reversed outcome (all p < 0.01) (Figure 2B). Next, detection on cell cellular processes found that overexpressed miR-130a enhanced cell proliferation, migration and invasion inhibited by silenced miR-130a (all p < 0.01) (Figure 2C–F). Meanwhile, with silencing miR-130a, expression of Vimentin, Snail and N-cadherin in CAL-27 cells was discouraged but E-cadherin level was encouraged. Meanwhile, SCC-4 cells with overexpressed miR-130a had opposite consequences (all p < 0.01) (Figure 2G).
 |
Table 3 Correlation Between miR-130a Expression and Clinical Parameters of Patients |
Silenced miR-130a Discourages Cell Biological Activity Enhanced by Overexpressed MYB
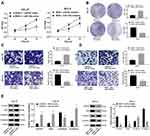
Given the better transfection outcome, si-MYB-2 was co-transfected with miR-130a to CAL-27 cells, and overexpressed MYB plasmids and miR-130a inhibitor were co-transfected to SCC-4 for measurement of cell activity. Then, it was concluded that miR-130a overexpression partly promoted cell growth inhibited by silenced MYB, but miR-130a knockdown contributed to opposite outcomes (all p < 0.01) (Figure 3A–D). Meanwhile, with silenced miR-130a, expression of Vimentin, Snail and N-cadherin in CAL-27 cells was discouraged but E-cadherin level was encouraged, but SCC-4 cells with overexpressed miR-130a had opposite consequences (both p < 0.01) (Figure 3E).
CYLD is Poorly Expressed in OTSCC and is Targeted by miR-130a
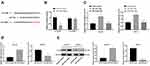
Through bioinformatics prediction, we found that miR-130a has specific binding sites with multiple mRNAs, and we focused on the CYLD. From previous studies,19–22 we found that CYLD is related to the proliferation and migration of a variety of tumor cells, so we chose CYLD for subsequent experiments. Bioinformatics analysis predicted the binding site between CYLD and miR-130a, and their target relation was further verified by dual-luciferase reporter gene assay and RNA pull-down assay (all p < 0.01) (Figure 4A–C). What’s more, CYLD expression was induced when miR-130a expression was suppressed in CAL-27 cells yet in SCC-4 cells, CYLD expression was reduced when miR-130a was overexpressed (both p < 0.01) (Figure 4D and E).
MYB Knockdown Activates CYLD to Suppress OTSCC in vivo by Downregulating miR-130a Expression
CAL-27 cells transfected with si-MYB-2 were subcutaneously transplanted into nude mice to observe the effects of silenced MYB on tumor growth. It was shown that tumor growth was inhibited (Figure 5A and B), with downregulated miR-130 expression and upregulated CYLD expression in tumor tissues (all p < 0.01) (Figure 5C and D).
Discussion
As one of the most serious cancers and a growing dilemma in health prevention globally, OSCC resulted in accelerated morbidity and mortality rates.23 MYB nuclear staining was strongly presented in SCC tissues and cells, implying MYB may be a tumor promoter in OSCC.24 In this study, we aimed to figure out the mechanism of MYB and miR-130a in OSCC development. And we concluded that silenced MYB suppressed OTSCC cell progression by inactivating miR-130a and upregulating CYLD.
Initially, this study found out that silenced MYB reduced cell progression, with decreased expression of EMT-related proteins Vimentin, Snail and N-cadherin but increased E-cadherin level. It was previously elucidated that both invasion and metastasis are pivotal reasons in augmenting ESCC progression.25 EMT is actively participated in a wide range of SCCs to trigger secondary tumors and enhance tumor stemness, metastasis and resistance to treatment.26 Importantly, a recent study clarified that in adenoid cystic carcinoma tumor samples, MYB expression elevation contributed to E-cadherin knockdown while its degradation tended to cause decreased Vimentin expression.27 Our data supported that in OTSCC, miR-130a expression was upregulated by MYB and could induce cell proliferation, migration and invasion. Manasa et al noted that in OC, different class of miRs were recognized as oncogenes or tumor suppressors as they were indispensably participated in cell survival, growth, death and differentiation, making them necessary biomarkers in OC diagnosis and prognosis.28 Highly expressed miR-130a was found in OCs, such as salivary gland adenoid cystic carcinoma and aggressive OSCC, implying its detrimental role in cancer cell metastasis.14,29 Our operations showed decreased levels of Vimentin, Snail and N-cadherin and increased E-cadherin level in OSCC cells with inhibited miR-130a, which indicated that silencing miR-130a was conducive in declining OTSCC malignancy enhanced by MYB overexpression. Vimentin, Snail and N-cadherin levels are all downgraded, which are coupled by an upgraded E-cadherin level in cells with low invasiveness.30 As an attractive diagnosis target in OSCC, N-cadherin was highly expressed in OSCC cell lines than that in adjacent non-tumor ones.31 Besides, snail served as a drive of EMT as well as a mediator in head and neck SCC malignancy.32 On the contrary, E-cadherin expression upregulation discouraged LSCC tumor metastasis and development.33 Likewise, in osteosarcoma patients, miR-130a was feasible to improve cell EMT, migration and invasion.34
Moreover, CYLD was poorly expressed in OSCC. Poor CYLD expression enhanced OSCC tumor invasion, suggesting low patient survival rates.20 What’s more, CYLD expression was reduced in SCC cells with drug resistance as compared with that in those with sensitivity.35 CYLD was identified as a target gene of miR-130b in our study. CYLD downregulation induced by miR-130b brought about glioma cell development and invasion.36 In dermal fibrosis malignancies, miR-130a augmented myofibroblast transformation, collagen secretion and cell activity by targeting CYLD, which would exaggerate fibrosis.37 Thus, the target relation between miR-130a and CYLD could be dramatically involved in OTSCC remedy. Additionally, knockdown of MYB activated CYLD to suppress OSCC in vivo by inactivating miR-130a expression. In cutaneous cylindroma, MYB expression and target genes were all promoted in tumors with loss of CYLD, implying these genes were negatively correlated.38 Aggressive OSCC tissues had more activated miR-130a than non-aggressive ones.14 Additionally, when expression of MYBL2, a member from MYB family, was reduced, ESCC tumor growth was also inhibited.39 All in all, our data exhibited that silenced MYB was a prospective approach in OTSCC treatment through the miR-130a/CYLD axis.
Conclusion
To sum up, our study supported that silenced MYB suppressed OTSCC cell proliferation and malignancy by inactivating miR-130a and upregulating CYLD. These results devoted a novel theoretical strategy for OTSCC treatment. In the future, we will further explore the underlying mechanism of other targets of MYB. More attention will be paid to seeking reliable therapeutic targets for OSCC. Although our findings provide therapeutic implication in OSCC treatment, it is just a preclinical research, and the experiment results and effective application into clinical practice need further validation. Through bioinformatics prediction, we found that miR-130a has specific binding sites with multiple mRNAs. In the future experiments, we will continue to pay attention to the role of other target genes.
Disclosure
The authors declared that they have no competing interests.
References
1. Huang CC, Tung TH, Huang CC, et al. Electrochemical assessment of anticancer compounds on the human tongue squamous carcinoma cells. Sensors. 2020;20(9). doi:10.3390/s20092632
2. Thomson PJ. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J Oral Pathol Med. 2018;47(9):803–807. doi:10.1111/jop.12733
3. Zhang H, Song Y, Du Z, et al. Exome sequencing identifies new somatic alterations and mutation patterns of tongue squamous cell carcinoma in a Chinese population. J Pathol. 2020. doi:10.1002/path.5467
4. Zidar N, Bostjancic E, Malgaj M, Gale N, Dovsak T, Didanovic V. The role of epithelial-mesenchymal transition in squamous cell carcinoma of the oral cavity. Virchows Arch. 2018;472(2):237–245. doi:10.1007/s00428-017-2192-1
5. Gharat SA, Momin M, Bhavsar C. Oral squamous cell carcinoma: current treatment strategies and nanotechnology-based approaches for prevention and therapy. Crit Rev Ther Drug Carrier Syst. 2016;33(4):363–400. doi:10.1615/CritRevTherDrugCarrierSyst.2016016272
6. Dumache R. Early diagnosis of oral squamous cell carcinoma by salivary microRNAs. Clin Lab. 2017;63(11):1771–1776. doi:10.7754/Clin.Lab.2017.170607
7. Liu X, Xu Y, Han L, Yi Y. Reassessing the potential of Myb-targeted anti-cancer therapy. J Cancer. 2018;9(7):1259–1266. doi:10.7150/jca.23992
8. Zhao X, Zhang W, Ji W. YB-1 promotes laryngeal squamous cell carcinoma progression by inducing miR-155 expression via c-Myb. Future Oncol. 2018;14(16):1579–1589. doi:10.2217/fon-2018-0058
9. Wang X, Li M, Wang Z, et al. Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. J Biol Chem. 2015;290(7):3925–3935. doi:10.1074/jbc.M114.596866
10. Zhang H, Jiang S, Guo L, Li X. MicroRNA-1258, regulated by c-Myb, inhibits growth and epithelial-to-mesenchymal transition phenotype via targeting SP1 in oral squamous cell carcinoma. J Cell Mol Med. 2019;23(4):2813–2821. doi:10.1111/jcmm.14189
11. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
12. Garcia-Sancha N, Corchado-Cobos R, Perez-Losada J, Canueto J. MicroRNA dysregulation in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20(9):2181. doi:10.3390/ijms20092181
13. Eichelmann AK, Matuszcak C, Lindner K, Haier J, Hussey DJ, Hummel R. Complex role of miR-130a-3p and miR-148a-3p balance on drug resistance and tumor biology in esophageal squamous cell carcinoma. Sci Rep. 2018;8(1):17553. doi:10.1038/s41598-018-35799-1
14. Hilly O, Pillar N, Stern S, et al. Distinctive pattern of let-7 family microRNAs in aggressive carcinoma of the oral tongue in young patients. Oncol Lett. 2016;12(3):1729–1736. doi:10.3892/ol.2016.4892
15. Wang Y, Zhang CY, Xia RH, et al. The MYB/miR-130a/NDRG2 axis modulates tumor proliferation and metastatic potential in salivary adenoid cystic carcinoma. Cell Death Dis. 2018;9(9):917. doi:10.1038/s41419-018-0966-2
16. Yang H, Zhang H, Ge S, et al. Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting C-MYB in vascular endothelial cells. Mol Ther. 2018;26(10):2466–2475. doi:10.1016/j.ymthe.2018.07.023
17. Suenaga N, Kuramitsu M, Komure K, et al. Loss of tumor suppressor CYLD expression triggers cisplatin resistance in oral squamous cell carcinoma. Int J Mol Sci. 2019;20(20):5194. doi:10.3390/ijms20205194
18. Zheng YJ, Zhao JY, Liang TS, et al. Long noncoding RNA SMAD5-AS1 acts as a microRNA-106a-5p sponge to promote epithelial mesenchymal transition in nasopharyngeal carcinoma. FASEB J. 2019;33(11):12915–12928. doi:10.1096/fj.201900803R
19. de Jel MM, Schott M, Lamm S, Neuhuber W, Kuphal S, Bosserhoff AK. Loss of CYLD accelerates melanoma development and progression in the Tg(Grm1) melanoma mouse model. Oncogenesis. 2019;8(10):56. doi:10.1038/s41389-019-0169-4
20. Ge WL, Xu JF, Hu J. Regulation of oral squamous cell carcinoma proliferation through crosstalk between SMAD7 and CYLD. Cell Physiol Biochem. 2016;38(3):1209–1217. doi:10.1159/000443069
21. Ni F, Zhao H, Cui H, et al. MicroRNA-362-5p promotes tumor growth and metastasis by targeting CYLD in hepatocellular carcinoma. Cancer Lett. 2015;356(2 Pt B):809–818. doi:10.1016/j.canlet.2014.10.041
22. Sanches JGP, Xu Y, Yabasin IB, et al. miR-501 is upregulated in cervical cancer and promotes cell proliferation, migration and invasion by targeting CYLD. Chem Biol Interact. 2018;285:85–95. doi:10.1016/j.cbi.2018.02.024
23. Panarese I, Aquino G, Ronchi A, et al. Oral and oropharyngeal squamous cell carcinoma: prognostic and predictive parameters in the etiopathogenetic route. Expert Rev Anticancer Ther. 2019;19(2):105–119. doi:10.1080/14737140.2019.1561288
24. Shi X, Wu S, Huo Z, Ling Q, Luo Y, Liang Z. Co-existing of adenoid cystic carcinoma and invasive squamous cell carcinoma of the uterine cervix: a report of 3 cases with immunohistochemical study and evaluation of human papillomavirus status. Diagn Pathol. 2015;10:145. doi:10.1186/s13000-015-0376-z
25. Tian X, Fei Q, Du M, et al. miR-130a-3p regulated TGF-beta1-induced epithelial-mesenchymal transition depends on SMAD4 in EC-1 cells. Cancer Med. 2019;8(3):1197–1208. doi:10.1002/cam4.1981
26. Latil M, Nassar D, Beck B, et al. Cell-type-specific chromatin states differentially prime squamous cell carcinoma tumor-initiating cells for epithelial to mesenchymal transition. Cell Stem Cell. 2017;20(2):191–204 e5. doi:10.1016/j.stem.2016.10.018
27. Xu LH, Zhao F, Yang WW, et al. MYB promotes the growth and metastasis of salivary adenoid cystic carcinoma. Int J Oncol. 2019;54(5):1579–1590. doi:10.3892/ijo.2019.4754
28. Manasa VG, Kannan S. Impact of microRNA dynamics on cancer hallmarks: an oral cancer scenario. Tumour Biol. 2017;39(3):1010428317695920. doi:10.1177/1010428317695920
29. Feng X, Matsuo K, Zhang T, et al. MicroRNA profiling and target genes related to metastasis of salivary adenoid cystic carcinoma. Anticancer Res. 2017;37(7):3473–3481. doi:10.21873/anticanres.11715
30. Kimura I, Kitahara H, Ooi K, et al. Loss of epidermal growth factor receptor expression in oral squamous cell carcinoma is associated with invasiveness and epithelial-mesenchymal transition. Oncol Lett. 2016;11(1):201–207. doi:10.3892/ol.2015.3833
31. Chandolia B, Rajliwal JP, Bajpai M, Arora M. Prognostic potential of N-Cadherin in oral squamous cell carcinoma via immunohistochemical methods. J Coll Physicians Surg Pak. 2017;27(8):475–478.
32. Ota I, Masui T, Kurihara M, et al. Snail-induced EMT promotes cancer stem cell-like properties in head and neck cancer cells. Oncol Rep. 2016;35(1):261–266. doi:10.3892/or.2015.4348
33. Cercelaru L, Stepan AE, Margaritescu C, et al. E-cadherin, beta-catenin and snail immunoexpression in laryngeal squamous cell carcinoma. Rom J Morphol Embryol. 2017;58(3):761–766.
34. Chen J, Yan D, Wu W, Zhu J, Ye W, Shu Q. MicroRNA-130a promotes the metastasis and epithelial-mesenchymal transition of osteosarcoma by targeting PTEN. Oncol Rep. 2016;35(6):3285–3292. doi:10.3892/or.2016.4719
35. Ratovitski EA. Phospho-DeltaNp63alpha-responsive microRNAs contribute to the regulation of necroptosis in squamous cell carcinoma upon cisplatin exposure. FEBS Lett. 2015;589(12):1352–1358. doi:10.1016/j.febslet.2015.04.020
36. Xiao ZQ, Yin TK, Li YX, Zhang JH, Gu JJ. miR-130b regulates the proliferation, invasion and apoptosis of glioma cells via targeting of CYLD. Oncol Rep. 2017;38(1):167–174. doi:10.3892/or.2017.5651
37. Zhang J, Zhou Q, Wang H, et al. MicroRNA-130a has pro-fibroproliferative potential in hypertrophic scar by targeting CYLD. Arch Biochem Biophys. 2019;671:152–161. doi:10.1016/j.abb.2019.07.003
38. Rajan N, Andersson MK, Sinclair N, et al. Overexpression of MYB drives proliferation of CYLD-defective cylindroma cells. J Pathol. 2016;239(2):197–205. doi:10.1002/path.4717
39. Qin H, Li Y, Zhang H, et al. Prognostic implications and oncogenic roles of MYBL2 protein expression in esophageal squamous-cell carcinoma. Onco Targets Ther. 2019;12:1917–1927. doi:10.2147/OTT.S190145
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.