Back to Journals » Drug Design, Development and Therapy » Volume 10
Sildenafil potentiates the antitumor activity of cisplatin by induction of apoptosis and inhibition of proliferation and angiogenesis
Authors El-Naa MM, Othman M, Younes S
Received 29 February 2016
Accepted for publication 11 April 2016
Published 16 November 2016 Volume 2016:10 Pages 3661—3672
DOI https://doi.org/10.2147/DDDT.S107490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Mona Mohamed El-Naa,1 Mohamed Othman,2,3 Sheren Younes4,5
1Faculty of Pharmacy, October University for Modern Sciences and Arts (MSA), 6 October City, Egypt; 2Preparatory Year College, University of Hail, Hail, Kingdom of Saudi Arabia; 3Faculty of Biotechnology, October University for Modern Science and Arts (MSA), 6 October City, Egypt; 4Pathology Department, Faculty of Medicine, Menoufia University, Shebin El-Kom, Egypt; 5College of Medicine, Princess Nora Bint Abdulrahman University, Riyadh, Kingdom of Saudi Arabia
Abstract: Sildenafil is the first phosphodiesterase-5 inhibitor used for the treatment of erectile dysfunction. However, recent studies have been suggesting an antitumor effect of sildenafil. The current study assessed the aforementioned activity of sildenafil in vivo and in vitro in solid-tumor-bearing mice and in a human cell line MCF-7, respectively. Moreover, we investigated the impact of sildenafil on cisplatin antitumor activity. The solid tumor was induced by inoculation of Ehrlich ascites carcinoma cells in female mice. The tumor-bearing mice were assigned randomly to control (saline), sildenafil (sildenafil 5 mg/kg/d, PO daily for 15 days), cisplatin (cisplatin 7.5 mg/kg, IP once on the 12th day of Ehrlich ascites carcinoma inoculation), and combination therapy (cisplatin and sildenafil) groups. The tumor volume was measured at the end of the treatment period along with the following parameters: angiogenin, vascular endothelial growth factor, tumor necrosis factor-α, Ki-67, caspase-3, DNA-flow cytometry analysis, and histopathological examination. The study results showed that sildenafil has significantly decreased the tumor volume by 30.4%, angiogenin and tumor necrosis factor-α contents, as well as vascular endothelial growth factor expression. Additionally, caspase-3 level significantly increased with sildenafil treatment, whereas Ki-67 expression failed to show any significant changes. Furthermore, the cell cycle analysis revealed that sildenafil was capable of improving the category of tumor activity from moderate to low proliferative. Sildenafil induced necrosis in the tumor. Moreover, the drug of interest showed cytotoxic activity against MCF-7 in vitro as well as potentiated cisplatin antitumor activity in vivo and in vitro. These findings shed light on the antitumor activity of sildenafil and its possible impact on potentiating the antitumor effect of conventional chemotherapeutic agents such as cisplatin. These effects might be related to antiangiogenic, antiproliferative, and apoptotic activities of sildenafil.
Keywords: sildenafil, phosphodiesterase-5, breast cancer, angiogenesis, proliferation, apoptosis
Introduction
Cyclic nucleotide phosphodiesterase enzymes (PDEs) are a large superfamily of enzymes that catalyze the hydrolytic breakdown of cyclic nucleotides cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) that regulate various biological processes such as cell growth, energy homeostasis, muscle relaxation, and neuronal signaling.1 There are at least eleven PDE isozyme family members having different substrate specificity, regulatory properties, tissue localization, and inhibitor sensitivity. PDE-5, -6, and -9 are selective for cGMP and PDE-4, -7, and -8 are cAMP selective, whereas PDE-1, -2, -3, -10, and -11 are dual substrate-degrading isozymes.2
Altered expression and/or activity of PDEs have been reported in various carcinomas and hematological malignancies.1 PDE-5 has been shown to be overexpressed in multiple human carcinomas including breast cancers, colon adenocarcinoma, bladder squamous carcinoma, and lung cancers that have significantly lower cGMP levels than normal cells.1,3–7 This implies that selective inhibition of PDE-5 may have antineoplastic effects and may increase the specificity of a given chemotherapeutic agent.
Sildenafil, a PDE-5 inhibitor, was initially developed as an antihypertensive agent. Due to the unexpected side effect of improvement of penile erection, in 1998, sildenafil citrate (Viagra®; Pfizer, Inc., New York, NY, USA) became the first PDE-5 inhibitor for the treatment of erectile dysfunction.8 Recently, sildenafil has shown benefits in experimental cardiac dysfunctions,9,10 tissue injury,11–13 cystic fibrosis,14 and ischemia/reperfusion injury.15,16 Additionally, sildenafil as well as other PDE-5 inhibitors were shown to induce apoptosis in different human tumors, such as colon carcinoma and chronic lymphocyte leukemia.17,18 Cisplatin is a cytotoxic agent commonly used in the treatment of solid tumors.19 It exerts its cytotoxic effect via formation of cisplatin–DNA adducts, which can ultimately result in cell cycle arrest20,21 and induction of apoptosis.22,23 However, its clinical usefulness is limited by the development of serious adverse reactions, including nephrotoxicity and ototoxicity, especially in high doses.24,25 Hence, the search has unfailingly continued to identify new antitumor agents or combinations that would be more potent and preferably exhibit less health hazards. The current study aimed at addressing the antitumor activity of the PDE-5 inhibitor sildenafil alone and in combination with cisplatin in Ehrlich solid-tumor-bearing mice. In addition, cytotoxicity of sildenafil against human mammary adenocarcinoma MCF-7 was assessed.
Materials and methods
Animals
Swiss albino female mice, 8 weeks old and weighing 20±2 g, were housed in the animal house, October University for Modern science and Arts (MSA), Egypt, under constant conditions (12 hours light, dark regimen, standard chow pellet food and water ad libitum). Procedures involving animals and their care were conducted in accordance with National Institute of Health regulations of animals care described in the “Guide for the Care and Use of Laboratory Animals”26 and with the approval of the local research ethical committee of Faculty of Pharmacy, October University for Modern Sciences and Arts, Egypt.
Experimental design
Ehrlich ascites carcinoma (EAC) cells were implanted subcutaneously into the left hind legs of the mice by inoculation of 0.2 mL of 2×105 tumor cells in physiological solution. Tumor-bearing mice were randomly divided into four groups (n=8). The control group comprised mice receiving saline only throughout the experiment. The sildenafil group comprised mice receiving oral sildenafil (5 mg/kg/d) dissolved in water for 15 consecutive days starting from 1st day of EAC cells inoculation. The cisplatin group comprised mice receiving a single intraperitoneal dose of cisplatin (7.5 mg/kg) on the 12th day after EAC cells inoculation. The combination therapy group comprised mice that were co-treated with the combination of cisplatin and sildenafil.
Assessment of tumor growth
The tumor volume was estimated by two-dimensional measurements using Vernier caliper. The measurements were taken on the day 15 after tumor implantation, the same day the mice were sacrificed. The tumor volume was calculated using the formula, V =0.4× a(b)2, where (a) and (b) are the longer and shorter diameters of the tumor, respectively.27
Determination of tumor content of angiogenin and tumor necrosis factor-alpha
For the assessment of tumor content of angiogenin and tumor necrosis factor-alpha (TNF-α), all tumors were punched out immediately after the mice were sacrificed. A part of each tumor was weighed and homogenized in phosphate-buffered saline (PBS) to prepare a 10% homogenate. Homogenates were centrifuged at 2,500 rpm for 15 minutes, and the supernatants were stored at −80°C. Quantitative measurements of tissue angiogenin and TNF-α were carried out by enzyme-linked immunosorbent assay technique, using mouse-specific kits purchased from BioVendor (Gunma, Japan) according to the protocol provided with each kit.
Histopathological examination of the tumor
Representative parts of each tumor were obtained and preserved in 10% neutral buffered formalin for histopathological evaluation. Tissues were embedded in paraffin blocks, and 4 μm thickness sections were sliced from the prepared paraffin blocks and stained with hematoxylin and eosin for assessment of necrosis. The necrotic area appeared as nonviable homogenous structureless material with degenerated or karyorrhectic nuclei.
Immunostaining for VEGF, Ki-67, and caspase-3 in the tumor
Immunohistochemical analysis of vascular endothelial growth factor (VEGF), Ki-67, and caspase-3 expression was performed on 4 μm thick serial tissue sections obtained from the prepared formalin-fixed paraffin-embedded tissue blocks and mounted on positive charged slides; manual staining was done according to the manufacturer’s instructions for each marker. All primary antibodies, clones, and dilutions are listed in Table 1.
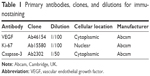  | Table 1 Primary antibodies, clones, and dilutions for immunostaining |
Immunohistochemical evaluation of staining was done semiquantitatively using H-score.28 H-score combines both intensity and percentage of positive cells in each section. The intensity of immunostaining was assessed as follows: 1 – mild, 2 – moderate, and 3 – strong. H-score was calculated by multiplying the intensity by the percentage of positive cells, creating a range of possible scores of 0–300.
DNA flow cytometry analysis
The tumor samples were minced with a scalpel in a cold PBS solution. The samples were filtered through a 70 m nylon mesh. After washing with PBS solution and centrifugation, cell cycle analysis was carried out using flow cytometer (Becto Dicknson, BD, FACScalbur, USA) according to the method of Smets et al.29
Calculation of inhibitory concentration 50% of sildenafil
Potential cytotoxicities of sildenafil and cisplatin were tested using the method of Skehan et al.30 Human mammary adenocarcinoma cells (MCF-7) were plated in 96 multiwell plates (104 cells/well) for 24 hours before treatment with the compounds to allow attachment of cell to the wall of the plate. Different concentrations (5, 12.5, 25, and 50 μg/mL) were prepared for each drug and added to the cell monolayer (performed in triplicate). Monolayer cells were incubated with the compounds for 48 hours at 37°C and in an atmosphere of 5% CO2. After 48 hours, cells were fixed with trichloroacetic acid, washed, and stained with sulforhodamine B stain. Excess stain was washed with acetic acid while attached stain was recovered with Tris–ethylenediaminetetraacetic acid buffer. Color intensity was measured using enzyme-linked immunosorbent assay. The relation between surviving fraction and drug concentrations was plotted to get the survival curve of the tumor cell line after treatment with the specific compounds. The curves were fit using linear equation, and inhibitory concentration 50% (IC50, dose of the drug which reduces survival to 50%) was calculated.
Statistical analysis
Data are expressed as mean ± standard error of mean. Comparisons between different groups were carried out by one-way analysis of variance followed by the Tukey–Kramer test. Correlations between different parameters were evaluated by Pearson’s correlation (r). The level of significance was set at probability P<0.05. The Graphpad Software Instat (version 6; GraphPad Software, Inc., La Jolla, CA, USA) was used to carry out the statistical analysis.
Results
Effect of sildenafil and/or cisplatin on tumor volume
Sildenafil and cisplatin treatment resulted in significant decrease (30.4% and 58.8%, respectively) in the tumor volume compared with control group (P<0.05; Figure 1). A further decrease in the tumor volume was observed in the cisplatin and sildenafil combination group compared with the corresponding single-treatment group as well as control group (P<0.05; Figure 1).
Effect of sildenafil and/or cisplatin on tumor content of angiogenin and TNF-α
Angiogenin and TNF-α levels in tumor tissue were significantly decreased (63.2% and 58.8%, respectively) in mice treated with sildenafil compared with control group (P<0.05; Table 2). Cisplatin treatment significantly decreased the amount angiogenin and TNF-α by 50.5% and 39.1%, respectively, compared with control group (P<0.05; Table 2). A significant decrease in angiogenin and TNF-α contents was observed in the cisplatin and sildenafil combination group compared with the group that received cisplatin alone (P<0.05; Table 2).
Effect of sildenafil and/or cisplatin on expression of VEGF, Ki-67, and caspase-3 in solid tumor
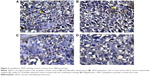
Immunohistochemical study revealed that the expression of VEGF, evaluated by H-score, was the highest (262.5±8.81) in tumors excised from mice that received no therapy. Levels of its expression were significantly reduced in tumors treated with sildenafil and cisplatin to 235±7.32 and 215±8.66, respectively. The lowest level of VEGF expression was found in tumors treated with combination therapy (176.25±8.01); this result was statistically significant when compared with cisplatin group (Table 3; Figure 2).
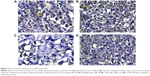
Ki-67 showed the lowest expression in tumors excised from mice that received the combination therapy (138.75±6.39), whereas the highest expression was detected in tumors excised from mice that received no therapy (250±12.96) (Table 3; Figure 3). Cisplatin significantly attenuated the expression of Ki-67 (191.25±6.39), whereas sildenafil did not induce any significant changes in Ki-67 expression (241±11.87) compared with control group (Table 3; Figure 3). However, combination therapy of sildenafil and cisplatin showed significant attenuation in Ki-67 expression compared with cisplatin group (Table 3; Figure 3).
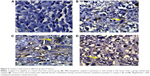
In contrast to VEGF and Ki-67, caspase-3, the apoptotic marker, was minimally expressed in control group (11.9±1.88); its level of expression was significantly increased in groups that received sildenafil, cisplatin, and combination therapy (45±6.81, 88.75±9.9, and 136.25±12.9, respectively) (Table 3; Figure 4). The combination therapy resulted in significantly higher caspase-3 expression compared with cisplatin group.
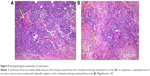
Effect of sildenafil and/or cisplatin on histopathological features of Ehrlich solid tumor
Histopathological examination of sections from solid tumor revealed typical malignant features including sheets of malignant cells infiltrating adjacent muscular tissue. The malignant cells showed pleomorphism, hyperchromatism, and mitotic activity. Assessment of the nonviable necrotic tissue revealed that tumors from the control group showed minimal areas of necrosis compared with those taken from other groups. The widest areas of necrosis were seen in tumors from mice that received combination therapy (Figure 5).
Effect of sildenafil and/or cisplatin on the cell cycle
DNA flow cytometry was used to study the effect of sildenafil, cisplatin, and their combination on the cell cycle distribution of tumor induced by inoculation of Ehrlich cell line in mice. Sildenafil and cisplatin produced nonsignificant changes in the nonproliferating cell fraction (G0/G1 phase), whereas the combination of both drugs significantly decreased G0/G1 phase by 40.1% compared with the control group (Figure 6A–E). Sildenafil, cisplatin, and combination therapy markedly decreased the S-phase cell population by 29.6%, 69.6%, and 73%, respectively, compared with the control group (Figure 6A–E). No apparent change in the G2/M-phase cell population was observed with sildenafil single treatment (Figure 6A–E), whereas cisplatin and combination therapy markedly decreased the G2/M-phase cell population by 63.2% and 56.6%, respectively, compared with the control group (Figure 6A–E). Sildenafil and cisplatin treatment resulted in significant accumulation of dead cells in sub-G1 phase by 58.5% and 65%, respectively, whereas the combination of cisplatin and sildenafil showed more accumulation of the dead cells in sub-G1 phase by 72.3% compared with 48.7% in control group (Figure 6A–F). The combination therapy showed potentiated effect on sub-G1 and G0/G1 phases compared with cisplatin, whereas it did not show any significant changes on S and G2/M-phases compared with cisplatin (Figure 6A–F).
On the other hand, there was a significant inhibition of the proliferation index (S% phase and G2/M% phase) by 30.8%, 68.2%, and 67.9% in sildenafil, cisplatin, and combination therapy, respectively, compared with control group (Figure 6G). However, combination therapy did not significantly affect the proliferation index compared with cisplatin group (Figure 6G).
IC50 of sildenafil
To explore the cytotoxicity of sildenafil and its impact on the cytotoxicity of cisplatin, concentration–response curves of both sildenafil and cisplatin were assessed, and the curve obtained with cisplatin was compared with that obtained after combination of both drugs. Sildenafil alone produced a dose-dependent inhibition of MCF-7 cell viability with IC50 values 14 μg/mL (Figure 7A). Furthermore, sildenafil at a concentration 14 μg/mL was able to synergistically increase the cytotoxicity of different concentrations of cisplatin (Figure 7B). The IC50 of cisplatin alone was 4.43 μg/mL, whereas the IC50 of cisplatin and sildenafil combination was decreased to 3.98 μg/mL.
The correlation between the tumor volume and the studied parameters
Figure 8 shows the scatter plots of the correlation between the tumor volume and the studied parameters in sildenafil-treated group. There were positive correlations between the tumor volume and angiogenin, TNF-α, VEGF, and proliferation index. On the other hand, there was a negative correlation between the tumor volume and caspase-3. There was no correlation between the tumor volume and Ki-67 with sildenafil treatment.
Discussion
This study showed for the first time that sildenafil significantly decreased the volume of the solid tumor induced by inoculation of Ehrlich cell line in mice. Moreover, mice that received the combination of sildenafil and cisplatin showed more reduction in tumor volume than those treated with each drug alone. Previously, Das et al31 showed that although sildenafil alone did not affect the tumor volume and weight of prostate tumor, it potentiated the inhibitory effect of doxorubicin on both tumor volume and weight.
Because the growth of most cancers, including breast cancer, is partly dependent on the local tissue microenvironment, apoptosis and proliferation of the tumor cells were assessed.
Caspase family is the key mediator involved in the apoptosis.32 As caspase-3 plays a central role in the execution phase of cell apoptosis33 and correlates with apoptotic rates in breast cancer,34,35 it is considered a potential marker for predicting the effect of chemotherapeutic agents in breast cancer.
In this study, tumor samples showed low expression of caspase-3 in untreated mice, whereas sildenafil induced significantly more expression of caspase-3 in the tumor cells. In accordance with this result, Sarfati et al18 reported that sildenafil suppressed tumor cell growth and induced apoptosis of chronic lymphocytic leukemia cells in vitro through activation of caspase-3. The tumor volume appeared to be negatively correlated with the caspase-3 expression level. Consequently, we hypothesized that caspase-dependent pathway was involved significantly in sildenafil-induced apoptosis of tumor cells. Furthermore, our results clarified that combination of sildenafil and cisplatin induced higher expression of caspase-3 compared with groups treated with cisplatin alone, indicating more apoptosis of the cancer cells. In line with these findings, DNA flow cytometry analysis revealed that all single and combination treatments increased the percentage of apoptotic cell (sub-G1) compared with untreated cells. Moreover, the combination therapy further increased the percentage of apoptotic cells compared with tumors treated with cisplatin only (Figure 6F). On the other hand, histopathological examination revealed that sildenafil, cisplatin, and the combination therapy produced a substantial area of necrosis in the tumor. These results indicated that both apoptosis and necrosis were involved in sildenafil-induced death of the Ehrlich tumor cells.
Proliferative activity was first assessed by DNA flow cytometry.36,37 With this method, cellular DNA content can be analyzed and cells can be quantified as percentages within the phases G0/G1 (presynthesis), S (DNA synthesis), and G2/M (premitosis and mitosis) of the cell cycle.38,39 Tumor cells in the S-phase and in the G2/M-phase were added together to give a “proliferation index”.40 In the present study, the proliferation index (S% phase and G2/M% phase) was significantly decreased with sildenafil treatment compared with control group (Figure 6G). Interestingly, according to Von Herbay et al’s40 Classification, sildenafil improved the category of tumor from moderate proliferative activity in control tumor (proliferating index 9.5%–15%) to low proliferative activity (proliferating index <9.5%).
In a second approach for determination of the proliferative activity, various antibodies to proliferation-associated antigens have been used to determine the proliferative activity of tumors.41,42 Of these antibodies, Ki-67, expressed in cycling cells, has gained considerable popularity for growth fraction determination. One of the most prevalent analysis methods of Ki-67 antigen is the immunohistochemical evaluation.41–43
In our study, there was a significant decrease in Ki-67 expression in the tumor tissues in cisplatin and combination therapy groups compared with control group; however, nonsignificant changes were observed in Ki-67 expression in sildenafil group compared with control group.
Based on these results, we assumed that the antiproliferative activity of sildenafil as indicated by the decrease in the proliferation index detected by flow cytometry was independent of Ki-67. This assumption is supported by the correlation study that showed that although the tumor volume of mice treated with sildenafil correlated significantly with proliferation index, it did not correlate significantly with Ki-67 expression. On the other hand, our results showed that combination of sildenafil and cisplatin significantly potentiated the decrease in Ki-67 expression compared with cisplatin.
Angiogenesis, the formation of new blood vessels from preexisting ones, is a crucial step in tumor growth, invasion, and metastasis and represents a promising therapeutic target for cancer therapy.44 Angiogenin and VEGF are probably the most widely acknowledged initiators of angiogenesis.45,46
Our results revealed that the tumor content of angiogenin and the expression of VEGF markedly decreased in tumor treated with sildenafil. The decrease in the angiogenin content and VEGF expression was positively correlated with the decrease in the tumor volume, which indicates that the beneficial effect of sildenafil on the tumor volume induced by Ehrlich cell line is mediated, in part, by decreasing angiogenin and VEGF levels. In addition, sildenafil potentiated the attenuating effect of cisplatin on both angiogenin and VEGF. Our data are in contrast with the observations of Zhang et al47 who reported that systemic administration of sildenafil increased the angiogenin content in the ischemic boundary regions in a rat model of stroke. A proangiogenic action of sildenafil was further supported by observations that this PDE-5 inhibitor promoted wound healing in dogs.48
TNF-α is an inflammatory mediator that has been implicated in carcinogenesis.49,50 TNF-α has a double-edged role in angiogenesis depending on the cell type.49 TNF-α stimulates angiogenesis in the cornea and chorioallantoic membrane in vivo.51,52 It increases tumor growth, induces angiogenesis of diverse tumors in mice, and enhances the expression of angiogenic ligands, such as IL-8 and VEGF.53 On the other hand, it blocks both basal and basic fibroblast growth factor-stimulated growth in bovine aortic and capillary endothelial cells and human umbilical endothelial cells in culture.54,55
In this study, the content of TNF-α significantly decreased in the tumor isolated from mice treated with sildenafil; the decrease in TNF-α was positively correlated with the tumor volume. In accordance with our findings, sildenafil was documented to decrease the level of TNF-α in different models of inflammatory diseases.56 In addition, Hube et al57 demonstrated that pretreatment with PDE inhibitors suppresses TNF-α expression in human adipocyte precursor cells. In addition, the combination of cisplatin and sildenafil resulted in more decrease in TNF-α compared with tumors isolated from mice treated with cisplatin alone.
Consequently, the decreased TNF-α along with the decreased angiogenin and VEGF expression reported in our study indicate the antiangiogenic potential of sildenafil that might underlie, in part, the antitumor effect of sildenafil.
In the current study, sildenafil showed a cytotoxic activity against human mammary adenocarcinoma cell line, MCF-7, with IC50 values 14 μg/mL. In addition, it synergistically potentiated the cytotoxicity of cisplatin. These results were in agreement with those of Tinsley et al,58,59 who showed that PDE-5 inhibitors produce apoptosis and growth arrest in human breast cancer cells through increasing the level of cGMP. A recent study by Fallahian et al60 concluded that increased activation of protein kinase G by cGMP induces apoptosis and growth arrest in breast cancer MCF-7 and MDA-MB-468 cells. Furthermore, the nonspecific PDE-5 inhibitor exisulind and its analogs selectively induce the apoptosis of various human prostate, colon, and breast cancer cells, and the effect that was attributed to inhibition of PDE-5 expression.7,61
Conclusion
These findings shed light on profound antitumor activities of sildenafil against solid tumor induced by inoculation of Ehrlich cell line in mice as well as cytotoxic activity against MCF-7 in vitro. In addition, our results revealed that sildenafil could potentiate the antitumor activity of traditional chemotherapeutic agents such as cisplatin. This effect can allow effective reduction of cisplatin doses with subsequent less adverse reactions. Improvement of the tumor microenvironment through controlling abnormal cell proliferation, apoptosis, and angiogenesis may underlie the antitumor activity of sildenafil.
Disclosure
The authors report no conflicts of interest in this work.
References
Fajardo AM, Piazza GA, Tinsley HN. The role of cyclic nucleotide signaling pathways in cancer: targets for prevention and treatment. Cancers (Basel). 2014;6(1):436–458. doi:10.3390/cancers6010436. | ||
Eskandari N, Mirmosayyeb O, Bordbari G, Bastan R, Yousefi Z, Andalib A. A short review on structure and role of cyclic-3′,5′-adenosine monophosphate-specific phosphodiesterase 4 as a treatment tool. J Res Pharm Pract. 2015;4(4):175–181. doi:10.4103/2279-042X.167043. | ||
Piazza GA, Thompson WJ, Pamukcu R, et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61(10):3961–3968. | ||
Whitehead CM, Earle KA, Fetter J, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2(5):479–488. | ||
Black KL, Yin D, Ong JM, et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302. doi:10.1016/j.brainres.2008.06.122. | ||
Abadi AH, Abouel-Ella DA, Ahmed NS, et al. Synthesis of novel tadalafil analogues and their evaluation as phosphodiesterase inhibitors and anticancer agents. Arzneimittelforschung. 2009;59(8):415–421. | ||
Barone I, Catalano S, Campana A, et al. Expression and function of phosphodiesterase type 5 in human breast cancer cell lines and tissues: implications for targeted therapy. Clin Cancer Res. 2016;22(9):2271–2282. doi:1078-0432.CCR-15-1900. | ||
Tahsili-Fahadan P, Yahyavi-Firouz-Abadi N, Orandi AH, Esmaeili B, Basseda Z, Dehpour AR. Rewarding properties of sildenafil citrate in mice: role of the nitric oxide-cyclic GMP pathway. Psychopharmacology. 2006;185(2):201–207. | ||
Lukowski R, Krieg T, Rybalkin SD, Beavo J, Hofmann F. Turning on cGMP-dependent pathways to treat cardiac dysfunctions: boom, bust, and beyond. Trends Pharmacol Sci. 2014;35(8):404–413. doi:10.1016/j.tips.2014.05.003. | ||
Behmenburg F, Dorsch M, Huhn R, et al. Impact of mitochondrial Ca2+-sensitive potassium (mBKCa) channels in sildenafil-induced cardioprotection in rats. PLoS One. 2015;10(12):e0144737. doi:10.1371/journal.pone.0144737. | ||
de Almeida LS, Barboza JR, Freitas FP, et al. Sildenafil prevents renal dysfunction in contrast media-induced nephropathy in Wistar rats. Hum Exp Toxicol. Epub 2016 Jan 20. pii:0960327115626582. | ||
Toblli JE, Cao G, Angerosa M, Rivero M. Long-term phosphodiesterase 5 inhibitor administration reduces inflammatory markers and heat-shock proteins in cavernous tissue of Zucker diabetic fatty rat (ZDF/fa/fa). Int J Impot Res. 2015;27(5):182–190. doi:10.1038/ijir.2015.13. | ||
Gokakin AK, Atabey M, Deveci K, et al. The effects of sildenafil in liver and kidney injury in a rat model of severe scald burn: a biochemical and histopathological study. Ulus Travma Acil Cerrahi Derg. 2014;20(5):319–327. doi:10.5505/tjtes.2014.39586. | ||
Noel S, Dhooghe B, Leal T. PDE5 inhibitors as potential tools in the treatment of cystic fibrosis. Front Pharmacol. 2012;3:167. | ||
Zahran MH, Hussein AM, Barakat N, et al. Sildenafil activates antioxidant and antiapoptotic genes and inhibits proinflammatory cytokine genes in a rat model of renal ischemia/reperfusion injury. Int Urol Nephrol. 2015;47(11):1907–1915. doi:10.1007/s11255-015-1099-5. | ||
Savvanis S, Nastos C, Tasoulis MK, et al. Sildenafil attenuates hepatocellular injury after liver ischemia reperfusion in rats: a preliminary study. Oxid Med Cell Longev. 2014;2014:161942. doi:10.1155/2014/161942. | ||
Mei XL, Yang Y, Zhang YJ, et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res. 2015;5(11):3311–3324. | ||
Sarfati M, Mateo V, Baudet S, et al. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101(1):265–269. | ||
Abdel Moneim AE, Othman MS, Aref AM. Azadirachta indica attenuates cisplatin-induced nephrotoxicity and oxidative stress. Biomed Res Int. 2014;2014:647131. doi:10.1155/2014/647131. | ||
Abdel Moneim AE. Azadirachta indica attenuates cisplatin-induced neurotoxicity in rats. Indian J Pharmacol. 2014;46(3):316–321. doi: 10.4103/0253-7613.132182. | ||
Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007;7(1):3–18. | ||
Ou YW, Zhao ZT, Wu CY, Xu BN, Song YM, Zhan QM. Mig-2 attenuates cisplatin-induced apoptosis of human glioma cells in vitro through AKT/JNK and AKT/p38 signaling pathways. Acta Pharmacol Sin. 2014;35(9):1199–1206. doi:10.1038/aps.2014.60. | ||
Novohradsky V, Zerzankova L, Stepankova J, et al. Antitumor platinum(IV) derivatives of oxaliplatin with axial valproato ligands. J Inorg Biochem. 2014;140:72–79. doi:10.1016/j.jinorgbio.2014.07.004. | ||
Oh GS, Kim HJ, Shen A, et al. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press. 2014;12(2):55–65. doi:10.5049/EBP.2014.12.2.55. | ||
Wensing KU, Ciarimboli G. Saving ears and kidneys from cisplatin. Anticancer Res. 2013;33(10):4183–4188. | ||
National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996:2–107. | ||
Lee I, Boucher Y, Jain RK. Nicotinamide can lower tumor interstitial fluid pressure: mechanistic and therapeutic implications. Cancer Res. 1992;52(11):3237–3240. | ||
Bilalovic N, Sandstad B, Golouh R, Nesland JM, Selak I, Torlakovic EE. CD10 protein expression in tumor and stromal cells of malignant melanoma is associated with tumor progression. Mod Pathol. 2004;17(10):1251–1258. | ||
Smets LA, Bout B, Brouwer M, Tulp A. Cytotoxic effects of dexamethasone restricted to noncycling, early G1-phase cells of L1210 leukemia. J Cell Physiol. 1983;116(3):397–403. | ||
Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–1112. | ||
Das A, Durrant D, Mitchell C, et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci U S A. 2010;107(42):18202–18207. doi:10.1073/pnas.1006965107. | ||
O’Donovan N, Crown J, Stunell H, et al. Caspase 3 in breast cancer. Clin Cancer Res. 2003;9(2):738–742. | ||
Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6(11):1067–1074. | ||
Blanc C, Deveraux QL, Krajewski S, et al. Caspase-3 is essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res. 2000;60(16):4386–4390. | ||
Yang XH, Sladek TL, Liu X, Butler BR, Froelich CJ, Thor AD. Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Res. 2001;61(1):348–354. | ||
Quinn CM, Wright NA. The clinical assessment of proliferation and growth in human tumours: evaluation of methods and applications as prognostic variables. J Pathol. 1990;160(2):93–102. | ||
Shapiro HM. Flow cytometry of DNA content and other indicators of proliferative activity. Arch Pathol Lab Med. 1989;113(6):591–597. | ||
Baserga R. The cell cycle. N Engl J Med. 1981;304(8):453–459. | ||
Golias CH, Charalabopoulos A, Charalabopoulos K. Cell proliferation and cell cycle control: a mini review. Int J Clin Pract. 2004;58(12):1134–1141. | ||
von Herbay A, Sieg B, Schurmann G, Hofmann WJ, Betzler M, Otto HF. Proliferative activity of neuroendocrine tumours of the gastroenteropancreatic endocrine system: DNA flow cytometric and immunohistological investigations. Gut. 1991;32(8):949–953. | ||
Steck K, el-Naggar AK. Comparative flow cytometric analysis of Ki-67 and proliferating cell nuclear antigen (PCNA) in solid neoplasms. Cytometry. 1994;17(3):258–265. | ||
Shiohara S, Shiozawa T, Miyamoto T, et al. Expression of cyclins, p53, and Ki-67 in cervical squamous cell carcinomas: overexpression of cyclin A is a poor prognostic factor in stage Ib and II disease. Virchows Arch. 2005;446(6):626–633. | ||
Inwald EC, Klinkhammer-Schalke M, Hofstadter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139(2):539–552. doi:10.1007/s10549-013-2560-8. | ||
Raica M, Cimpean AM, Ribatti D. Angiogenesis in pre-malignant conditions. Eur J Cancer. 2009;45(11):1924–1934. doi:10.1038/sj.bjc.6604733. | ||
Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52(2):237–268. | ||
Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56(3):794–814. | ||
Zhang R, Wang L, Zhang L, et al. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92(3):308–313. | ||
Taş A, Atasoy N, Özbek H, et al. Effects of sildenafil citrate (Viagra) in the early phase of healing process in open wounds induced experimentally in dogs. Acta Vet Brno. 2003;72:273–277. doi:10.2754/avb200372020273. | ||
Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140(3):539–544. | ||
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi:10.1155/2014/149185. | ||
Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Bohlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987;84(15):5277–5281. doi:10.1073/pnas.84.15.5277. | ||
Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329(6140):630–632. | ||
Li B, Vincent A, Cates J, Brantley-Sieders DM, Polk DB, Young PP. Low levels of tumor necrosis factor alpha increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res. 2009;69(1):338–348. doi:10.1158/0008-5472.CAN-08-1565. | ||
Sato N, Fukuda K, Nariuchi H, Sagara N. Tumor necrosis factor inhibiting angiogenesis in vitro. J Natl Cancer Inst. 1987;79(6):1383–1391. | ||
Schweigerer L, Malerstein B, Gospodarowicz D. Tumor necrosis factor inhibits the proliferation of cultured capillary endothelial cells. Biochem Biophys Res Commun. 1987;143(3):997–1004. doi:10.1016/0006-291X(87)90350-0. | ||
Karakoyun B, Uslu U, Ercan F, et al. The effect of phosphodiesterase-5 inhibition by sildenafil citrate on inflammation and apoptosis in rat experimental colitis. Life Sci. 2011;89(11–12):402–407. doi:10.1016/j.lfs.2011.07.005. | ||
Hube F, Lee YM, Rohrig K, Hauner H. The phosphodiesterase inhibitor IBMX suppresses TNF-alpha expression in human adipocyte precursor cells: a possible explanation for its adipogenic effect. Horm Metab Res. 1999;31(6):359–362. | ||
Tinsley HN, Gary BD, Keeton AB, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8(12):3331–3340. doi:10.1158/1535-7163.MCT-09-0758. | ||
Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/beta-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila). 2011;4(8):1275–1284. doi:10.1158/1940-6207.CAPR-11-0095. | ||
Fallahian F, Karami-Tehrani F, Salami S, Aghaei M. Cyclic GMP induced apoptosis via protein kinase G in oestrogen receptor-positive and -negative breast cancer cell lines. FEBS J. 2011;278(18):3360–3369. doi:10.1111/j.1742-4658.2011.08260.x. | ||
Abadi AH, Gary BD, Tinsley HN, Piazza GA, Abdel-Halim M. Synthesis, molecular modeling and biological evaluation of novel tadalafil analogues as phosphodiesterase 5 and colon tumor cell growth inhibitors, new stereochemical perspective. Eur J Med Chem. 2010;45(4):1278–1286. doi:10.1016/j.ejmech.2009.10.046. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.