Back to Journals » Cancer Management and Research » Volume 11
Significance of the neutrophil-to-lymphocyte ratio in young patients with oral squamous cell carcinoma
Authors Zhang B, Du W, Gan K, Fang Q , Zhang X
Received 11 April 2019
Accepted for publication 10 July 2019
Published 9 August 2019 Volume 2019:11 Pages 7597—7603
DOI https://doi.org/10.2147/CMAR.S211847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Baixia Zhang,1 Wei Du,2 Kang Gan,1 Qigen Fang,2 Xu Zhang2
1Department of Stomatology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, People’s Republic of China; 2Department of Head Neck and Thyroid, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, Henan Province, People’s Republic of China
Background: The main goal of this study was to evaluate the prognosis of young patients with oral squamous cell carcinoma (SCC) with a focus on the value of the pretreatment neutrophil-to-lymphocyte ratio (NLR).
Materials and methods: Young (≤40 years old) patients with oral SCC were retrospectively enrolled, and each young patient was matched with an old (≥60 years old) oral SCC patient. Associations between the NLR and clinicopathological variables were analyzed by the chi-square test, and the Kaplan–Meier method was used to analyze recurrence-free survival (RFS) and disease-specific survival (DSS) rates.
Results: A total of 103 young patients were enrolled, and compared to the old group, the young group had a significantly lower NLR value (p=0.012). In the young group, the 5-year RFS and DSS rates were 82% and 85%, respectively. In the old group, the 5-year RFS and DSS rates were 65% and 71%, respectively, and the differences between the groups were significant (both p<0.05). In the young patients with an NLR≤2.56, the 5-year DSS rate was 93%, while in the young patients with an NLR >2.56, the 5-year DSS rate was 76%. This difference was significant (p=0.020). A further Cox model analysis confirmed that the NLR was an independent prognostic factor for DSS.
Conclusion: Young patients with oral SCC have a better prognosis than old oral SCC patients, and the NLR is significantly associated with DSS in young patients.
Keywords: oral squamous cell carcinoma, young patient, head and neck cancer, neutrophil-to-lymphocyte ratio, survival analysis
Introduction
Oral squamous cell carcinoma (SCC) is a common and often lethal form of head and neck SCC; traditionally, it is thought to affect men older than 60 years old after extensive alcohol and tobacco use. However, an alarming increase in the incidence of oral SCC among young patients has been noted by other authors.1,2 Generally, young patients are less likely to have a history of alcohol or tobacco exposure, and the common risk factors might act as inflammation promoters that upregulate the neutrophil-to-lymphocyte ratio (NLR).3–7 The NLR is a well-known inflammatory marker, several cytokines, and angiogenic factors can be produced by neutrophils, and these agents play important roles in promoting tumor development, and lymphocytes are associated with immune surveillance and act by eliminating cancer cells, a decreased lymphocyte level is related to poorer ability to eliminate cancer cells. Increasingly stronger evidence has indicated the prognostic role of the NLR in traditional head and neck SCC,3–8 but no researchers have analyzed whether there is a similar phenomenon in young patients. Moreover, the prognosis in young patients remains controversial, although many comparable outcomes have been reported in young and old patients. A recent study suggested that young patients may actually have worse recurrence rates and a worse prognosis,8 which suggests that young oral SCC patients might be different from traditional oral SCC patients. Therefore, the current study evaluated the prognosis of young patients with oral SCC with a focus on the value of the pretreatment NLR.
Patients and methods
The Zhengzhou University institutional research committee approved our study, all participants provided written informed consent for medical research prior to their initial treatment, this study was conducted in accordance with the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations.
Consecutive patients from January 1995 to July 2018 surgically treated for primary oral SCC were retrospectively reviewed. Eligible patients were those who were ≤40 or ≥60 years old, of any sex, able to provide written informed consent, and not previously treated for oral SCC. Data regarding age, sex, TNM stage, the pretreatment NLR, pathological characteristics, surgical treatment, and follow-up were collected and analyzed. All pathological sections were re-reviewed, and all patients were routinely followed up by out-patient clinic, telephone, email, or we-chat.
In our cancer center, neck dissection was routinely suggested for any stage of oral SCC; in extreme cases, the wait-and-see approach was reserved only for patients with a very small primary lesion. Adjuvant treatments including radiotherapy or chemoradiotherapy were suggested if there was presence of high-risk factors including T3 to T4 or N2 to N3 disease, positive surgical margins, extracapsular extension, lymphovascular invasion, and perineural invasion. The regimens of chemotherapy were mainly platinum-based drugs.
Systematic examination was routinely performed with the help of ultrasound, CT, MRI, and/or PET-CT examinations. TNM staging was performed based on the AJCC 7th edition classification.
The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count measured within 2 weeks before the initial treatment.3–9 The cutoff values calculated from the ROC curve, mean, tertiles, or median in previous studies varied from 1.98 to 5,3–9 and the standard cutoff value remains undetermined. In the current study, the cutoff value was defined as the mean value of the NLR based on our previous studies.8,9
The patients ≤40 years old were enrolled as a young group. Matched patients ≥60 years old were selected. In this study, each young patient was matched to an old patient, and the patients were matched by sex, smoking status, drinking status, primary site, margin status, disease stage (stage I/II vs stage III/IV), and neck node status. The matched patients ≥60 years old were enrolled as the old group.9,10 Drinkers were defined as patients who consumed at least one alcoholic drink per day for at least 1 year,11,12 and smokers were defined as patients who smoked on a daily basis.12
The chi-square test was used to analyze the associations between the NLR and clinicopathological variables. Recurrence-free survival (RFS) and disease-specific survival (DSS) were calculated from the date of surgery to the date of the event or the last follow-up. The Kaplan–Meier approach was used to calculate the RFS and DSS rates. Factors that were significant in univariate analysis (log-rank method) were then analyzed by Cox model analysis to identify the independent risk factors for the RFS and DSS rates. All statistical analyses were performed by using SPSS 20.0, and p<0.05 was considered significant.
Results
A total of 103 (53 female and 50 male) young patients were enrolled, all patients underwent a neck dissection operation, a negative margin was achieved in all patients, and no patients had chronic steroid use or an autoimmune disease. The clinicopathological characteristics of the two groups are presented in Table 1. More patients received adjuvant therapy in young group, including radiotherapy and chemotherapy, than those in the old group (p<0.001), and the NLR value was significantly lower in the young patients than in the old patients (p=0.012). There was no apparent difference regarding adverse pathological characteristics (all p>0.05).
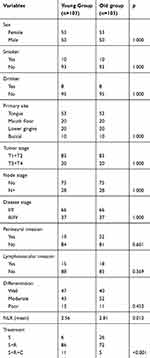 |
Table 1 Clinical pathologic information of the two groups |
During our follow-up, which had a mean time of 89.9 (range: 7–205) months, recurrence occurred in 18 and 34 patients in the young and old groups, respectively. In the young group, there were 5 local recurrences, 7 regional recurrences, 3 locoregional recurrences, and 3 distant recurrences, while in the old group, there were 9 local recurrences, 10 regional recurrences, 12 locoregional recurrences, and 3 distant recurrences; the difference between the groups was not significant (p=0.501). The 5-year RFS rates in the young and old groups were 82% and 65%, respectively, and the difference was significant (Figure 1, p=0.014). Disease-related death occurred in 11 and 27 patients in the young and old groups, respectively. The 5-year DSS rates in the young and old groups were 85% and 71%, respectively, and the difference was significant (Figure 2, p=0.010).
 |
Figure 1 Comparison of recurrence-free survival between young and old groups (p=0.014). |
 |
Figure 2 Comparison of disease-specific survival between young and old groups (p=0.010). |
The associations between the NLR and clinicopathological variables are presented in Table 2. A high NLR was significantly related to advanced-stage disease and poor differentiation. There were no positive associations between the NLR and sex, tumor stage, node stage, perineural invasion, or lymphovascular invasion.
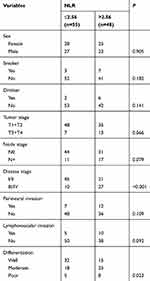 |
Table 2 Association between neutrophil-to-lymphocyte ratio (NLR) and clinical pathologic characteristics in young patients with oral squamous cell carcinoma |
Univariate analysis (log-rank analysis) of RFS in the young group showed that in the patients with an NLR≤2.56, the 5-year RFS rate was 83%, while in the patients with an NLR >2.56, the 5-year RFS rate was 80%, and the difference was not significant (p=0.643). Other factors including the primary site, disease stage, tumor differentiation, radiotherapy use, and lymphovascular invasion status were significantly associated with RFS (all p<0.05). In a further Cox model analysis, the primary site, disease stage, and lymphovascular invasion status were independent predictors of RFS (Table 3).
 |
Table 3 Predictors for recurrence-free survival in young patients with oral squamous cell carcinoma |
Univariate analysis (log-rank analysis) of DSS in the young group showed that in the patients with an NLR≤2.56, the 5-year DSS rate was 93%, while in the patients with an NLR >2.56, the 5-year DSS rate was 76%, and the difference was significant (Figure 3, p=0.020). Other factors including the primary site, disease stage, tumor differentiation, perineural invasion status, and radiotherapy use were also significantly associated with DSS (all p<0.05). In a further Cox model analysis, a high NLR was also an independent predictor of worse DSS (Table 4).
 |
Figure 3 Disease-specific survival in patients with different neutrophil-to-lymphocyte ratio (NLR) (p=0.020). |
 |
Table 4 Predictors for disease-specific survival in young patients with oral squamous cell carcinoma |
Discussion
Oral SCC is uncommon in patients less than 40 years old, and no overall consensus concerning the difference in prognosis between old and young patients has been reached. One of the main findings in the current study was that the prognosis of young patients was significantly better than that of old patients, and this finding conflicted some with the findings of previous reports. Farquhar et al,13 found that although there was no difference in overall mortality, young patients were more likely to recur within 3 years than old patients, and the authors concluded that oral SCC among young patients may be distinct from traditional oral SCC. Hilly et al,14 described recurrence in similar proportions of patients in young and old groups (38% and 29.9%, respectively), and a Kaplan–Meier analysis yielded no between-group differences in disease-free or overall survival. Pitman et al,15 noted that the 3-year disease-free survival rate of a group of patients older than 40 years old was 55.0%, while the 3-year disease-free survival rate was 53.3% in young groups, and the authors suggested that the outcomes of treatment for tongue SCC in young patients were similar to those in patients older than 40 with a similar extent of disease. Friedlander et al,16 reported that younger patients with tongue SCC had a higher rate of locoregional recurrence than older patients, but this difference did not translate into a survival difference. However, the results of these reports were potentially influenced by one or more confounding factors that affected the interpretation of the outcomes. First, the definition of young patients was inconsistent, and the cutoff age varied from 30 years to 40 years. Second, the factors of smoking and alcohol use were never matched to prevent interference in previous studies;13–16 the incidences of smokers and drinkers were usually higher in the older groups, and alcohol use and smoking have immunosuppressive effects and are associated with a worse prognosis,17,18 which would lead to decreased disease control in the older, control groups. In the current study, as many confounding factors as possible were matched for increasing the study reliability, but there were still some differences regarding the baseline data of the two groups, which might partially explain the prognosis variation. Owing to the better toleration of side effects, more adjuvant treatments were performed in the young patients, and this increased treatment surely could improve patient prognosis. On the other hand, it was interesting to find that the NLR value was significantly lower in the young patients than in the old patients, and previous strong evidence has shown that a high NLR value is a predictor of a worse prognosis in head and neck SCC.3–7 Additionally, although we did not have the HPV status of the patients, because of the higher sexual activity in young patients, there might be higher rate of HPV infection in young patients, and several authors had shown the correlation between HPV + and better prognosis in young people.19,20 Furthermore, other unassessed factors, including poor oral hygiene and poor dentures in the old patients, might also be responsible for this finding.
The significance of the NLR in traditional head and neck SCC has been widely analyzed. Yu et al,21 described that head and neck cancer patients with an elevated pretreatment NLR in the peripheral blood were prone to local invasion and distant recurrence and had a poor prognosis. Kano et al,22 analyzed data from 285 patients with head and neck cancer treated with concurrent chemotherapy and found that there were significant relationships between a high NLR and hypopharyngeal or oropharyngeal cancer, N2b to N3 stages, T3 to T4 stages, and clinical stages III to IV and that in further survival assessments, a high NLR was significantly associated with decreases in disease-free survival and overall survival. However, whether there are similar findings in young oral SCC patients remains unknown. The current study is the first to report that a high NLR is related to adverse pathological characteristics and DSS but not RFS.
The exact mechanisms underlying the association between the NLR and the prognosis remain unknown, but some possible explanations can be inferred from previous evidence. On the one hand, an elevated neutrophil level is a sign of local and systemic inflammatory responses. Several cytokines and angiogenic factors can be produced by neutrophils, and these agents play important roles in promoting tumor development.23 These hematological markers are also surrogate markers of cancer cachexia, which is related to poor survival.24,25 On the other hand, lymphocytes are associated with immune surveillance and act by eliminating cancer cells,26 a decreased lymphocyte level is related to poorer ability to eliminate cancer cells. Therefore, a high NLR is considered to predict a worse prognosis.
We failed to note there is a positive relationship between NLR and the RFS in young patients, the finding was conflicted with previous researchers,9,21,22 possible explanation was our different subjects, only young patients were enrolled in the current study, young oral SCC patients might be a distinct entity from traditional oral SCC patients owing to different pathogenic factors, additionally, as our result described, lower NLR in young patients might mean relatively milder negative effect than higher NLR in old patients.
The limitations of the current study must be acknowledged. First, the number of events in the younger cohort was relatively small, which might decrease the statistical power of our research. A study with a larger sample size is needed to clarify some questions. Second, this is a retrospective study, and there is an inherent bias that might decease the statistical power. Third, it should be recognized that neutrophil and lymphocyte counts are nonspecific parameters because they can be influenced by concomitant conditions, such as infections or inflammation.
In summary, young patients with oral SCC have a better prognosis than old patients, and the NLR is significantly associated with DSS in young patients.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The primary data can be obtained from the corresponding author.
Ethics Statement
The Zhengzhou University institutional research committee approved our study, all participants provided written informed consent for medical research prior to their initial treatment, this study was conducted in accordance with the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations. All the material came from our cancer center, and the consent to publish was obtained from all patients.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ng JH, Iyer NG, Tan M-H, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck. 2017;39:297–304. doi:10.1002/hed.24589
2. Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29:1488–1494. doi:10.1200/JCO.2010.31.7883
3. Chen MF, Tsai MS, Chen WC, Chen PT. Predictive value of the pretreatment neutrophil-to-lymphocyte ratio in head and neck squamous cell carcinoma. J Clin Med. 2018;20:7.
4. Mascarella MA, Mannard E, Silva SD, Zeitouni A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: a systematic review and meta-analysis. Head Neck. 2018;40:1091–1100. doi:10.1002/hed.25075
5. Rosculet N, Zhou XC, Ha P, et al. Neutrophil-to-lymphocyte ratio: prognostic indicator for head and neck squamous cell carcinoma. Head Neck. 2017;39:662–667. doi:10.1002/hed.24658
6. Sumner WA, Stokes WA, Oweida A, et al. Survival impact of pre-treatment neutrophils on oropharyngeal and laryngeal cancer patients undergoing definitive radiotherapy. J Transl Med. 2017;15:168. doi:10.1186/s12967-017-1268-7
7. Valero C, Pardo L, López M, et al. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck. 2017;39:219–226. doi:10.1002/hed.24561
8. Fang Q, Liu F, Seng D. Oncologic outcome of parotid mucoepidermoid carcinoma in pediatric patients. Cancer Manag Res. 2019;11:1081–1085. doi:10.2147/CMAR.S192788
9. Fang Q, Li P, Qi J, Luo R, Chen D, Zhang X. Value of lingual lymph node metastasis in patients with squamous cell carcinoma of the tongue. Laryngoscope. 2019. doi:10.1002/lary.27927
10. Blanchard P, Belkhir F, Temam S, et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: a single-institution case-matched analysis. Eur Arch Otorhinolaryngol. 2017;274:1683–1690. doi:10.1007/s00405-016-4419-1
11. Fang QG, Shi S, Liu FY, Sun CF. Squamous cell carcinoma of the oral cavity in ever smokers: a matched-pair analysis of survival. J Craniofac Surg. 2014;25:934–937. doi:10.1097/SCS.0000000000000710
12. Bachar G, Hod R, Goldstein DP, et al. Outcome of oral tongue squamous cell carcinoma in patients with and without known risk factors. Oral Oncol. 2011;47:45–50. doi:10.1016/j.oraloncology.2010.11.003
13. Farquhar DR, Tanner AM, Masood MM, et al. Oral tongue carcinoma among young patients: an analysis of risk factors and survival. Oral Oncol. 2018;84:7–11. doi:10.1016/j.oraloncology.2018.06.014
14. Hilly O, Shkedy Y, Hod R, et al. Carcinoma of the oral tongue in patients younger than 30 years: comparison with patients older than 60 years. Oral Oncol. 2013;49:987–990. doi:10.1016/j.oraloncology.2013.07.005
15. Pitman KT, Johnson JT, Wagner RL, Myers EN. Cancer of the tongue in patients less than forty. Head Neck. 2000;22:297–302.
16. Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck. 1998;20:363–368.
17. Gerson SJ. Oral cancer. Crit Rev Oral Biol Med. 1990;1:153–166. doi:10.1177/10454411900010030101
18. Chen AM, Chen LM, Vaughan A, et al. Head and neck cancer among lifelong never-smokers and ever-smokers: matched-pair analysis of outcomes after radiation therapy. Am J Clin Oncol. 2011;34:270–275. doi:10.1097/COC.0b013e3181dea40b
19. Wang F, Zhang H, Xue Y, et al. A systematic investigation of the association between HPV and the clinicopathological parameters and prognosis of oral and oropharyngeal squamous cell carcinomas. Cancer Med. 2017;6:910–917. doi:10.1002/cam4.1045
20. Abbate V, Dell’Aversana Orabona G, Salzano G, et al. Pre-treatment Neutrophil-to-Lymphocyte Ratio as a predictor for occult cervical metastasis in early stage (T1-T2 cN0) squamous cell carcinoma of the oral tongue. Surg Oncol. 2018;27:503–507. doi:10.1016/j.suronc.2018.06.002
21. Yu Y, Wang H, Yan A, et al. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: a meta-analysis. BMC Cancer. 2018;18:383. doi:10.1186/s12885-018-4242-8
22. Kano S, Homma A, Hatakeyama H, et al. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck. 2017;39:247–253. doi:10.1002/hed.24576
23. Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol. 2013;23:159–170. doi:10.1016/j.semcancer.2013.02.004
24. Kawakita D, Tada Y, Imanishi Y, et al. Impact of hematological inflammatory markers on clinical outcome in patients with salivary duct carcinoma: a multi-institutional study in Japan. Oncotarget. 2017;8:1083–1091. doi:10.18632/oncotarget.13565
25. Liu F, Cheng G, Fang Q, Sun Q. Natural history of untreated squamous cell carcinoma of the head and neck. Clin Otolaryngol. 2018; Epub ahead of print. doi:10.1111/coa.13260.
26. Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2012;107:864–873. doi:10.1038/bjc.2012.347
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
