Back to Journals » Cancer Management and Research » Volume 10
Significance of circulating tumor cells in osteosarcoma patients treated by neoadjuvant chemotherapy and surgery
Authors Wu ZJ , Tan JC, Qin X, Liu B, Yuan ZC
Received 6 June 2018
Accepted for publication 24 July 2018
Published 10 September 2018 Volume 2018:10 Pages 3333—3339
DOI https://doi.org/10.2147/CMAR.S176515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Zhen-Jie Wu,* Jia-Chang Tan,* Xiong Qin,* Bin Liu, Zhen-Chao Yuan
Department of Bone and Soft Tissue Surgery, Affiliated Tumor Hospital, Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Purpose: By examining and identifying circulating tumor cell (CTC) counts and subtypes of peripheral blood in osteosarcoma patients, we evaluated the relationship between CTCs and characteristics of osteosarcoma patients, as well as CTC changes after neoadjuvant chemotherapy and surgery.
Methods: CanPatrol™ CTC technology was used to detect CTCs in peripheral blood before and after treatment in 32 osteosarcoma patients. Peripheral blood samples from 10 healthy volunteers were included as controls and examined for the presence of CTCs.
Results: Of the 32 osteosarcoma patients, CTCs were detected in 30 patients before treatment, and the average CTC count was 14.06±9.08. No CTCs were detected in the 10 healthy volunteers. The detected CTCs were divided into epithelial CTCs, mesenchymal CTCs (M-CTCs), and biophenotypic epithelial/mesenchymal CTCs. The average number of pretreatment CTCs was higher in stage III patients than in stage IIB patients (P=0.012). Twenty-eight patients were screened for changes in CTC count at 1 week after neoadjuvant chemotherapy and at 4 weeks after surgery. We divided these 28 patients into two groups according to the changes in the percentage of M-CTCs before and after treatment, and the results showed that the disease-free survival (DFS) was significantly shorter in the M-CTC percentage-increased group than in the M-CTC percentage-decreased or no-change group (P=0.032). Five patients with stage II osteosarcoma were examined for CTCs at the appearance of lung metastases, and the total number of CTCs was found to be higher at the appearance of lung metastases than before treatment in these patients.
Conclusion: The rate of presence of CTCs in the peripheral blood of osteosarcoma patients is high, and patients with an increased percentage of M-CTCs after treatment have a shorter DFS. The dynamic monitoring of changes in CTC counts after treatment has clinical significance for the timely detection of recurrence or metastasis.
Keywords: circulating tumor cells, osteosarcoma, neoadjuvant chemotherapy, surgery
Introduction
Osteosarcoma is a rapidly growing malignant tumor and accounts for about 60% of primary malignant bone tumors in adolescents.1 Local recurrence and distant metastasis are the most important factors causing death in patients with osteosarcoma. The local recurrence rate of osteosarcoma after surgery is approximately 10%–20%. Once the tumor recurs, the survival rate is significantly reduced.2 About 10%–20% of patients have distant metastases at the first visit, of which 90% are lung metastases, and about 50% of patients receiving regular treatment have lung metastases. Once patients with osteosarcoma develop distant metastases, their 5-year survival rate drops to 20%–30%.3 However, since the advent of combined neoadjuvant chemotherapy4 and extensive tumor resection,5 the 5-year survival rate has increased from 20% to 70%, and the limb salvage rate has increased from <20% to >80%.
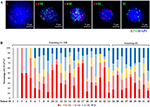
Circulating tumor cells (CTCs) are a subset of cells derived from a primary tumor and are released into the peripheral blood circulation under specific conditions. They possess the biological characteristics of the tumor and become the seeds of metastases in vital distant organs. Hence, CTCs are considered to be one of the important causes of tumor recurrence and distant metastasis.6 CTCs include five subtypes according to the epithelial-to-mesenchymal transition (EMT) markers, namely epithelial CTCs (E-CTCs), mesenchymal CTCs (M-CTCs), and biophenotypic epithelial/mesenchymal CTCs (E<M-CTCs, E>M-CTCs, and E≈M-CTCs), which are closely related to tumor progression.
The number of CTCs in the peripheral blood is extremely small. In recent years, with the rapid development of immunomagnetic beads, biological cell-sorting chips, microporous membranes, and multicolor fluorescent labels, the enrichment, separation, and detection of CTCs have become a reality. Detection of CTCs in the peripheral blood of patients with cancer is of great significance for the diagnosis and prognosis evaluation. Detection of CTCs can indicate recurrence and micrometastasis of tumor cells earlier than imaging methods and can be used to monitor the development status of tumors, as well as to evaluate the prognosis of patients.7
In 2004, the Food and Drug Administration approved the CellSearch® system for the clinical detection of CTCs, which is a representative product for the positive enrichment of CTCs based on immunomagnetic bead assays and has been widely verified by many studies.8,9 EpCAM is expressed exclusively in epithelia and epithelial-derived neoplasms.10 The CellSearch® system specifically enriches the E-CTCs through an EpCAM antibody. However, CTCs act as a bridge between primary tumors and metastases, undergoing EMT during metastasis and giving them greater metastatic potential. Tumor cells that have acquired the mesenchymal phenotype after EMT are more likely to enter the circulatory system. Therefore, the limitation of using the CellSearch® detection system is that it cannot enrich the M-CTCs.11,12
CanPatrol™ CTC second-generation capture technology was used to isolate and characterize CTCs from the peripheral blood of cancer patients. The red blood cells in the peripheral blood are first lysed, and then the CTCs are separated and enriched by nanotechnology according to the difference in the size of CTCs and white blood cells. Subsequently, EMT-related markers and RNA in situ hybridization (RNA-ISH) were used to identify the five subtypes of CTCs, including M-CTCs, which make up for the shortcomings of the CellSearch® system.13,14
In this study, CanPatrol™ CTC second-generation capture technology was used to detect the phenotypes of CTCs in the peripheral blood of patients with osteosarcoma at different stages. We evaluated the relationship between CTCs and characteristics of osteosarcoma patients, as well as CTC changes after neoadjuvant chemotherapy and surgery.
Methods
Patients and blood samples
This study was approved by the Committee on Reasoning at the Affiliated Tumor Hospital of Guangxi Medical University. All subjects signed an informed consent form, and written informed consents were signed by a parent or legal guardian for participants under the age of 18 years. For the study, 32 osteosarcoma patients were recruited and their diagnosis was confirmed by pathological examination at the Affiliated Tumor Hospital of Guangxi Medical University from January 2015 to December 2017. None of these patients had undergone any prior treatment. Ten healthy volunteers from Guangxi Medical University were included in the study as controls. The following information was collected from participants: age, gender, Enneking stage, tumor location, tumor size, and clinicopathological type. All enrolled patients received four cycles of neoadjuvant chemotherapy after the diagnosis of osteosarcoma, followed by surgery 4 weeks later and chemotherapy 4 weeks after surgery.
Blood samples were collected at the following three time points: before neoadjuvant chemotherapy, 1 week after neoadjuvant chemotherapy, and 4 weeks after surgery. To avoid cell contamination due to venipuncture of the skin, the first 2 mL of the collected peripheral blood was discarded, and then 7.5 mL of blood was collected into a 10 mL tube containing 2.5 mL of EDTA anticoagulant. All blood samples were tested within 4 hours of collection using the CanPatrol™ System (SurExam Biotech, Guangzhou, People’s Republic of China).13
Enneking staging system
Musculoskeletal tumors are staged according to the Enneking system,15 which includes two staging systems for benign and malignant tumors. The Enneking system for benign musculoskeletal tumors is based on radiographic characteristics of the tumor host margin: latent, active, and aggressive. The Enneking surgical staging system for primary malignant mesenchymal tumors is based on the tumor grade (G, a histologic evaluation of cellular atypia and relates to the risk of metastasis), anatomical location (T), and metastasis (M), and classifies tumors into three stages (Table 1).15,16
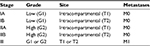  | Table 1 Enneking staging system for primary malignant mesenchymal tumors Note: M0, no regional or distant metastasis; M1, regional or distant metastasis. |
Isolation of CTCs from peripheral blood
We used the previously reported CanPatrol™ CTC enrichment technique14 to identify CTCs. Briefly, red blood cell lysis buffer was used to remove erythrocytes from the blood sample, and a size-based filtration system with an 8 µm pore filter membrane was used to remove leukocytes. RNA-ISH, as described below, and EMT markers were used to identify CTC subpopulations according to differential expressions of EpCAM, vimentin, and the leukocyte marker CD45. E-CTCs are only positive for epithelial-related markers (EpCAM and cytokeratins 8/18/19), M-CTCs are only positive for mesenchymal markers (vimentin and twist), and biophenotypic epithelial/mesenchymal CTCs simultaneously express epithelial and mesenchymal genes.
RNA-ISH assay
The detailed RNA-ISH assay procedure has been previously published.13 Briefly, enriched cells from the CanPatrol™ technique were permeabilized and digested with protease (Qiagen, Hilden, Germany). The digested cell suspension was subjected to a series of hybridization reactions with captured probes specific for EpCAM, vimentin, and CD45. Finally, DAPI was used to stain the cell nucleus. The samples were analyzed with a fluorescent microscope.
Statistical analysis
The data were analyzed using SPSS 21.0 (IBM Corp., Armonk, NY, USA). Data were recorded as median and range (or mean and SD) for continuous variables or as frequencies and percentages for categorical variables. The correlations of CTC counts with clinical parameters were evaluated using a two-sided chi-squared test or one-way ANOVA. A P-value of <0.05 was considered statistically significant.
Results
Patient characteristics and classification of CTCs
This study enrolled 32 patients with osteosarcoma (mean age: 23 years, range: 10–56 years) which included 25 patients (78%) with intramedullary osteosarcoma and seven patients (22%) with intracortical osteosarcoma. According to the Enneking staging system, two cases (6%) were classified as IB, two cases (6%) were classified as IIA, 19 cases (60%) were classified as IIB, and nine cases (28%) were classified as III. The detected CTCs were divided into E-CTCs, E>M-CTCs, E≈M-CTCs, E<M-CTCs, and M-CTCs (Figure 1A). Of the 32 patients with osteosarcoma, CTCs were detected in the peripheral blood of 30 patients (94%) before treatment, and the average CTC count was 14.06±9.08 (Figure 1B). Two patients (stage IB) did not have any CTCs. No CTCs were present in the peripheral blood of the 10 healthy volunteers. Furthermore, the average number of pretreatment CTCs was significantly related to the Enneking stage and was higher in stage III patients than in stage IIB patients (P=0.012). There was no significant relationship between the number of CTCs and clinicopathological variables of osteosarcoma, including age, gender, tumor location, tumor size, and clinicopathological type (Table 2).
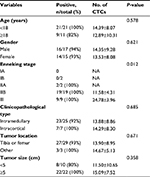  | Table 2 Relationship between characteristics of osteosarcoma patients and number of CTCs before treatment Abbreviations: CTC, circulating tumor cell; NA, not applicable. |
CTCs and therapeutic response
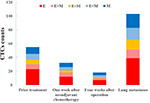
Twenty-eight patients with osteosarcoma (19 in stage IIB and nine in stage III) were screened for changes in CTC counts at 1 week after neoadjuvant chemotherapy and at 4 weeks after surgery, which included limb salvage surgery and amputations. Statistical results showed that at both of these time points, the total number of CTCs, the percentage of E-CTCs, and the percentage of mixed CTCs were all decreased in 28 patients, but the percentage of M-CTCs was increased (Figure 2). Furthermore, the average number of CTCs was higher in stage III patients than in stage IIB patients both at 1 week after neoadjuvant chemotherapy (P=0.026) and at 4 weeks after surgery (P=0.010) (Figure 3). We divided the 28 patients into two groups according to the changes in the total number of CTCs before treatment and 4 weeks after surgery, and the Kaplan–Meier method was used to analyze the differences in the disease-free survival (DFS) between the two groups. The results showed that there was no statistical difference in the DFS between the two groups (P=0.315). We then divided the 28 patients into two groups according to the changes in the percentage of the M-CTCs before treatment and 4 weeks after surgery. The results showed that the DFS was significantly shorter in the M-CTC percentage-increased group than in the M-CTC percentage-decreased or no-change group (P=0.032) (Figure 4). All included patients completed four cycles of neoadjuvant chemotherapy, limb salvage surgery or amputation, and an average of 12.5±3.3 cycles of postoperative chemotherapy. Five of these patients with stage II osteosarcoma were examined for CTCs at the appearance of lung metastases (median 2.1 years after surgery) on computed tomography. The total number of CTCs was higher at the appearance of lung metastases than before treatment in these five patients (Figure 5).
Discussion
Compared with traditional tissue biopsy, CTC detection and characterization have the advantages of simplicity, noninvasiveness, and real-time monitoring, and can obtain a large amount of tumor-related information, earning it the nickname “liquid biopsy”. Current CTC capture techniques involve the use of physical properties and employing cell surface antigens for separation. In the current study, the CanPatrol™ CTC detection and phenotyping system, which includes CTC separation and typing identification, was tested on 32 osteosarcoma patients. In the peripheral blood, the CTCs were separated from the lysed red blood cells and enriched by nanotechnology according to the difference in the size of CTCs and white blood cells. RNA-ISH and EMT markers were then used to identify five different subtypes of CTCs, including the “M-CTCs”, which is not possible using CellSearch®.
CTCs were detected in 30 of 32 osteosarcoma patients before treatment, and the rate of presence of CTCs was as high as 94%. This study also revealed that the total number of CTCs and the percentage of M-CTCs were significantly higher in stage III patients than in stage IIB patients. This suggests that CTCs, particularly M-CTCs, may play a crucial role in the metastasis of osteosarcoma. The results of this study are consistent with those reported by Zhong et al;17 the CTC counts correlated significantly with the Enneking stage (P<0.001), and the ratio of M-CTCs correlated with the distant metastases (P<0.001).
Studies have shown that changes in CTCs can reflect the tumor’s growth activity and sensitivity to chemotherapy, and can be used as a reference to evaluate the effect of individualized tumor therapy.18 In this study, 28 patients with osteosarcoma were retested for CTCs after treatment (including neoadjuvant chemotherapy and surgical treatment). In these 28 patients, the total number of CTCs after treatment decreased compared with that before treatment, but the total percentage of M-CTCs increased compared with that before treatment. Subsequent subgroup analysis revealed no significant difference in DFS between the CTC count-decreased and CTC count-increased groups. Patients with an increased percentage of M-CTCs after surgery had a shorter DFS. The specific reason for this outcome is not yet clear, but may be related to the strong metastatic invasion and the antiapoptotic ability of M-CTCs.19
After a primary tumor has been surgically resected, an increase in the total number of CTCs in the peripheral blood may indicate that the primary tumor has undergone colonization and metastasis in some target organs. This important indicator of metastasis may not be detected by traditional test methods.20 In this study, five patients with osteosarcoma who suffered lung metastasis after treatment were tested for peripheral blood CTCs, and it was found that the total number of CTCs was higher at the appearance of lung metastases than before treatment. The results suggest that changes in CTCs can be detected on a regular basis, and that metastases may be discovered in time for successful treatment.
Nevertheless, the current study has some limitations. First, additional CTC testing methods should be investigated to determine whether our results are affected by the detection method. Second, the number of patients in our study is small, and the results should be interpreted with caution.
In conclusion, the rate of presence of CTCs in the peripheral blood of osteosarcoma patients is high, and patients with an increased percentage of M-CTCs after treatment have a shorter DFS. The dynamic monitoring of changes in CTC levels and types after treatment has clinical significance for the timely detection of recurrence or metastasis.
Acknowledgments
The authors would like to thank SurExam Biotech, Guangzhou, People’s Republic of China, for technical support. This work was supported, in part, by the Guangxi Traditional Chinese Medicine and Ethnic Medical Inheritance and Innovation Project (GZLC16-38).
Author contributions
Z-JW and Z-CY were involved in the concept and design of this manuscript. XQ and J-CT were involved in the acquisition of subjects and data. Z-JW, XQ, J-CT, and BL were involved in the collection of CTC data. Z-JW, XQ, and J-CT were involved in data analysis. All authors contributed toward drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. | ||
Bacci G, Forni C, Longhi A, et al. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007;96(2):118–123. | ||
Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, Bacci G. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol. 2010;19(4):193–199. | ||
Collins M, Wilhelm M, Conyers R, et al. Benefits and adverse events in younger versus older patients receiving neoadjuvant chemotherapy for osteosarcoma: findings from a meta-analysis. J Clin Oncol. 2013;31(18):2303–2312. | ||
Aksnes LH, Bauer HC, Jebsen NL, et al. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90(6):786–794. | ||
Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. | ||
Heller G, Mccormack R, Kheoh T, et al. Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison With Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials. J Clin Oncol. 2018;36(6):572–580. | ||
Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol. 2006;33(3 Suppl 9):9–14. | ||
Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920–928. | ||
Armstrong A, Eck SL. EpCAM: A new therapeutic target for an old cancer antigen. Cancer Biol Ther. 2003;2(4):320–325. | ||
Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23(5):573–581. | ||
Plaks V, Koopman CD, Werb Z, Cancer WZ. Cancer. Circulating tumor cells. Science. 2013;341(6151):1186–1188. | ||
Wu S, Liu S, Liu Z, et al. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10(4): e0123976. | ||
Wu S, Liu Z, Liu S, Lin L, Yang W, Xu J. Enrichment and enumeration of circulating tumor cells by efficient depletion of leukocyte fractions. Clin Chem Lab Med. 2015;53(2):337. | ||
Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. | ||
Jawad MU, Scully SP. In brief: classifications in brief: enneking classification: benign and malignant tumors of the musculoskeletal system. Clin Orthop Relat Res. 2010;468(7):2000–2002. | ||
Zhong GX, Feng SD, Shen R, Wu ZY, Chen F, Zhu X. The clinical significance of the Ezrin gene and circulating tumor cells in osteosarcoma. Onco Targets Ther. 2017;10:527–533. | ||
Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. | ||
Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. | ||
Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9(4):302–312. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.