Back to Journals » Patient Preference and Adherence » Volume 13
Side Effects, Self-Management Activities, and Adherence to Oral Anticancer Agents
Authors Jiang Y , Wickersham KE , Zhang X, Barton DL , Farris KB , Krauss JC, Harris MR
Received 25 July 2019
Accepted for publication 27 November 2019
Published 3 January 2020 Volume 2019:13 Pages 2243—2252
DOI https://doi.org/10.2147/PPA.S224496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Yun Jiang,1 Karen E Wickersham,2 Xingyu Zhang,1 Debra L Barton,1 Karen B Farris,3 John C Krauss,4 Marcelline R Harris1
1University of Michigan School of Nursing, Ann Arbor, MI, USA; 2University of South Carolina College of Nursing, Columbia, SC, USA; 3University of Michigan College of Pharmacy, Ann Arbor, MI, USA; 4University of Michigan Medical School, Michigan Medicine, Ann Arbor, MI, USA
Correspondence: Yun Jiang
University of Michigan School of Nursing, 400 North Ingalls Street, Ann Arbor, MI 48109, USA
Email [email protected]
Purpose: There are growing concerns about patients’ adherence to oral anticancer agents (OAAs), and the need for patients to engage in self-management of OAA-related side effects. We assessed associations among adherence, severity of side effects, and effectiveness of self-management of side effects in patients taking capecitabine.
Methods: Adherence to capecitabine at 6 weeks was measured by the Medication Event Monitoring System among 50 patients with gastrointestinal cancers. Severity of side effects related to capecitabine and effectiveness of self-management of side effects were captured using the Modified Self-Care Diary at the time of enrollment and weekly for 6 weeks. Spearman’s correlation, Mann–Whitney U-tests, and multiple linear regression were conducted, p<0.05.
Results: Overall mean adherence rate was 85.4±14.1%. Adherence rate was not significantly correlated to the mean severity of total side effects at any time point and was correlated with the mean effectiveness of self-management of total side effects only at week 2 (rho=0.29, p=0.04). However, adherence rate was associated with the mean severity of one specific side effect, diarrhea, at 6 weeks (rho=0.36, p=0.01) and marginally correlated to the mean effectiveness of self-management of diarrhea at 6 weeks (rho=0.28, p=0.05). Mean severity of diarrhea at 6 weeks was an independent predictor of adherence rate (b=4.97, p=0.01), with the control of age (b=0.52, p=0.002), number of outpatient medications (b=1.12, p=0.007), health literacy (b=2.53, p=0.04), diagnosis of colorectal cancer (b=11.6, p=0.03), and capecitabine in combination with other chemotherapies (b=16.8, p=0.001) in the model.
Conclusion: This pilot study suggests ongoing examination of both severity and effectiveness of self-management of side effects in future studies of adherence to OAAs is merited. There is a need for future studies with larger sample sizes that explore the complex relationships among adherence, severity of side effects, and effectiveness of self-management of side effects in OAA therapy.
Keywords: oral anticancer agents, adherence, side effects, self-management
Introduction
With the increasing use of oral anticancer agents (OAAs), there are growing concerns around patients’ non-adherence to OAAs.1–3 The International Society for Pharmacoeconomics and Outcome Research defines adherence as “the degree or extent of conformity to the recommendations about day-to-day treatment by the provider with respect to the timing, dosage, and frequency”.4 Adherence to OAAs can be challenging due to certain unique characteristics of OAAs. For example, OAAs often have complex regimens (such as 2-weeks on and 1-week off); a high potential for drug–drug and drug–food interactions, and frequent dose changes due to intolerable side effects.4–6 In addition, OAAs are more frequently prescribed for patients with metastatic cancer rather than early stage of cancers; thus, patients’ expectations for treatment outcomes and their health status may affect their adherence to OAA treatment.1,3
There is no gold standard definition of “adequate” adherence to OAAs, but 80% is commonly used as the cutoff.1,7,8 For molecularly targeted therapies, complete adherence (100%) may be more appropriate given that small deviations from a treatment plan may result in treatment resistance or failure.7 Depending on the agent and method of measurement, adherence to OAAs is reported to range from 20% to 100%.1,9–11
Capecitabine, the single OAA discussed in this study, is an oral chemotherapy widely used to treat metastatic breast, colorectal, and other gastrointestinal cancers.8 Adherence to capecitabine is generally high, ranging from 73.3% to 97.9%.10–13 Although many patients consider capecitabine therapy necessary, about one-third have serious concerns about its side effects.11 Diarrhea, nausea, and vomiting are identified as main side effects contributing to decreased adherence to capecitabine.14 Severe hand-food syndrome (HFS) has been reported as a key side effect contributing to capecitabine dose reduction or interruption.5 OAA side effect experience can vary significantly by individual,15 and it is thought that effective self-management of OAA-related side effects may directly or indirectly impact patient adherence to OAAs.3
We did not identify studies that have explored the relationship between side effect self-management and adherence to OAAs. For example, Zahrina and colleagues found that patients who did not experience severe side effects of capecitabine had significantly higher adherence rates than those who experienced side effects, but they did not examine patients’ ability to self-manage those side effects.14 Spoelstra and colleagues compared a telephone-based intervention with nurse support for side effect self-management and adherence of OAAs over 8 weeks.16 Although the intervention group reported improved severity of side effects compared to the control group, there was no significant difference in adherence between the groups. The relationship between effectiveness of side effect self-management and adherence was not addressed in the study. In general, medication adherence research highlights patients’ adherence with medication regimen and administration, but not on the influence of side effect self-management on medication adherence.17,18
A better understanding of the complex relationships between medication adherence, medication side effects, and patients’ self-management of side effects is needed to design and develop targeted interventions for improving adherence to OAAs. The purpose of this pilot study was to explore how patient self-reported severity of OAA-related side effects and perceived effectiveness of side effect self-management were associated with adherence to OAAs, using capecitabine as the example. Relationships of adherence with other potential factors, such as patient socio-demographics, clinical characteristics, and psychosocial status, were also explored.
Materials and Methods
Study Design, Sample, and Setting
An observational, single group, longitudinal study was conducted among patients who were: (1) diagnosed with a gastrointestinal (GI) malignancy; (2) prescribed capecitabine for cancer treatment; (3) completed at least 1 prior cycle of capecitabine treatment, (4) 18 years of age or older; and (5) able to read and speak English. Patients who took capecitabine as part of chemoradiation therapy were excluded due to the different regimens and side effect experiences attributed to concurrent radiation therapies. All patients were identified through chart review and recruited with support of the oncologists from the comprehensive GI oncology clinics at Michigan Medicine. Each patient received $20 for completing assessments at the time of enrollment and every week for 6 weeks (total amount of $140). The study was reviewed and approved by the University of Michigan Institutional Review Broad and conducted in accordance with the Declaration of Helsinki. Written informed consent documents were obtained from all individual patients participating in this study.
Data Collection and Measurement
Data were collected at seven time points: study enrollment and each week for 6 weeks following enrollment. The enrollment visit was in person at the clinic, and the subsequent weekly contacts were via phone. The 6-week period was necessary in order to capture adherence for at least one full cycle of capecitabine therapy, which can be as long as 4 weeks, and because of varying OAA cycles, some patients could have 2 weeks off before starting a new cycle (Table 1).
 |
Table 1 Participant Characteristics and Other Psychosocial Measures at the Time of Enrollment (n = 50) |
Patient socio-demographic variables were collected at the time of enrollment, including age, race, ethnicity, education status, employment status, marital status, and income status, while gender was extracted from the medical record (Table 1). Patient clinical characteristics were extracted from medical records, including cancer diagnosis, cancer stage, treatment type, daily dose, cycle pattern, dose change during the study, number of comorbidities, number of outpatient medications, and number of days on capecitabine prior to the study (Table 1).
Psychosocial factors were measured at the time of enrollment using questionnaires, including (1) cognitive functioning, measured by the 7-item Blessed Orientation–Memory-Concentration Test (Correlation with the MMSE: r = 0.71–0.83.);19 (2) health literacy, measured by the 6-item Newest Vital Sign (Cronbach α = 0.76);20 (3) depression, measured by the 2-item Patient Health Questionnaire-2 (Sensitivity: 83%; Specificity: 92%);21 (4) self-efficacy for chronic disease management, measured by the 6-item Stanford Self-efficacy for Managing Chronic Disease (Cronbach α = 0.88–0.95);22 (5) social support, measured by the 12-item Interpersonal Evaluation List (Cronbach α = 0.82);23 and (6) beliefs in chemotherapy, including Necessity and Concern subscales, modified from the Beliefs about Medicines Questionnaire (12 items, Cronbach α = 0.51–0.86).24
Adherence to capecitabine over 6 weeks was measured by the Medication Event Monitoring System (MEMS®, AARDEX Group, Belgium) which was given to the patient at the time of study enrollment. The built-in microchip in MEMS caps recorded every time the patient opened the bottle for capecitabine pills. All patients agreed to use the MEMS caps to track their taking capecitabine. Some patients preferred to use their own pillboxes rather than a pill bottle with a MEMS cap but agreed to open the cap of the MEMS bottle every time they took capecitabine. Patients returned the MEMS at the end of 6 weeks. MEMS data were downloaded into a database, adherence rates over 6 weeks were calculated for each patient. The formula used to calculate adherence rates was adapted from Walter et al:25
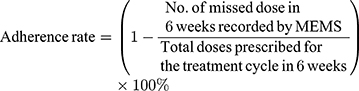
In addition, at the time of enrollment and at each week’s phone call, patients were asked “Did you forget to take your capecitabine at any time last week?” (yes/no). Patient responses to this question were used to impute the missing adherence data measured by the MEMS.
Severity of side effects and effectiveness of side effect self-management were measured at the time of enrollment, and each week thereafter for a total of 6 weeks. Patients’ were asked to self-report the severity of eight common side effects of capecitabine over the past week (fatigue, constipation, diarrhea, nausea, vomiting, hand-foot syndrome [HFS], mouth sores, and sleep difficulties). We modified the Self-care Diary (SCD) to collect severity and self-management data.26 Specifically, we replaced the SCD side effect severity questions with questions from the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAETM).27 The severity of each side effect was reported on a 5-point Likert scale from 0=none to 4=very severe. We retained the SCD activities and indications of use of self-management activities for each side effect (note: activities for nausea and vomiting are combined). As the SCD does not include HFS, the list of HFS self-management activities (e.g., “used emollients or moist creams”) was added based on evidence found in the literature.28–32 For each side effect, the patient was asked to add any self-management activities that were not included in the list. Patients were asked to rate the effectiveness of each self-management activity on a scale from 0 to 5, with 0=not used, 1=used but not relief, 2=used and a little relief, 3=used and some relief, 4=used and quite a bit of relief, and 5=used and completely relieved.
Data Analysis
Descriptive statistics were used to describe the sample and summarize all measures. For each participant, at enrollment and at each week, mean severity of total eight side effects and the mean effectiveness of self-management of total side effects were calculated. Binary correlation analysis (Spearman's correlation) and Mann–Whitney U-tests were used to assess univariate associations between adherence and all potential factors including the severity of side effects and effectiveness of side effect self-management. As discussed previously, there is no standard for defining “adequate” adherence of OAAs; however, for capecitabine, 80% is a commonly used threshold; temporary interruptions of capecitabine do not reduce its overall efficacy.1,5,7–9,33
Multiple linear regression with backward selection was used to explore potential factors that predicted 6-week adherence rates. The potential factors included socio-demographics, clinical characteristics, psychosocial factors, and severity of side effects and effectiveness of self-management of side effects. Potential factors that reached p<0.20 in the univariate analyses were included in the initial regression models.
A power analysis was conducted using the statistical package PASS 14 (NCSS, LLC. Kaysville, Utah, USA). A sample size of 44 was estimated to achieve 80% power to establish a multiple linear regression model with R2 value of 0.3, using 8 independent variables to predict adherence rates, when the two-side significance level is 0.05.
Results
Patient Characteristics
Fifty patients were recruited from March to November 2017 at the Michigan Medicine Rogel Cancer Center. Three patients withdrew from the study at the end of first week and one withdrew at the end of fourth week due to a change in their treatment plan. Forty-six patients completed the 6-week follow-up. The CONSORT flow diagram is presented in Figure 1.
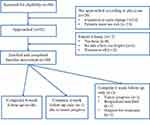 |
Figure 1 CONSORT flow diagram. |
Patients had a mean age of 63.8±11.9 years old. The majority were male (60%), Caucasian (88%), currently married (80%), not currently working (68%), and with a college degree or higher (52%) (Table 1). Most patients had diagnoses of advanced or metastatic pancreatic cancer, were taking capecitabine in combination with other chemotherapeutic agents, and had a daily dose that ranged from 1000 to 5000 mg. Mean scores of cognitive functioning, health literacy, perceived high self-efficacy, social support, and depression did not indicate any impairment in psychosocial functions (Table 1). Eleven of 50 patients indicated that they would prefer to use their own pillboxes but agreed to open the MEMS caps when they took capecitabine from their own pillboxes.
Summary of Adherence, Side Effect Severity, and Side Effect Self-Management Activities
Among the total sample of 50 patients enrolled in the study, 6-week adherence rate data were incomplete for eight patients (missing MEMS data at 6 weeks and/or missing self-report data of whether they forgot to take their capecitabine at any time in the last week). Of these eight patients, six did not have 6-week MEMS caps data (three did not return devices, one patient who used his/her own pillbox forgot to open the MEMS cap when taking their capecitabine, and two patients withdrew from the study at the end of the first week). For those six patients, we used their most recent response to the self-reported question about taking capecitabine to categorize the patients as adherent or not. Thus, 3 patients who answered that they did not forget to take their capecitabine were categorized as adherent, and 3 who answered they did forget were categorized as non-adherent. As among patients with complete 6-week MEMS adherence rate data (n=42), the non-adherent group (adherence rate < 80%) had a mean adherence rate of 66.7%, compared to a mean adherence rate of 93.4% for the adherent group (≥80%), we then imputed the mean group adherence rates (66.7% and 93.4%) for the missing values of those six patients. The two other patients with incomplete adherence rate data withdrew before the completion of the study and therefore had only partial MEMS cap data available. For those two patients, we used the MEMS cap data available at the time of their withdrawal to estimate adherence rate. The mean adherence rates across all participants (n=50) over 6 weeks were 85.4±14.1% (range 50–100%). With 70% of patients (n=35) were categorized into the adherent group (≥80%), there were 76% (n=38) patients who answered “no” to the single self-report question all the times, indicating that they had never forgotten to take capecitabine in 6 weeks.
Severity of Side Effect
The mean severity of all eight side effects at each time point ranged from a high of 0.81±0.56 at the time of enrollment (n=50) to a low of 0.56±0.42 at Week 2 (n=47). Regarding specific side effect, the three side effects with the highest severity ratings at the time of enrollment were fatigue (1.52±0.95), diarrhea (1.12±1.12), and constipation (1.02±1.24); and the three side effects with the highest mean severity at 6 weeks (n=46) were fatigue (1.45±0.76), diarrhea (0.73±0.85), and sleep difficulties (0.69±0.74). During the 6 weeks, the severity ≥ 3 (“severe” or “very severe”) was rated at least once by 20 patients (40%) for fatigue, 10 patients (20%) for constipation, 13 patients (26%) for diarrhea, 8 patients (16%) for nausea, 3 patients (6%) for vomiting, 10 patients (20%) for HFS, 4 patients (8%) for mouth sores, and 5 patients (10%) for sleep difficulties.
Effectiveness of Side Effect Self-Management Activities
Mean effectiveness of self-management of total side effects ranged from 2.25±1.24 at the time of enrollment (n=50) to 0.96±0.65 at week 6 (n=46). At the time of enrollment (n=50), the three specific side effects with the highest rates of mean effectiveness of self-management were fatigue (3.44±1.23), nausea/vomiting (2.50±2.08), and diarrhea (2.48±2.04). At 6 weeks (n=46), the three side effects with the highest mean effectiveness were fatigue (2.90±1.10), sleep difficulties (1.72±1.31), and nausea/vomiting (1.69±1.54). During 6 weeks, the effectiveness of self-management activities ≥ 4 (“quite a bit relief” or “completely relieved”) was rated at least once by 10 (20%) patients for fatigue, 4 (8%) for constipation, 4 (8%) for diarrhea, 4 (8%) for nausea/vomiting, 2 (4%) for HFS, 1 (2%) for mouth sores, and none for sleep difficulties.
Univariate Associations Between Adherence and Severity of Side Effects and Effectiveness of Self-Management of Side Effects
Adherence and Severity of Side Effects
Patients with more severe side effects were more likely in the adherent group (≥80%) than the non-adherent group at weeks 2, 4 and 6 (n=50, p=0.005; n=47, p=0.01; and n=46, p=0.04, respectively) (Figure 2). When looking at individual side effect, only the mean severity of diarrhea at 6 weeks was significantly positively correlated with adherence rates (Spearman’s rho=0.36, p=0.01), and patients who rated the severity of diarrhea at least once as “severe” or “very severe” during 6 weeks had significantly higher adherence rates than those who had never rated diarrhea as “severe” or “very severe” (U=116.0, p=0.006).
 |
Figure 2 Adherence groups with different severity of side effect at 6 weeks. Notes: **p<0.01; *p<0.05. |
Adherence and Effectiveness of Self-Management of Side Effects
Patients who perceived more effective self-management of total side effects were more likely in the adherent group than the non-adherent group at week 1 (n=50, U=166.5, p=0.04). There was a trend toward significant associations at weeks 2 and 4 (n=50, U=152.0, p=0.06; n=47, U=147.5, p=0.05, respectively) (Figure 3). When evaluating each side effect individually during 6 weeks, a trend toward significant correlation was seen between mean effectiveness of self-management of diarrhea at 6 weeks and adherence rates (Spearman’s rho=0.28, p=0.05); and patients who rated the effectiveness of self-management of diarrhea at least once as “quite a bit relief” or “completely relieved” during 6 weeks had a trend of higher adherence rates than those who had never rated self-management of diarrhea as “quite a bit relief” or “completely relieved” (U=217.5, p=0.07).
 |
Figure 3 Adherence groups with different effectiveness of self-management of side effects at 6 weeks. Note: *p<0.05. |
Potential Predictors of Adherence Rates
In addition to the mean severity of diarrhea and mean effectiveness of self-management of diarrhea at 6 weeks, univariate analyses also identified 10 other factors that were potentially associated with adherence with p<0.20. These 10 factors were age (years), race (white/non-white), colorectal cancer diagnosis (yes/no), treatment type (monotherapy/in combination with other chemotherapies), daily dose, number of days on capecitabine prior to the study, number of outpatient medications, comorbidity of diabetes (yes/no), preference of using MEMS (yes/no), and health literacy. Thus, the initial multiple regression models included 12 independent factors.
Eight factors remained in the final regression model after the backward selection, produced adjusted R2=0.76, F(8, 38)=6.49, p<0.001, indicating that adherence rates were significantly predicted by the mean severity of diarrhea at 6 weeks (b=4.97, p=0.01), with the control of age (b=0.56, p=0.001), number of outpatient medications (b=1.01, p=0.001), health literacy (b=2.43, p=0.03), the diagnosis of colorectal cancer (b=11.38, p=0.02), taking capecitabine treatment in combination with other chemotherapies (b=18.61, p<0.001), daily dose (b=0.004, p=0.03), and preference of using MEMS (b=14.5, p=0.002) in the model (Table 2).
 |
Table 2 Predictors of Adherence Rate to Capecitabine in 6 Weeks |
Discussion
This study provides new insights into potential factors associated with adherence to OAAs and specifically explores associations among severity of side effects, and self-management of side effects with adherence. Findings indicated higher severity of side effects and more effective self-management of side effects tended to be associated with higher adherence rates. Significantly, adherence rate was predicted by the 6-week mean severity of diarrhea.
Prior research has reported adherence rates measured by MEMS tend to underestimate adherence.34 In our study, 6-week mean adherence to capecitabine (85.4±14.1%) was similar to rates reported in the literature, measured by a variety of approaches (73.3–97.9%).10–13 We did find that participants who used MEMS in our study had higher adherence rates and it is possible that their adherence was positively influenced by the use of MEMS.35 In addition, our participants took capecitabine for a mean of 47 days prior to enrolling onto the study; given this and that 80% of participants took capecitabine in combination with another therapy, it is possible that our participants had already well-established adherence practices.
We found the positive direction of potential associations between adherence rates and the severity of side effects, which was in the opposite direction of what has been reported in the literature.14 There are several possible reasons for this. For example, people who take their full dose of capecitabine may experience more side effects. Alternatively, as our participants had a low severity of side effects, these side effects may not have been bothersome enough to impact adherence. In addition, patients may interpret the occurrence of side effects as a sign that the medication is working and be inspired to be more adherent.36
Diarrhea is a specific severe side effect commonly reported by patients taking capecitabine.4 Findings from our study confirmed the impact of the severity of diarrhea on capecitabine adherence rates,14 although the average rate of the severity of diarrhea at 6 weeks was not high. We did find that patients who ever rated their experience with diarrhea as severe or very severe during 6 six weeks had significantly higher adherence rates. Since there were only a few patients who reported “very severe” side effects, we were not able to look at the further subgroup differences. Experience with very severe side effects is a common reason for patient taking OAAs to have dose reduction.5 Our univariate analysis revealed that patients who had dose reduction during 6 weeks tended to have lower adherence rates than those without dose reductions. Although this difference was not statistically significant due to the small sample size, such a negative direction of the association is congruent with the literature. It may be interesting to further investigate whether patients have different OAA adherence behavior when they experience not-very severe vs. very severe side effects, in addition to the types of side effects.
Our study also examined associations between adherence and the effectiveness of side effect self-management. Similar to the pattern of severity of side effects, adherent patients in general reported more effective self-management activities (Figure 3). This finding may be related to the fact that those who had more side effects were more adherence and therefore, utilized more successfully self-management activities. From a self-management theoretical perspective, patients’ internal self-care agency may play a role in the consistency of their OAA adherence (medication self-management behavior) and self-management of OAA-related side effects. It is possible there is a more complex relationship among severity of side effects, effectiveness of self-management of side effects, and OAA adherence, than we are able to tease out in this small study.
The other predictors of adherence to OAAs in our study were similar to those reported in the literature, that being age and the number of concomitant medications.37 Our patients had significantly higher adherence when taking capecitabine in combination with other intravenous chemotherapies, which may be because those people getting intravenous therapy at the same time frame had more contacts with providers who could encourage and remind them in person about taking their OAAs.38 It is important to note that the daily dose remained in the final regression models, indicating that dosing was related to adherence to OAAs. Previous studies have reported that women are more adherent than men.39 We did not find a gender difference in adherence to capecitabine. Also, we did not find any association between adherence and self-efficacy for chronic disease management. Potential interpretations of this finding could be (1) our self-efficacy measurement was not specifically on self-efficacy for adherence to OAAs; and (2) perceived self-efficacy may not accurately reflect patients’ actual adherence behavior considering the common ceiling effects of self-efficacy self-reporting among cancer patients.40
We recognize the limitations of this study. First, as a pilot study, our sample size was small and included only one OAA; therefore, results are not generalizable to all patients taking OAAs; it is possible that results would be different for different agents such as tyrosine kinase or multi-kinase inhibitors. In addition, our sample lacks variation in patient income (98% had household incomes met their basic needs) and financial concerns around paying for capecitabine pills (92% never had a hard time for paying), which may potentially affect adherence rates. We used MEMS to objectively measure adherence rates, and at the same time, we also used patient self-reports of adherence. Self-report is wrought with validity issues such as memory and wanting to provide socially acceptable/pleasing answers.41 However, in this study, we found that there was congruence between self-reported adherence and MEMS measured adherence. MEMS, when used correctly, is a more objective measure of adherence,1,42 but difficulties have been reported concerning using pillboxes at the same time, as we did in our study.
Conclusions
This pilot study identified factors associated with adherence to OAAs, including the potential associations between severity of side effects, effectiveness of self-management of side effects, and OAA adherence. Future prospective, longitudinal studies with larger sample sizes and long-term follow-up may help reveal more details around the complex and dynamic relationships between adherence, side effects, side effect management and other important factors such as self-efficacy, self-regulation, psychosocial characteristics, and clinical characteristics. Such research is needed to guide effective intervention development to improve adherence to OAA’s with a range of toxicities.
Compliance with Ethical Standards
The study was reviewed and approved by the University of Michigan Institutional Review Broad, Ann Arbor, MI, USA, and was conducted in accordance with the Declaration of Helsinki. Written informed consent documents were obtained from all individual patients participating in this study.
Acknowledgments
The authors thank all patients and their caregivers for their participation in the study. The authors also want to acknowledge research staff and students for their work and clinicans at the Michigan Medicine Rogel Cancer Center for their support.
Author Contributions
All authors made significant contributions to the study design, analysis and interpretation of the data, and preparing and reviewing the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be submitted.
Funding
This work was supported by the National Institute of Nursing Research (5 P20 NR015331-03) at the National Institutes of Health.
Disclosure
Karen B Farris reports personal fees from QuiO, outside the submitted work. The authors report no other conflicts of interest to this work.
References
1. Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist. 2016;21:354–356. doi:10.1634/theoncologist.2015-0405
2. Wood L. A review on adherence management in patients on oral cancer therapies. Eur J Oncol Nurs. 2012;16:432–438. doi:10.1016/j.ejon.2011.10.002
3. Salgado TM, Mackler E, Severson JA, et al. The relationship between patient activation, confidence to self-manage side effects, and adherence to oral oncolytics: a pilot study with Michigan oncology practices. Support Care Cancer. 2017;25:1797–1807. doi:10.1007/s00520-017-3584-0
4. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi:10.1111/j.1524-4733.2007.00213.x
5. Marsé H, Van Cutsem E, Grothey A, Valverde S. Management of adverse events and other practical considerations in patients receiving capecitabine (Xeloda®). Eur J Oncol Nurs. 2004;8:S16–S30. doi:10.1016/j.ejon.2004.06.006
6. Lester J. Safe handling and administration considerations of oral anticancer agents in the clinical and home setting. Clin J Oncol Nurs. 2012;16:E192–E197. doi:10.1188/12.CJON.E192-E197
7. Geynisman DM, Wickersham KE. Adherence to targeted oral anticancer medications. Discov Med. 2013;15:231–241.
8. Banning M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur J Cancer Care. 2012;21:10–19. doi:10.1111/ecc.2012.21.issue-1
9. Partridge AH, Archer L, Kornblith AB, et al. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J Clin Oncol. 2010;28:2418–2422. doi:10.1200/JCO.2009.26.4671
10. Simons S, Ringsdorf S, Braun M, et al. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Support Care Cancer. 2011;19:1009–1018. doi:10.1007/s00520-010-0927-5
11. Bhattacharya D, Easthall C, Willoughby KA, Small M, Watson S. Capecitabine non-adherence: exploration of magnitude, nature and contributing factors. J Oncol Pharm Pract. 2012;18:333–342. doi:10.1177/1078155211436022
12. Figueiredo AG
13. Winterhalder R, Hoesli P, Delmore G, et al.; SAEDA Investigators Group (Swiss prospective cohort group. Self-reported compliance with capecitabine: findings from a prospective cohort analysis. Oncology. 2011;80:29–33. doi:10.1159/000328317
14. Zahrina AK, Norsa’adah B, Hassan NB, et al. Adherence to capecitabine treatment and contributing factors among cancer patients in Malaysia. Asian Pac J Cancer Prev. 2014;15(21):9225–9232. doi:10.7314/APJCP.2014.15.21.9225
15. Amlani A, Kumar A, Ruan JY, Cheung WY. Compliance with adjuvant capecitabine in patients with stage II and III colon cancer: comparison of administrative versus medical record data. Cancer Med. 2016;5(8):1776–1782. doi:10.1002/cam4.2016.5.issue-8
16. Spoelstra SL, Given BA, Given CW, et al. An intervention to improve adherence and management of symptoms for patients prescribed oral chemotherapy agents: an exploratory study. Cancer Nurs. 2013;36:18–28. doi:10.1097/NCC.0b013e3182551587
17. Vioral A, Leslie M, Best R, Somerville D. Patient adherence with oral oncolytic therapies. Semin Oncol Nurs. 2014;30:190–199. doi:10.1016/j.soncn.2014.05.007
18. Kavookjian J, Wittayanukorn S. Interventions for adherence with oral chemotherapy in hematological malignancies: a systematic review. Res Social Adm Pharm. 2015;11:303–314. doi:10.1016/j.sapharm.2014.08.006
19. Fillenbaum GG, Heyman A, Wilkinson WE, Haynes CS. Comparison of two screening tests in alzheimer’s disease. The correlation and reliability of the mini-mental state examination and the modified blessed test. Arch Neurol. 1987;44:924–927. doi:10.1001/archneur.1987.00520210026014
20. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514–522. doi:10.1370/afm.405
21. Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi:10.1097/01.MLR.0000093487.78664.3C
22. Ritter PL, Lorig K. The English and Spanish self-efficacy to manage chronic disease scale measures were validated using multiple studies. J Clin Epidemiol. 2014;67:1265–1273. doi:10.1016/j.jclinepi.2014.06.009
23. Merz EL, Roesch SC, Malcarne VL, et al. Validation of interpersonal support evaluation list-12 (ISEL-12) scores among english-and Spanish-speaking Hispanics/Latinos from the HCHS/SOL Sociocultural Ancillary Study. Psychol Assess. 2014;26:384. doi:10.1037/a0035248
24. Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–557. doi:10.1016/S0022-3999(99)00057-4
25. Walter T, Wang L, Chuk K, Ng P, Tannock IF, Krzyzanowska MK. Assessing adherence to oral chemotherapy using different measurement methods: lessons learned from capecitabine. J Oncol Pharm Pract. 2013;20:249–256. doi:10.1177/1078155213501100
26. Nail LM, Jones LS, Greene D, Schipper DL, Jensen R. Use and perceived efficacy of self-care activities in patients receiving chemotherapy. Oncol Nurs Forum. 1991;18:883–887.
27. National Cancer Institute. Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). 2019. Available from: https://healthcaredelivery.cancer.gov/pro-ctcae/.
28. Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda). Eur J Oncol Nurs. 2004;8(Suppl 1):S31–S40. doi:10.1016/j.ejon.2004.06.007
29. Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract. 2006;12(3):131–141. doi:10.1177/1078155206069242
30. Zhao Y, Ding Y, Lu Y, Zhang J, Gu J, Li M. Incidence and self-management of hand-foot syndrome in patients with colorectal cancer. Clin J Oncol Nurs. 2013;17(4):434–437. doi:10.1188/13.CJON.434-437
31. Braik T, Yim B, Evans AT, et al. Randomized trial of vitamin B6 for preventing hand-foot syndrome from capecitabine chemotherapy. J Community Support Oncol. 2014;12(2):65–70. doi:10.12788/jcso.0017
32. Murugan K, Ostwal V, Carvalho MD, et al. Self-identification and management of hand-foot syndrome (HFS): effect of a structured teaching program on patients receiving capecitabine-based chemotherapy for colon cancer. Support Care Cancer. 2016;24(6):2575–2581. doi:10.1007/s00520-015-3061-6
33. Thivat E, Van Praagh I, Belliere A, et al. Adherence with oral oncologic treatment in cancer patients: interest of an adherence score of all dosing errors. Oncol. 2013;84:67–74. doi:10.1159/000342087
34. Buono EW, Vrijens B, Bosworth HB, Liu LZ, Zullig LL, Granger BB. Coming full circle in the measurement of medication adherence: opportunities and implications for health care. Patient Prefer Adherence. 2017;11:1009–1017. doi:10.2147/PPA
35. Devices EH. Electronic tools to measure and enhance medication adherence. US Pharm. 2010;36:6–10.
36. Wickersham KE, Happ MB, Bender CM, Engberg SJ, Tarhini A, Erlen JA. Surviving with lung cancer: medication-taking and oral targeted therapy. Geriatr Nurs. 2014;35:S49–S56. doi:10.1016/j.gerinurse.2014.02.020
37. Verbrugghe M, Verhaeghe S, Lauwaert K, Beeckman D, Van Hecke A. Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: a systematic review. Cancer Treat Rev. 2013;39:610–621. doi:10.1016/j.ctrv.2012.12.014
38. Seal BS, Anderson S, Shermock KM. Factors associated with adherence rates for oral and intravenous anticancer therapy in commercially insured patients with metastatic colon cancer. J Manag Care Spec Pharm. 2016;22:227–235. doi:10.18553/jmcp.2016.22.3.227
39. Jacobs JM, Pensak NA, Sporn NJ, et al. Treatment satisfaction and adherence to oral chemotherapy in patients with cancer. J Oncol Pract. 2017;13:e474–e485. doi:10.1200/JOP.2016.019729
40. Williams DM, Rhodes RE. The confounded self-efficacy construct: conceptual analysis and recommendations for future research. Health Psychol Rev. 2016;10(2):113–128. doi:10.1080/17437199.2014.941998
41. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5:470–482. doi:10.1007/s13142-015-0315-2
42. El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82(1):268–279. doi:10.1111/bcp.12942
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
