Back to Journals » Patient Preference and Adherence » Volume 16
Shortness of Breath on Day 1 After Surgery Alerting the Presence of Early Respiratory Complications After Surgery in Lung Cancer Patients
Authors Yu Q , Yu H , Xu W , Pu Y, Nie Y , Dai W , Wei X , Wang XS , Cleeland CS, Li Q , Shi Q
Received 11 November 2021
Accepted for publication 4 March 2022
Published 19 March 2022 Volume 2022:16 Pages 709—722
DOI https://doi.org/10.2147/PPA.S348633
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Naifeng Liu
Qingsong Yu,1 Hongfan Yu,1 Wei Xu,1 Yang Pu,1 Yuxian Nie,2 Wei Dai,3 Xing Wei,3 Xin Shelley Wang,4 Charles S Cleeland,4 Qiang Li,3 Qiuling Shi1,2,5
1School of Public Health and Management, Chongqing Medical University, Chongqing, People’s Republic of China; 2State Key Laboratory of Ultrasound in Medicine and Engineering, Chongqing Medical University, Chongqing, People’s Republic of China; 3Department of Thoracic Surgery, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan, People’s Republic of China; 4Department of Symptom Research, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 5Center for Cancer Prevention Research, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan, People’s Republic of China
Correspondence: Qiuling Shi, School of Public Health and Management, Chongqing Medical University, No. 1, Medical School Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86-18290585397, Fax +86-28-85420116, Email [email protected]
Purpose: Patient-reported outcome (PRO)-based symptom assessment with a threshold can facilitate the early alert of adverse events. The purpose of this study was to determine whether shortness of breath (SOB) on postoperative day 1 (POD1) can inform postoperative pulmonary complications (PPCs) for patients after lung cancer (LC) surgery.
Methods: Data were extracted from a prospective cohort study of patients with LC surgery. Symptoms were assessed by the MD Anderson Symptom Inventory-lung cancer module (MDASI-LC) before and daily after surgery. Types and grades of complications during hospitalization were recorded. SOB and other symptoms were tested for a possible association with PPCs by logistic regression models. Optimal cutpoints of SOB were derived, using the presence of PPCs as an anchor.
Results: Among 401 patients with complete POD1 MDASI-LC and records on postoperative complications, 46 (11.5%) patients reported Clavien–Dindo grade II-IV PPCs. Logistic regression revealed that higher SOB score on POD1 (odds ratio [OR]=1.13, 95% CI=1.01– 1.27), male (OR=2.86, 95% CI=1.32– 6.23), open surgery (OR=3.03, 95% CI=1.49– 6.14), and lower forced expiratory volume in one second (OR=1.78, 95% CI=1.66– 2.96) were significantly associated with PPCs. The optimal cutpoint was 6 (on a 0– 10 scale) for SOB. Patients reporting SOB < 6 on POD1 had shorter postoperative length of stay than those reporting 6 or greater SOB (median, 6 vs 7, P =0.007).
Conclusion: SOB on POD1 can inform the onset of PPCs in patients after lung cancer surgery. PRO-based symptom assessment with a clinically meaningful threshold could alert clinicians for the early management of PPCs.
Keywords: lung cancer, surgery, postoperative pulmonary complications, patient-reported outcomes, shortness of breath
Introduction
Postoperative pulmonary complications (PPCs) are one of the major problems in patients following lung cancer (LC) surgery.1,2 It has been reported that the incidence of PPCs reaches 14% to 40% after LC resection, which is greater than that in other major procedures.3 PPCs typically occurred within the first week after surgery and nearly 25% of the postoperative deaths that occur in this time are related to PPCs.4,5 In addition, PPCs can result in delayed recovery, greater Medical expense,6 and obvious respiratory symptom burden, such as dyspnea and cough, for patients with LC.7 Thus, accurate monitoring, alerting, and preparing an appropriate plan for PPCs as early as possible are needed to reduce patient mortality and morbidity during the perioperative period.
After LC surgery, patients commonly suffer from numerous symptoms, such as pain, coughing, dyspnea, and fatigue.8 Including heterogeneous surgery types, a previous study associated an increased risk of respiratory complications such as atelectasis and pulmonary infections with postoperative pain.9 The positive association between early postoperative pain and 30-day postoperative complications demonstrated that symptom monitoring in the early recovery period (ie, the first several days after surgery) can alert negative events related to the procedure and then promote postoperative recovery. Unfortunately, for patients undergoing cancer surgery, pain is a general symptom induced by all kinds of surgery,10 and complications vary from procedures.11 For patients with LC surgery, there is a need for PPCs-specified symptoms to be clearly defined for early intervention.
Dyspnea is a highly frequent and disruptive symptom posttreatment in LC patients, with a prevalence up to 90% in advanced disease.12 Different from other symptoms, the severity of dyspnea worsens partly because of a loss of 10–15% of lung function after surgery,13,14 and it can substantially add to the onset of fatigue,15 which has been shown to have a strong correlation with quality of life (QOL) and finally to increase the risk of PPCs.16 Fagundes et al reported that the occurrence of dyspnea was associated with postoperative tissue injury and medications induced metabolic stress response.17 The degree of dyspnea was commonly measured by the modified Medical Research Council (mMRC) dyspnea scale and transition dyspnea Index (TDI) in chronic obstructive pulmonary disease.18,19 American Thoracic Society defines dyspnea as a subjective experience of breathing discomfort and can be accurately obtained from a patient report on the severity and frequency of “shortness of breath” (SOB).20,21
Of note, the patient-reported outcome (PRO) has been widely used for surgical outcome reporting22 and can provide clinicians precise information on patients’ experience with aggressive cancer treatments.23 Recently, several investigators have demonstrated that using a web-based symptom monitoring system can reduce symptom burden and improve the overall survival of patients with advanced cancer.24,25 This process is done through a mechanism that using the weekly PRO assessment of index symptoms with an alert threshold can inform the clinician of the early onset of adverse events. Therefore, there is a great possibility of using PRO-based symptom monitoring to generate alerts to inform clinicians about the potential risk of PPCs after lung resection.
However, despite increasing awareness of the serious negative effects on SOB, it is still an undetected symptom and should be accurately assessed, recorded, and related to postoperative complications.26 Moreover, using PRO-based symptoms to alert severe postoperative outcomes early is hampered by the lack of data on how those symptoms change over the course of recovering from LC surgery and at what level of symptom severity the clinician should be informed for further clinical actions.
With the availability of prospectively collected symptom and PPCs data during hospitalization after thoracic surgery, we conducted an analysis with longitudinal PRO-based symptom assessment in patients with LC surgery. The objectives of the current analysis were (1) to describe the trajectory of SOB based on a group-based trajectory model (GBTM) from preoperation to one week after surgery; (2) to determine whether SOB on postoperative day 1 (POD1) can inform PPCs after thoracic surgery; (3) to establish cutpoint of SOB, anchored by the presence of PPCs; and (4) to demonstrate the sensitivity of SOB cutpoints in differentiating clinical outcomes, such as postoperative length of stay (PLOS) and functional status.
Materials and Methods
Study Design and Patients
Patients for this study were from a prospective observational cohort study (NCT03341377) investigating the perioperative symptom burden for lung cancer patients from Nov, 2017 to Jan, 2020 in 6 tertiary hospitals in China. The study protocol was published in 2019.27 Eligible patients of this study were at least over 18 years old, can understand the study requirements, and participate in the study (Figure 1). Patients were not included in this analysis if (1) the postoperative pathological type was benign pulmonary disease, (2) the 16 symptom severity items or 6 symptom interference items of the MDASI-LC were missing, and (3) complications of subcutaneous pneumatosis or postoperative blood transfusion occurred during hospitalization. The study was approved by the Ethics Committee of each hospital. Written informed consent was obtained from all participating patients.
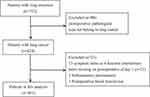 |
Figure 1 Study flow diagram for analytical sample. |
Measurements
Symptoms were assessed using the MD Anderson Symptom Inventory-lung cancer module (MDASI-LC),28 a brief, validated and lung cancer-specific PRO instrument. The MDASI-LC consists of SOB and other 12 common cancer-related symptoms and 6 items of symptom interference with daily function. The MDASI-LC also assesses 3 symptoms (coughing, constipation and sore throat) specific to lung cancer. Each item is rated on a 0–10 numerical scale, with 0 being “not present” or “did not interfere” and 10 presenting “as bad as you can imagine” or “interfered completely.” The recall period was 24 hours. MDASI-LC was administered within 3 days prior to the operation, daily after surgery (up to 14 days), and weekly after discharge until 4 weeks or the start of postoperative adjuvant treatment. Since the majority of PPCs were diagnosed within 1 week after surgery,4 data from preoperation to one week after surgery were used in this analysis. Other data, such as demographic characteristics, preoperative characteristics, surgery, types and grades of complications during hospitalization of all patients, were collected and recorded in the REDCap database.29 The surgical resection types including lobectomy, sublobectomy and others. Lobectomy was a procedure with the entire lobe of lung resection, requiring dissection and ligation of vascular and bronchial structure; Sublobectomy was consisted of segmentectomy and wedge resection and regarded as the resection extent less than a lobe either requiring dissection of sectional vascular and bronchus or not.30 Others were included bilobectomy, sleeve lobectomy, and sublobectomy plus sublobectomy or lobectomy.31
Postoperative Pulmonary Complications
We used the Clavien-Dindo classification (CDC)32 criteria of surgical complications to score the severity of PPCs in 5 grades. PPCs were defined as the occurrence of one or more of the following complications with Clavien-Dindo grade ≥ II from the day of surgery to hospital discharge:33–38 (1) pneumonia, associated with 3 of the 4 following criteria or one of the first three and the fourth criterion: 1) fever with temperature >38.0°C after 72 hours postoperatively, 2) leukocytosis with white blood cell count > (12–15) × 109/L or returned to normal value but then increased again > 10 × 109/L, 3) lung consolidation or increasing patchy opacity on chest imaging, and 4) purulent sputum or positive sputum culture; (2) prolonged air leak, defined as persistent air leak from the drainage tube for more than 7 days after surgery and bronchopleural fistula were excluded; (3) postoperative pneumothorax, the accumulation of effusion or air in the pleural space of the operative side after surgery, with symptoms such as dyspnea and requiring treatments such as reinsertion; (4) pneumothorax, the accumulation of air in the pleural space of the non-operative side during or after surgery, with the lungs were compressed by more than 30% and requiring treatments; (5) bronchopleural fistula, the abnormal passage between the bronchus and the pleura confirmed by bronchoscope; (6) respiratory failure, postoperative PaO2 <60mmHg on room air, a ratio of PaO2 to inspired oxygen fraction <300 mmHg, or pulse oximetry <90% and requiring oxygen therapy.
Statistical Methods
Descriptive statistics were used to summarize demographic and clinical characteristics. Normally distributed continuous variables are expressed as the mean (± standard deviation, SD), and skewed continuous variables are expressed as the median (interquartile range, IQR 25th–75th percentile), while the differences were compared with the t-test and the Wilcoxon rank sum test. Categorical variables are expressed as a number and percentage (%) and were analyzed using the χ2 test or Fisher’s exact test for categorical variables where the numbers per cell were 5 or less.
Group-based trajectory modeling (GBTM) was used to identify patient subgroups with distinct symptom trajectories over the course of recovering from thoracic surgery, with the SOB score considered the dependent variable. The SAS macro PROC TRAJ was used to estimate trajectories on the basis of data collected at 8 time points from before surgery to postoperative day 7.39 We generated a two-group model with the prior of simplicity and clinical interpretability, representing either a high or low symptom burden over time during the 7-day study because the two-group model could identify subgroups of the symptom burden in patients who received aggressive cancer treatment.40
Factors, such as sex (male vs female), history of smoking (smoking vs never smoking), and approach of operations (Open vs VATs), age (> 55 vs ≤ 55) and forced expiratory volume in one second (FEV1), were tested using univariate analysis with the presence of grade II or greater PPCs as the dependent variable. Those variables with P values less than or equal to 0.05 were entered into the multivariate logistic analyses to determine the risk factors for PPCs.
To establish clinically meaningful thresholds of symptoms to inform the onset of major complications, we used a single dependent variable of PPCs as the anchor to drive symptom interference cutpoints. Binary logistic analysis was used to identify the optimal cutpoint for categorizing symptom scales into 2 levels. We examined 9 different sets of cutpoints (CPs) (CP1, CP2, CP3. CP4, CP5, CP6, CP7, CP8, and CP9). Each group was considered a new variable and used as an independent variable to simulate a logistic regression model with the occurrence of PPCs as the dependent variable. Using the minimum P-value approach, the cutpoints that produced the maximum Wald chi-squared statistic would be selected as the optimal cutpoints for each symptom scale.41 Bootstrap resampling with 2000 samples was used to check that the choice of optimal cutpoints was robust.
To validate the optimal cutpoint, we assessed how the derived symptom categories differed in functional interference and postoperative length of hospital stay (PLOS). We used mixed-effects models to compare the MDASI-LC symptom interference scores from pre-operation to 7 days after surgery during hospitalization in patients with different SOB symptom levels by the cutpoints. In these models, the score of symptom interference (continuous variable) was considered the dependent variable; the independent variables were the time and the interaction between time and SOB symptom severity. The maximum likelihood estimation method was used to calculate the fixed effects of all dependent variables and random effects for the intercept and time.
All statistical analyses were conducted using JMP software version 13.0 (SAS Institute Inc., Cary, NC, USA), and a two-sided p-value < 0.05 was considered significant.
Results
Patient Characteristics
A total of 401 patients with complete POD1 MDASI-LC and records on postoperative complications were included and analyzed in this study. Baseline demographic and clinical features are summarized in Table 1. The average age of the patients was 56.1 ± 10.4 years, and 47.9% (n=192) were women. Of patients included in the study, 252 (62.8%) were never smokers, 106 (26.4%) were current smokers, and 43 (10.7%) were former smokers. More than half of the patients were staged with TNM I/II (63.3%). The surgical approach consisted of open surgery (19.5%) and VATS (80.6%). Median [IQR] days of PLOS was 7 [5–8] days.
 |
Table 1 Demographics and Clinical Characteristics |
Postoperative Pulmonary Complications
A total of 11.5% (n=46) of patients reported grade II–IV PPCs during hospitalization after surgery, and 18 (4.5%) patients reported two or more types of PPCs (Table S1). Total 55 PPCs events with Clavien-Dindo grade ≥ II were reported while postoperative pneumonia (5.7%) was the most frequent PPCs, followed by prolonged air leak (>7d) (5.5%), postoperative pneumothorax (1.7%), pneumothorax (0.2%), and bronchopleural fistula (0.5%).
Risk Factors for PPCs
The univariate analyses revealed that male, approach of operations, FEV1, and SOB on POD1 were significantly associated with PPCs (all P < 0.05). Multivariate logistic regression model identified that variables significantly associated with PPCs, including male (odds ratio [OR]: 2.86, 95% CI: 1.32–6.23; P=0.008); open surgery (OR: 3.03; 95% CI: 1.49–6.14; P=0.002); lower FEV1 (OR: 1.78; 95% CI: 1.66–2.96; P=0.025); and SOB on POD1 (OR: 1.13, 95% CI: 1.01–1.27; P=0.034) (Table 2).
 |
Table 2 Multivariate Logistic Regression Analysis of Risk Factors for PPCs During Hospitalization After Surgery |
Postoperative Symptom Trajectory by GBTM Selection and Evaluation of SOB
According to the results of GBTM, we defined the two-group symptom trajectories as patients-reported persistently with lower severity on SOB (52% of patients) or higher SOB (48%) from POD1 to POD7. Estimated means (dashed lines) and observed means (solid lines) at each time point for all subgroups are plotted in Figure 2. In the two-group model, both groups demonstrated significantly linear and quadratic terms, with a mild symptom peak (mean 2.33, SD 2.17) at POD1 for the low group and a moderate symptom peak (mean 5.34, SD 2.61) for the high group (Figure 2). The Wilcoxon rank sum test showed that the difference in POD1 SOB between the low and high groups was significant (P < 0.001).
Optimal Cutpoints for SOB
Table 3 demonstrates the chi-square and p-values for the 9 sets of cutpoint (CP1 to CP9, ordered by the chi-square values). Using presence of PPCs as the anchor, CP6 and CP5 demonstrated the two highest Wald chi-square values. Among the 2000 samples of bootstrap resampling, 82.9% confirmed CP6 as the optimal cutpoint. (Table 4) Thus, we defined score < 6 as none/mild SOB and score (6 to 10) as severe SOB.
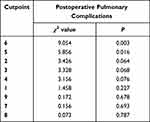 |
Table 3 Optimal Cutpoint Analysis Using Anchor of Postoperative Pulmonary Complications |
 |
Table 4 Bootstrap with 2000 Resamplings for Cutpoints of Shortness of Breath |
Validating the Cutpoint of SOB
On day 1 after surgery, compared with patients reporting none/mild SOB, those severe SOB scores reported more symptom interference of general activity [median (IQR), 7 (3–9) vs 4 (1–7), P < 0.001], work [9 (3–10) vs 6 (1–10), P < 0.05], walking [8.5 (5–10) vs 5 (2–9), P < 0.001], mood [5 (1–8) vs 1 (0–4), P < 0.001], relations with others [6 (1–9) vs 2 (0–5), P < 0.001], and enjoyment of life [1 (0–6) vs 0 (0–2), P < 0.001] (Figure 3).
Longitudinally, patients with none/mild SOB experienced significantly less symptom interference with daily functioning (general activity, work, walking, mood, relations with others and enjoyment of life) during first 7 days after surgery than those in the severe SOB group (all P < 0.05) (Figure 4).
The severity of PPCs had a positive correlation with PLOS (r=0.64, P < 0.001). As shown in Figure S1, the median PLOS for patients with severe SOB was 7 (95% CI=6-7), which was significantly higher than those who reported none/mild SOB (6 [95% CI=6-7], P = 0.007).
Discussion
For the first time, our analysis demonstrated that patient reported-SOB on POD1 was related to the onset of PPCs in patients with LC surgery, and half of the patients reported a high level of SOB during hospitalization. Using the presence of PPCs as an anchor, we then determined and validated that the optimal cutpoint of SOB for alerting PPCs is 6 on the 0–10 numeric scale. This cutpoint might serve as the threshold when using POD1 SOB to identify patients who might experience PPCs after lung cancer surgery. Our result supported that using the PRO-based SOB on POD1 can alert PPCs after thoracic surgery with the optimal threshold of 6.
Lung cancer resection is associated with a considerable risk of postoperative pulmonary complications (PPCs), which have a significant health and economic impact on patients and health care services.42 The PPCs need a multidisciplinary approach and careful monitoring to optimize the survival and quality of life of LC patients.43 For decades, efforts to decrease the incidence of PPCs have started with a comprehensive preoperative risk assessment.11 Various risk factors associated with PPCs and prediction models have been identified to aid in predicting PPCs. For example, preoperative pulmonary function tests were regarded as the potential risk factors for PPCs.44 Consistent with previous studies,2,33 we found that male and lower FEV1 were significantly associated with PPCs. These significant associations may due to smoking, because most smokers were male in our study. It has been reported that smoking as one of well-known risk factors for lung cancer and persistent cigarette exposure had a negative effect on lung function with spirometric parameters decreased and exercise performance of patients impaired.45
In recent years, numerous studies have established corresponding clinical scores based on these risk factors, which can quickly determine the degree of risk for a patient to develop PPCs.46 By adding PROs to traditional factors, our study showed that POD1 SOB was related to PPCs during hospitalization. Similarly, for patients following upper abdominal surgery, preoperative SOB was associated with the development of severe respiratory complications.47 For a busy surgical department, PRO-measured SOB may provide a feasible and interpretable method to identify those patients who have a risk of the occurrence of PPCs after lung surgery.
Symptoms of SOB, fatigue, and pain were commonly reported by patients with LC surgery, even though they suffered from SOB after completion of therapy.48 In our study, the two-group GBTM demonstrated that although all patients started with a low SOB symptom burden, more than half of the patients reported significantly more severe SOB symptoms during hospitalization, peaking on POD1. In addition, SOB could interfere with patients’ activities of daily living, with more symptom interference occurring as symptom severity increases.49 Cheville et al described that symptom of dyspnea was strongly associated with poor clinical outcomes (ie, employment status, physical activity, and overall QoL) in LC survivors.8 These results suggested that routine and proactive monitoring of the presence and severity of SOB as early as possible might be needed in practice. Adequate assessment and cutpoint-assisted interpretation of patient-reported SOB will support on-time patient care and promote optimal outcomes in the clinic.
Several symptom management guidelines have been developed based on symptom ratings so far, typically including severity thresholds. The National Comprehensive Cancer Network (NCCN) suggests that clinical attention was needed when these scores of symptoms, such as pain, fatigue, and distress, were rated ≥ 4 on a 0–10 scale.50 Our study suggested that 6 was the optimal cutpoint of SOB on POD1 for identifying patients who might develop PPCs after LC surgery. Selby et al used a receiver operating characteristic (ROC) curve, and the severity of SOB expressed on a verbal scale as a reference reported CP6 for severe SOB.51 The results are comparable to the cutoff values recorded in our study, although Selby et al used a verbal scale with patient options of none, mild, moderate, or severe as the anchor to determine cutpoints of symptoms in patients with palliative care.
Investigations have also used anchor-based methods to describe grades of symptom severity as the basis for establishing cutpoints between mild, moderate, and severe levels for various symptoms.52 Although there is no overall recommendation for choosing the anchors for the determination of cutpoints, the symptom interference items of the MDASI are usually used as anchors for symptom severity items, such as pain and fatigue.53,54 However, we used PPCs as the anchor in our study for the purpose of generating a clinically meaningful cutpoint for the early alerting of PPCs. The anchor (PPCs) we used significantly correlated with postoperative length of stay, which is commonly used in oncology practice for reflecting surgical skill and quality of care.55 Development of any PPCs, even if mild, is associated with reduced survival, prolonged stay in the hospital and increased in-hospital mortality.56 Additionally, our analysis demonstrated that the CP6-defined severe SOB level was associated with higher MDASI-LC symptom interference levels, suggesting that CP6 can be interpreted for functional impairment, similar as the pain and fatigue cutpoints anchored on the MDASI symptom interference.
This study had several limitations. First, few lung cancer patients underwent open surgery, resulting in a lower frequency of PPCs, which may preclude the observation of a large group in a timely manner and affect the comparability and generalization of the study results. However, VATs have been increasingly applied for LC surgery during the past 10 years,57 and recent report showed the proportion of VATs was reached 74.9% in LC patients,58 suggesting that this result can be interpreted for current clinical practice. Second, we used an anchor-based method to find the optimal cutpoint considering the relation between symptom severity and PPCs. Choosing different anchors and methods in other patient cohorts might produce different “optimal” cutpoint and variability in optimal cutpoint is to be expected,59 depending on the purpose of the test in a specific context as well as the costs of misses and false alarms.60 Third, we only used internal samples to validate the cutpoint of SOB but lacked external validation. External validation of the SOB cutpoint generated from our study is needed for a more generalized interpretation. Finally, we used 0–10 scale to measure dyspnea instead of using mMRC or TDI, further validation of our results are needed with those commonly used measurements.
Conclusion
In summary, the current analysis identified high POD1 dyspnea score (the patient-reported SOB), low FEV1, male and open surgery as predictive factors to the onset of PPCs after lung cancer surgery. The clinically meaningful cutpoint of SOB might facilitate the future evaluation of risk for PPCs, improve postoperative patient care and decrease clinician burden.
Abbreviations
CDC, Clavien–Dindo classification; CI, confidence interval; FEV1, forced expiratory volume in 1 second; GBTM, group-based trajectory modeling; LC, lung cancer; MDASI, MD Anderson Symptom Inventory; MDASI-LC, MD Anderson Symptom Inventory-lung cancer module; PLOS, postoperative length of stay; POD1, postoperative day 1; PPCs, postoperative pulmonary complications; PRO, patient-reported outcome; SOB, shortness of breath.
Data Sharing Statement
The datasets generated and analyzed during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Sichuan Cancer Hospital (Approval number: SCCHEC-02-2017-042). Trials registration number: NCT03341377. I confirm that this study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank Yaqin Wang, Jia Liao, and Shaohua Xie for providing data collection expertise in the critical appraisal of the included studies. We would also like to thank Editage for English language edit.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 81872506).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. de la Gala F, Piñeiro P, Reyes A, et al. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. Br J Anaesth. 2017;119(4):655–663. doi:10.1093/bja/aex230
2. Stéphan F, Boucheseiche S, Hollande J, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest. 2000;118(5):1263–1270. doi:10.1378/chest.118.5.1263
3. Efficace F, Cartoni C, Niscola P, et al. Predicting survival in advanced hematologic malignancies: do patient-reported symptoms matter? Eur J Haematol. 2012;89(5):410–416. doi:10.1111/ejh.12004
4. Odor PM, Bampoe S, Gilhooly D, Creagh-Brown B, Moonesinghe SR. Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ. 2020;368:m540. doi:10.1136/bmj.m540
5. Lakshminarasimhachar A, Smetana GW. Preoperative evaluation: estimation of pulmonary risk. Anesthesiol Clin. 2016;34(1):71–88. doi:10.1016/j.anclin.2015.10.007
6. Brunelli A, Drosos P, Dinesh P, Ismail H, Bassi V. The severity of complications is associated with postoperative costs after lung resection. Ann Thorac Surg. 2017;103(5):1641–1646. doi:10.1016/j.athoracsur.2016.10.061
7. McCannon J, Temel J. Comprehensive management of respiratory symptoms in patients with advanced lung cancer. J Support Oncol. 2012;10(1):1–9. doi:10.1016/j.suponc.2011.07.002
8. Cheville AL, Novotny PJ, Sloan JA, et al. The value of a symptom cluster of fatigue, dyspnea, and cough in predicting clinical outcomes in lung cancer survivors. J Pain Symptom Manage. 2011;42(2):213–221. doi:10.1016/j.jpainsymman.2010.11.005
9. van Boekel RLM, Warlé MC, Nielen RGC, et al. Relationship between postoperative pain and overall 30-day complications in a broad surgical population: an observational study. Ann Surg. 2019;269(5):856–865. doi:10.1097/SLA.0000000000002583
10. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi:10.1093/annonc/mdm056
11. Chandler D, Mosieri C, Kallurkar A, et al. Perioperative strategies for the reduction of postoperative pulmonary complications. Best Pract Res Clin Anaesthesiol. 2020;34(2):153–166. doi:10.1016/j.bpa.2020.04.011
12. Janssen DJ, Wouters EF, Spruit MA. Psychosocial consequences of living with breathlessness due to advanced disease. Curr Opin Support Palliat Care. 2015;9(3):232–237. doi:10.1097/SPC.0000000000000146
13. Feinstein MB, Krebs P, Coups EJ, et al. Current dyspnea among long-term survivors of early-stage non-small cell lung cancer. J Thorac Oncol. 2010;5(8):1221–1226. doi:10.1097/JTO.0b013e3181df61c8
14. Sarna L, Cooley ME, Brown JK, Chernecky C, Elashoff D, Kotlerman J. Symptom severity 1 to 4 months after thoracotomy for lung cancer. Am J Crit Care. 2008;17(5):
15. Bauml J, Haas A, Simone CB, et al. Acupuncture for dyspnea in lung cancer: results of a feasibility trial. Integr Cancer Ther. 2016;15(3):326–332. doi:10.1177/1534735415624138
16. Ha D, Ries AL. Characterization of dyspnea in veteran lung cancer survivors following curative-intent therapy. J Cardiopulm Rehabil Prev. 2020;40(2):120–127. doi:10.1097/HCR.0000000000000464
17. Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg. 2015;150(3):613–619 e612. doi:10.1016/j.jtcvs.2015.05.057
18. Ban W, Lee JM, Ha JH, et al. Dyspnea as a prognostic factor in patients with non-small cell lung cancer. Yonsei Med J. 2016;57(5):1063. doi:10.3349/ymj.2016.57.5.1063
19. Obi ON, Judson MA, Birring SS, et al. Assessment of dyspnea in sarcoidosis using the Baseline Dyspnea Index (BDI) and the Transition Dyspnea Index (TDI). Respir Med. 2022;191:106436. doi:10.1016/j.rmed.2021.106436
20. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi:10.1164/rccm.201111-2042ST
21. Watkins ML, Wilcox TK, Tabberer M, et al. Shortness of breath with daily activities questionnaire: validation and responder thresholds in patients with chronic obstructive pulmonary disease. BMJ open. 2013;3(10):e003048. doi:10.1136/bmjopen-2013-003048
22. Reponen E, Korja M, Tuominen H. Simple preoperative patient-reported factors predict adverse outcome after elective cranial neurosurgery. Neurosurgery. 2018;83(2):197–202. doi:10.1093/neuros/nyx385
23. Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362(10):865–869. doi:10.1056/NEJMp0911494
24. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. doi:10.1200/JCO.2015.63.0830
25. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6(3):537–546. doi:10.1002/cam4.1002
26. Johnson MJ, Currow DC, Booth S. Prevalence and assessment of breathlessness in the clinical setting. Expert Rev Respir Med. 2014;8(2):151–161. doi:10.1586/17476348.2014.879530
27. Dai W, Xie S, Zhang R, et al. Developing and validating utility parameters to establish patient-reported outcome-based perioperative symptom management in patients with lung cancer: a multicentre, prospective, observational cohort study protocol. BMJ open. 2019;9(10):e030726. doi:10.1136/bmjopen-2019-030726
28. Mendoza TR, Wang XS, Lu C, et al. Measuring the symptom burden of lung cancer: the validity and utility of the lung cancer module of the M. D. Anderson Symptom Inventory. Oncologist. 2011;16(2):217–227. doi:10.1634/theoncologist.2010-0193
29. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
30. Yang X, Zhan C, Li M, et al. Lobectomy versus sublobectomy in metachronous second primary lung cancer: a propensity score study. Ann Thorac Surg. 2018;106(3):880–887. doi:10.1016/j.athoracsur.2018.04.071
31. Dai W, Feng W, Zhang Y, et al. Patient-reported outcome-based symptom management versus usual care after lung cancer surgery: a multicenter randomized controlled trial. J Clin Oncol. 2022;JCO2101344. doi:10.1200/JCO.21.01344
32. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
33. Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65(9):815–818. doi:10.1136/thx.2009.123083
34. Li S, Fan J, Liu J, et al. Neoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: a systematic meta-analysis of 14 912 patients. Jpn J Clin Oncol. 2016;46(6):534–546. doi:10.1093/jjco/hyw037
35. Shinohara S, Kobayashi K, Kasahara C, et al. Long-term impact of complications after lung resections in non-small cell lung cancer. J Thorac Dis. 2019;11(5):2024–2033. doi:10.21037/jtd.2019.04.91
36. Mahajan AK, Doeing DC, Hogarth DK. Isolation of persistent air leaks and placement of intrabronchial valves. J Thorac Cardiovasc Surg. 2013;145(3):626–630. doi:10.1016/j.jtcvs.2012.12.003
37. Savardekar A, Gyurmey T, Agarwal R, et al. Incidence, risk factors, and outcome of postoperative pneumonia after microsurgical clipping of ruptured intracranial aneurysms. Surg Neurol Int. 2013;4:24. doi:10.4103/2152-7806.107894
38. Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32(2):88–105. doi:10.1097/EJA.0000000000000118
39. Jones BL. TRAJ: group-based modeling of longitudinal data; 2005. Available from: http://www.andrew.cmu.edu/user/bjones/.
40. Shi Q, Mendoza TR, Gunn GB, Wang XS, Rosenthal DI, Cleeland CS. Using group-based trajectory modeling to examine heterogeneity of symptom burden in patients with head and neck cancer undergoing aggressive non-surgical therapy. Qual Life Res. 2013;22(9):2331–2339. doi:10.1007/s11136-013-0380-2
41. Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000;19(1):113–132. doi:10.1002/(SICI)1097-0258(20000115)19:1<113::AID-SIM245>3.0.CO;2-O
42. Kim ES, Kim YT, Kang CH, et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1317–1326. doi:10.2147/COPD.S105206
43. Pezzuto A, Terzo F, Graziani ML, Ricci A, Bruno P, Mariotta S. Lung cancer requires multidisciplinary treatment to improve patient survival: a case report. Oncol Lett. 2017;14(3):3035–3038. doi:10.3892/ol.2017.6511
44. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):581–595. doi:10.7326/0003-4819-144-8-200604180-00009
45. Pezzuto A, Spoto C, Vincenzi B, Tonini G. Short-term effectiveness of smoking-cessation treatment on respiratory function and CEA level. J Comp Eff Res. 2013;2(3):335–343. doi:10.2217/cer.13.25
46. Gallart L, Canet J. Post-operative pulmonary complications: understanding definitions and risk assessment. Best Pract Res Clin Anaesthesiol. 2015;29(3):315–330. doi:10.1016/j.bpa.2015.10.004
47. Barisione G, Rovida S, Gazzaniga GM, Fontana L. Upper abdominal surgery: does a lung function test exist to predict early severe postoperative respiratory complications? Eur Respir J. 1997;10(6):1301–1308. doi:10.1183/09031936.97.10061301
48. Whisenant MS, Williams LA, Garcia Gonzalez A, et al. What do patients with non-small-cell lung cancer experience? Content domain for the MD Anderson Symptom Inventory for lung cancer. JCO Oncol Pract. 2020;16(10):e1151–e1160. doi:10.1200/JOP.19.00577
49. Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer. J Pain Symptom Manage. 2002;23(5):417–423. doi:10.1016/S0885-3924(02)00376-7
50. Shi Q, Lee JW, Wang XS, et al. Testing symptom severity thresholds and potential alerts for clinical intervention in patients with cancer undergoing chemotherapy. JCO Oncol Pract. 2020;16(9):e893–e901.
51. Selby D, Cascella A, Gardiner K, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2010;39(2):241–249. doi:10.1016/j.jpainsymman.2009.06.010
52. Chow E, Ding K, Parulekar WR, et al. Revisiting classification of pain from bone metastases as mild, moderate, or severe based on correlation with function and quality of life. Support Care Cancer. 2016;24(4):1617–1623. doi:10.1007/s00520-015-2957-5
53. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi:10.1016/0304-3959(94)00178-H
54. Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–432. doi:10.1002/cncr.28434
55. Hu XL, Xu ST, Wang XC, et al. Development and validation of nomogram estimating post-surgery hospital stay of lung cancer patients: relevance for predictive, preventive, and personalized healthcare strategies. EPMA J. 2019;10(2):173–183. doi:10.1007/s13167-019-00168-z
56. Serpa Neto A, Hemmes SN, Barbas CS, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med. 2014;2(12):1007–1015. doi:10.1016/S2213-2600(14)70228-0
57. Yun J, Lee J, Shin S, et al. Video-assisted thoracoscopic lobectomy versus open lobectomy in the treatment of large lung cancer: propensity-score matched analysis. J Cardiothorac Surg. 2022;17(1):2. doi:10.1186/s13019-021-01749-8
58. Sun D, Chen P, Liu L, et al. The 5-year survival rate of 11958 postoperative non-small cell lung cancer patients in stage I-IIIA by two different follow-up patterns: a multi-center, real-world study. Chin J Clin Thorac Cardiovasc Surg. 2021;28(6):615–622.
59. Hirschfeld G, Zernikow B. Variability of ”optimal” cut points for mild, moderate, and severe pain: neglected problems when comparing groups. Pain. 2013;154(1):154–159. doi:10.1016/j.pain.2012.10.008
60. Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45(6):1083–1093. doi:10.1016/j.jpainsymman.2012.06.007
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



