Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Short-term treatment of irbesartan and hydrochlorothiazide decreases plasma N-terminal pro-brain natriuretic peptide levels in subjects with acute exacerbations of COPD
Received 10 September 2018
Accepted for publication 4 December 2018
Published 20 December 2018 Volume 2019:14 Pages 73—80
DOI https://doi.org/10.2147/COPD.S186872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chunxue Bai
Gui-yun Jiang,1 Qun Li,2 Yun-xiang Lv2,3
1Department of Clinical Laboratory, The Second People’s Hospital of Bengbu, Bengbu, Anhui 233000, People’s Republic of China; 2Department of Respiratory Medicine, The Second Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui 233000, People’s Republic of China; 3Department of Pulmonary Medical, Anhui Geriatric Institute, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui 230022, People’s Republic of China
Background: Plasma levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) are elevated in subjects with COPD, and high plasma NT-proBNP levels are correlated with a poor prognosis. Thus, it is crucial to decrease the plasma NT-proBNP levels at the early stage of disease. We aimed to assess the effects of short-term treatment of irbesartan and hydrochlorothiazide on plasma NT-proBNP levels and health-related quality of life (HRQOL) in subjects with acute exacerbations of COPD (AECOPD).
Subjects and methods: Eighty subjects with AECOPD and high plasma NT-proBNP levels, without any clinical evidence of cor pulmonale, were enrolled. The subjects were randomly allocated into two groups of 40 subjects. In addition to standard treatment for AECOPD, the subjects in group I were treated with irbesartan alone, and those in group II were treated with irbesartan and hydrochlorothiazide for a week. Forty subjects with stable COPD were enrolled as a control group. Plasma NT-proBNP concentrations were measured on admission and on the first, fourth, and seventh days. The subjects’ health-related quality of life was evaluated applying the 36-item short-form questionnaire on the first day before treatment and on the seventh day after treatment.
Results: Treatment of irbesartan and hydrochlorothiazide significantly decreased plasma NT-proBNP levels in subjects with AECOPD, and this reduction was more significant in group II than that in group I. There were no significant differences in 36-item short-form domain scores between subjects with stable COPD and those with AECOPD who were treated with irbesartan and hydrochlorothiazide.
Conclusion: Treatment of irbesartan and hydrochlorothiazide rapidly decreased plasma NT-proBNP levels in subjects with AECOPD, and the treatment did not impair their physical status.
Keywords: COPD, health-related quality of life, hydrochlorothiazide, irbesartan, NT-proBNP
Introduction
COPD is a common, preventable, and difficult to long-time controlled disease, and acute exacerbations of COPD (AECOPD) may directly affect rates of morbidity and mortality.1–3 The main cause of death in subjects with COPD is cardiac rather than respiratory complications.4 Moreover, secondary pulmonary hypertension and cor pulmonale are also causes of death in subjects with COPD.5 Thus, early diagnosis and intervention to treat comorbidities are critical for improving the prognosis of these subjects.1
B-type natriuretic peptide (BNP) and 76-amino acid N-terminal fragment of pro-brain natriuretic peptide (NT-proBNP) are derived from the pro-hormone proBNP, an intracellular, 108-amino acid precursor protein, which are known as biomarkers of heart failure (HF) and are mainly used for diagnosis, risk stratification, and management.6 It plays a role in the control of sodium excretion and blood pressure, and has a compensatory role in cardiorenal homeostasis.7,8 Elevated NT-proBNP levels have been observed in hypoxemic subjects with COPD and it is significantly elevated, particularly in subjects with cor pulmonale when compared to subjects with COPD alone.9–11 Raised plasma NT-proBNP levels may be a useful early indicator of right ventricular (RV) systolic dysfunction in subjects with COPD, and monitoring changes in plasma NT-proBNP concentration may provide a critical way for evaluating RV function during follow-up.12,13 Moreover, other stimuli that induce cardiac production of NT-proBNP include hypoxia, ischemia, and nephrosis and cirrhosis associated with an increase in central blood volume.14,15
Since high plasma levels of NT-proBNP are correlated with increased strain on the ventricles, subjects with COPD of high NT-proBNP concentration should have some degree of RV strain, resulting in raised NT-proBNP levels even in the absence of clinical findings of RV dysfunction. Thus, subjects suffering with AECOPD and high plasma NT-proBNP levels might benefit from reduction of volumetric strain on the RV. In an emphysema mouse model, administration of irbesartan, an angiotensin II type-1 receptor (AT1) blocker, improved the lung biomechanics and exercise capacity.16 Treatment of losartan increased elastic fiber production in subjects with emphysema, which suggests that losartan may have salutary effects in subjects with emphysema.17 Furthermore, not only diuretic therapy alone reduced high plasma BNP levels18 but also combination therapy of a diuretic and a vasodilator decreased its levels in subjects with AECOPD.19 However, the effects of irbesartan and hydrochlorothiazide, a fixed-dose combination for treatment of hypertension, on plasma NT-proBNP levels and health-related quality of life (HRQOL) have not been investigated in subjects with AECOPD. Here, we aimed to evaluate the effects of these drugs on plasma NT-proBNP levels and HRQOL in subjects with AECOPD.
Subjects and methods
Subjects
Eighty consecutive subjects with AECOPD (groups I and II) were diagnosed in accordance with the 2013 guidelines of the Global Initiative for Chronic Obstructive Lung Disease.20 Forty subjects with stable COPD (control, group III) also were enrolled in the study from January 2016 to June 2017. We excluded subjects with cor pulmonale, pneumonia, diabetes mellitus, renal failure, lung cancer, atherosclerotic or congenital cardiac disease, or left ventricular failure. All subjects underwent clinical and radiological examinations, pulmonary function testing, arterial blood gas analysis, and echocardiographical examination. All procedures were approved by the local ethics committee, and each subject gave written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Randomization and concealment of allocations
Eligible subjects were identified and randomly enrolled to each group using boxes that were consecutively numbered, sealed, and adiaphanous. The drugs were randomly placed in the boxes by a statistician who was not involved in the study. Doctors and nurses were blinded to what drugs the subjects received. Subjects selected a box that was opened to determine which treatment they would receive. Outside of the box was a questionnaire to be finished by the attending doctor, who was blind to the contents of the box.
NT-proBNP test and laboratory tests
Venous blood samples from subjects were collected by venipuncture into EDTA tubes. The samples were centrifuged and plasmas were then analyzed within 2 hours using a One Step Test for NT-proBNP kit, performed on automated FIA8000 Quantitative Immunoassay Analyzer (Getein Biotechnology Co., Ltd., Nanjing, Jiangsu, People’s Republic of China). Blood samples were also taken within 24 hours of admission to analyze plasma NT-proBNP, serum creatinine, and arterial blood gas.
Health-related quality of life
To assess the effects of treatment of irbesartan and hydrochlorothiazide on subjects with COPD, we applied Medical Outcomes Study 36-item short-form (SF-36) survey to evaluate HRQOL.21 The SF-36 has been usually used to estimate the health status of subjects with COPD.22 The SF-36 is defined for health status by WHO and contains eight domains: physical functioning, role physical, bodily pain, general health, social functioning, role emotional, vitality, and mental health. The score for each domain ranges from 0 to 100, with higher scores indicating better HRQOL. Each subject read the 36 questions and marked an answer for each on the sheet. This process took <10 minutes for each patient.
Study design
We detected plasma NT-proBNP levels of all subjects before further randomization and excluded those subjects with normal plasma NT-proBNP levels in the study. Eighty subjects with AECOPD and high plasma NT-proBNP levels satisfied our criteria and were randomly allocated into groups I and II. In addition to the standard therapy for AECOPD, the 40 subjects in group I received irbesartan (150 mg/day) for 7 days. The 40 subjects in group II were treated with a fixed dose irbesartan (150 mg/day) and hydrochlorothiazide (12.5 mg/day) for 7 days. Group III comprised 40 subjects with stable COPD.
Measurements of plasma NT-proBNP levels were detected on the first, fourth, and seventh days of therapy. The subjects’ HRQOL was evaluated again using the SF-36 on the seventh day.
The subjects in groups I and II received standard therapy for AECOPD as following: all subjects received oxygen through nasal catheter to sustain an oxygen saturation of 88%–92%. According to the blood gas analysis results, subjects with hypoxemia received 3–5 L/min, and those with hypercapnia received 1–2 L/min. Doxofylline (0.2 g/12 h) was the preferred bronchodilator. Corticosteroids (intravenous methylprednisolone, 40 mg/day for 3 days, followed by decreasing doses of oral methylprednisolone for 7 days) were administered to all subjects. When bacterial infections were diagnosed, antibiotics were administered and prescribed according to the patient’s sputum etiology test results. Nebulized terbutaline (5 mg/8 h) or nebulized ipratropium bromide (500 mg/8 h) was used as bronchodilators for appropriate subjects. Subjects in group III received only dry powder inhalations of budesonide (200 mg/day) and tiotropium (18 mg/day).
Echocardiography was performed to assess RV function on the first and seventh days. The echocardiographic evidence of RV hypokinesia with or without dilatation was used to define RV dysfunction. RV dysfunction and pulmonary arterial pressures on echo examination of all subjects were noted. We used electrocardiograms to collect the levels of RV voltage. The subjects with RV5 ≥2.5 mV were considered as a higher RV strain than those with RV5 <2.5 mV.
Statistical analyses
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Data are expressed as the mean (±SD). To examine differences between the two groups, Student’s t-test or Mann–Whitney U-test was used to compare continuous variables depending on the distribution of the data. To compare differences among the three groups, a Kruskal–Wallis test for continuous variables was performed. Correlation coefficients were calculated with the Spearman rank test. The values for domains of the SF-36 were categorized into tertiles for parsimony and were subsequently dichotomized.23 The range of scores on domains from 0 to 100 was signed. The scores on domains are positively correlated with the health status; the higher scores represent the better health status.
Results
Characteristics and differences of study subjects
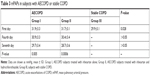
A total of 80 subjects with AECOPD and 40 subjects with stable COPD met the study criteria were finally enrolled. The general characteristics of the three groups are shown in Table 1. There was no significant correlation between plasma NT-proBNP levels and arterial blood gas measurements (P>0.05). There were no differences in age, gender, smoking history, arterial blood gas measurements, and the presence of RV dysfunction on echocardiographical among subjects in the three groups. The plasma levels of NT-proBNP of the first-day measurement were lower in the subjects with stable COPD than that in those with acute exacerbations (1,083.3±308.5 vs 517.4±80.1 pg/mL, P<0.05). Nevertheless, there were no significant differences between subjects with AECOPD who were enrolled in group I and those in group II (P>0.05). Thus, the allocation of subjects into these groups could be thought to be proper.
There was a gradual reduction in plasma NT-proBNP levels from the first day to the fourth and seventh days in groups I and II, and this reduction was more significant in group II than that in group I (Table 2). The plasma NT-proBNP levels were significantly decreased by treatment of irbesartan and hydrochlorothiazide compared to treatment of irbesartan in subjects with AECOPD (Table 2). These findings suggested that treatment of irbesartan and hydrochlorothiazide has a rapider effect on decrease of plasma NT-proBNP levels than irbesartan alone. Moreover, in groups I and II, the decrease of plasma NT-proBNP levels was independent of the presence or absence of RV dysfunction that was determined by echocardiographical evaluation. In addition, there was also a gradual decrease in mean pulmonary arterial pressures (mPAPs) from the first day to the fourth and seventh days in groups I and II; however, the significant decrease was only observed between the first day and the seventh day. Furthermore, there were no significant differences between the two groups (Table 3).
In addition, we also found 97.5% AECOPD subjects enrolled in our study with an mPAP ≥25 mmHg by echocardiography measurement. In the Pearson correlation analysis (Figure 1), there was a significant, strongly positive relationship between plasma NT-proBNP levels and mPAP (r=0.857, P<0.001). The reduction of NT-proBNP concentrations was correlated with the reduction of mPAPs.
The effects of irbesartan and hydrochlorothiazide therapy on HRQOL in subjects with AECOPD
First, we used questionnaires to assess the mean scores of the SF-36 domains between subjects with AECOPD in group II and subjects with stable COPD in group III on the first day before treatment of irbesartan and hydrochlorothiazide. In the present research, the scores from questionnaires on the role physical, general health, mental health, and vitality domains in subjects with AECOPD were significantly poor relative to that in subjects with stable COPD (Table 4). Second, we also assessed the effects of treatment of irbesartan and hydrochlorothiazide on HRQOL on the seventh day. Interestingly, although this treatment significantly increased the mean scores for the role physical, general health, mental health, and vitality domains compared to that before treatment, there were no significant differences between subjects in group II after treatment and subjects in group III (Table 4).
Discussion
The increased plasma NT-proBNP concentrations have been observed in subjects with AECOPD compared to that those with stable COPD.24,25 Moreover, the plasma NT-proBNP levels are significantly higher in subjects with stage IV and stage III COPD than individuals with stage II COPD.26 One reason for this elevated plasma NT-proBNP levels may be the presence of secondary pulmonary hypertension and/or left ventricular failure in subjects with COPD.27 Thus, in our study, we excluded the subjects with a history of symptoms and/or physical evidence of cor pulmonale. Similarly, we also found that subjects involved in our research were similar to previous studies that subjects with AECOPD had higher levels of pulmonary arterial pressure than those with stable COPD.17,18 Here, the short-term treatment of irbesartan and hydrochlorothiazide, a fixed-dose combination, or of irbesartan alone significantly reduced the plasma NT-proBNP levels in subjects with AECOPD on the fourth and seventh days. Furthermore, the effects of combined treatment on this reduction were more striking than that of irbesartan alone. More importantly, the treatment did not impair health status of subjects with AECOPD.
NT-proBNP is produced and released from the ventricles in response to increased wall stretch and tension.28 High plasma NT-proBNP levels are significantly correlated with increased RV strain in subjects with stable COPD.26 Moreover, elevated plasma NT-proBNP levels are related to increased risk of all-cause mortality in COPD subjects both with and without exacerbation and NT-proBNP is considered as a strong and independent biomarker of mortality after AECOPD.29,30 In line with previous studies, we found that there was a strong positive correlation between plasma NT-proBNP levels and mPAP, which further supported the result of increased RV strain and can lead to high plasma NT-proBNP levels.6,24 Interestingly, we also found that the significant decrease of mPAPs was only observed between the first day and the seventh day in groups I and II, which may suggest that it is a relatively chronic process to regulate pulmonary arterial pressures through treatment of irbesartan and hydrochlorothiazide. In our study, adding hydrochlorothiazide treatment in subjects with AECOPD may have an effect on reducing elevated plasma NT-proBNP levels and thus the RV strain. It may be considered that plasma BNP levels may reduce more rapidly in those subjects who have received combined treatment than those only received irbesartan. RV insufficiency, polycythemia, and sodium and water retention lead to increased intravascular volume. An increased RV afterload strengthens RV contractility, which results in the increased production of NT-proBNP levels. If the intravascular volume is decreased, pulmonary hemodynamics, RV performance, and gas exchange may improve. Hydrochlorothiazide can reduce volume overload by enhancing salt and water excretion. Therefore, treatment of irbesartan and hydrochlorothiazide has a more striking reduction of NT-proBNP levels than hydrochlorothiazide alone.
The sympathetic nervous system, as well as the renin-angiotensin system, is activated in subjects with COPD.31 AT1 receptors are highly expressed within the lung and regulate alveolar epithelial cell apoptosis and lung fibroblast growth.32–34 Treatment of irbesartan, an AT1 receptor blocker, resulted in a significant decrease in hematocrit and total lung capacity in subjects with COPD.35 This role of irbesartan can improve the pulmonary hemodynamics and the gas exchanges. In addition, treatment of irbesartan improved lung biomechanics and exercise capacity, which further supported its role of improving gas exchanges in an emphysema mouse model.16 Treatment of irbesartan has reduced the plasma NT-proBNP levels in subjects with chronic HF.36,37 As we expected, treatment of irbesartan led to the reduction of plasma NT-proBNP levels in subjects with AECOPD in our study. These results suggest that irbesartan may have a role in reducing plasma NT-proBNP levels through improvement of pulmonary hemodynamics and gas exchange in subjects with AECOPD.
The elevated plasma NT-proBNP levels have been observed in most subjects with AECOPD even when there is no clinical or echocardiographical evidence of pulmonary arterial hypertension and/or cor pulmonale. Treatment of irbesartan has reduced the plasma NT-proBNP levels in subjects with chronic HF.36,37 The significant decrease of plasma NT-proBNP levels was also observed in subjects with AECOPD when administration of irbesartan alone, which further demonstrated its beneficial effects in subjects with AECOPD. Furthermore, not only diuretic alone reduced high plasma BNP levels17 but also combination therapy of a diuretic and a vasodilator decreased its levels in subjects with AECOPD.18 In the present study, our novel findings indicated that combined therapy of irbesartan and hydrochlorothiazide also decreased plasma NT-proBNP levels in subjects with AECOPD. These results suggested that treatment of irbesartan and hydrochlorothiazide may be helpful for improvement of RV condition and delay of RV failure by reduction of plasma BNP levels in subjects with AECOPD.
In regard to optimistic results in the present study, we assessed if the combined therapy will impair HRQOL of those subjects. HRQOL is applied to measure the subjects with chronic diseases.38 In previous studies, HRQOL was used to evaluate the impact of medical treatment that potentially prolongs survival, for example, treatment with β-blockers in COPD.39 In such cases, HRQOL measurement provides important information that is complementary to lung function parameters. In our study, the results showed that therapy of irbesartan and hydrochlorothiazide did not impair HRQOL of subjects with AECOPD. More importantly, this therapy significantly increased the scores for the role physical, general health, mental health, and vitality domains of the SF-36. Consistent with a previous research, treatment of a diuretic and a vasodilator also did not impair HRQOL of subjects with AECOPD.18 Although scores for the role physical, general health, and vitality were significantly increased, significant increased scores for mental health ere not observed in previous study.18 The differences may be explained for two points as following: on the one hand, varying drugs brought diverse effects; on the other hand, we have enrolled more subjects, which represented the less bias may be absent in our study. We also showed that combined treatment could be safe to subjects with AECOPD and it did not impair their lung functions at least in the short term. These extended findings suggest that combined treatment of irbesartan and hydrochlorothiazide did not impair health status of subjects with AECOPD.
Conclusion
Short-term treatment of irbesartan and hydrochlorothiazide rapidly reduced the plasma NT-proBNP levels in subjects with AECOPD. Furthermore, the treatment did not impair health status of subjects with AECOPD. These results may provide a novel therapeutic strategy leading to early clinical improvement in subjects with AECOPD.
Acknowledgment
This project was supported by the Natural Science Foundation of Bengbu Medical College (BYKY1633ZD).
Disclosure
The authors report no conflicts of interest in this work.
References
Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. | ||
Fruchter O, Yigla M. Predictors of long-term survival in elderly patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Respirology. 2008;13(6):851–855. | ||
Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117(5 Suppl 2):398S–401S. | ||
Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. | ||
Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107(5):1193–1198. | ||
Andreassen AK, Wergeland R, Simonsen S, Geiran O, Guevara C, Ueland T. N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol. 2006;98(4):525–529. | ||
Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. | ||
Yap LB, Mukerjee D, Timms PM, Ashrafian H, Coghlan JG. Natriuretic peptides, respiratory disease, and the right heart. Chest. 2004;126(4):1330–1336. | ||
Lang CC, Coutie WJ, Struthers AD, Dhillon DP, Winter JH, Lipworth BJ. Elevated levels of brain natriuretic peptide in acute hypoxaemic chronic obstructive pulmonary disease. Clin Sci. 1992;83(5):529–533. | ||
Morrison LK, Harrison A, Krishnaswamy P, Kazanegra R, Clopton P, Maisel A. Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol. 2002;39(2):202–209. | ||
Arjamaa O, Nikinmaa M. Hypoxia regulates the natriuretic peptide system. Int J Physiol Pathophysiol Pharmacol. 2011;3(3):191–201. | ||
Tulevski II, Groenink M, van der Wall EE, et al. Increased brain and atrial natriuretic peptides in patients with chronic right ventricular pressure overload: correlation between plasma neurohormones and right ventricular dysfunction. Heart. 2001;86(1):27–30. | ||
Bando M, Ishii Y, Sugiyama Y, Kitamura S. Elevated plasma brain natriuretic peptide levels in chronic respiratory failure with cor pulmonale. Respir Med. 1999;93(7):507–514. | ||
D’Souza SP, Yellon DM, Martin C, et al. B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol. 2003;284(5):H1592–H1600. | ||
Tóth M, Vuorinen KH, Vuolteenaho O, et al. Hypoxia stimulates release of ANP and BNP from perfused rat ventricular myocardium. Am J Physiol. 1994;266(4 Pt 2):H1572–H1580. | ||
Raupach T, Lüthje L, Kögler H, et al. Local and systemic effects of angiotensin receptor blockade in an emphysema mouse model. Pulm Pharmacol Ther. 2011;24(2):215–220. | ||
Lehman A, Mattman A, Sin D, et al. Emphysema in an adult with galactosialidosis linked to a defect in primary elastic fiber assembly. Mol Genet Metab. 2012;106(1):99–103. | ||
Kanat F, Vatansev H, Teke T. Diuretics, plasma brain natriuretic peptide and chronic obstructive pulmonary disease. Neth J Med. 2007;65(8):296–300. | ||
Zhang J, Zhao G, Yu X, Pan X. Intravenous diuretic and vasodilator therapy reduce plasma brain natriuretic peptide levels in acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2012;17(4):715–720. | ||
Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–1068. | ||
Desikan R, Mason HL, Rupp MT, Skehan M. Health-related quality of life and healthcare resource utilization by COPD patients: a comparison of three instruments. Qual Life Res. 2002;11(8):739–751. | ||
Rumsfeld JS, Magid DJ, Plomondon ME, et al. History of depression, angina, and quality of life after acute coronary syndromes. Am Heart J. 2003;145(3):493–499. | ||
Adrish M, Nannaka VB, Cano EJ, Bajantri B, Diaz-Fuentes G. Significance of NT-pro-BNP in acute exacerbation of COPD patients without underlying left ventricular dysfunction. Int J Chron Obstruct Pulmon Dis. 2017;12:1183–1189. | ||
Vallabhajosyula S, Haddad TM, Sundaragiri PR, et al. Role of B-type natriuretic peptide in predicting in-hospital outcomes in acute exacerbation of chronic obstructive pulmonary disease with preserved left ventricular function: a 5-year retrospective analysis. J Intensive Care Med. 2018;33(11):635–644. | ||
Chi SY, Kim EY, Ban HJ, et al. Plasma N-terminal pro-brain natriuretic peptide: a prognostic marker in patients with chronic obstructive pulmonary disease. Lung. 2012;190(3):271–276. | ||
Shujaat A, Minkin R, Eden E. Pulmonary hypertension and chronic cor pulmonale in COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(3):273–282. | ||
Luchner A, Stevens TL, Borgeson DD, et al. Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am J Physiol. 1998;274(5 Pt 2):H1684–H1689. | ||
Høiseth AD, Omland T, Hagve TA, Brekke PH, Søyseth V. NT-proBNP independently predicts long term mortality after acute exacerbation of COPD – a prospective cohort study. Respir Res. 2012;13(1):97. | ||
Pavasini R, Tavazzi G, Biscaglia S, et al. Amino terminal pro brain natriuretic peptide predicts all-cause mortality in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis. Chron Respir Dis. 2017;14(2):117–126. | ||
Andreas S, Anker SD, Scanlon PD, Somers VK. Neurohumoral activation as a link to systemic manifestations of chronic lung disease. Chest. 2005;128(5):3618–3624. | ||
Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension. 1994;24(5):538–548. | ||
Wang R, Zagariya A, Ibarra-Sunga O, et al. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol. 1999;276(5 Pt 1):L885–L889. | ||
Molteni A, Ward WF, Ts’ao CH, et al. Cytostatic properties of some angiotensin I converting enzyme inhibitors and of angiotensin II type I receptor antagonists. Curr Pharm Des. 2003;9(9):751–761. | ||
Andreas S, Herrmann-Lingen C, Raupach T, et al. Angiotensin II blockers in obstructive pulmonary disease: a randomised controlled trial. Eur Respir J. 2006;27(5):972–979. | ||
Brack J, Sindone A, Funston R, et al. Is addition of angiotensin receptor blockade superior to increasing ACE inhibitor dose in patients with heart failure? Int J Cardiol. 2010;139(3):309–312. | ||
Yip GW, Wang M, Wang T, et al. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart. 2008;94(5):573–580. | ||
Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–629. | ||
van Gestel YR, Hoeks SE, Sin DD, et al. Beta-blockers and health-related quality of life in patients with peripheral arterial disease and COPD. Int J Chron Obstruct Pulmon Dis. 2009;4:177–183. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.