Back to Journals » Journal of Pain Research » Volume 11
Short-term incubation of gabapentin or pregabalin does not affect chemically induced injury in neuronal cell models in vitro
Authors Baldewig M, Goldbaum O , Richter-Landsberg C , Weyland A , Bantel C
Received 12 January 2018
Accepted for publication 1 April 2018
Published 20 June 2018 Volume 2018:11 Pages 1181—1190
DOI https://doi.org/10.2147/JPR.S162322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Malte Baldewig,1 Olaf Goldbaum,2 Christiane Richter-Landsberg,2 Andreas Weyland,1 Carsten Bantel1
1Department of Anesthesiology, Klinikum Oldenburg, Oldenburg, Germany; 2Molecular Neurobiology, Department of Neuroscience, University of Oldenburg, Oldenburg, Germany
Purpose: Gabapentinoids are currently the mainstay of pharmacological treatments for patients with neuropathic pain. Little is known about the effects of this therapy on the integrity of neuronal networks, especially in patients with an already-damaged nervous system. Since gabapentinoids can worsen cognitive functions and recent studies have shown alterations in the brains of patients with neuropathic pain, it may be possible that these drugs have neurotoxic effects.
Methods: Rat clonal PC12 pheochromocytoma (autonomic) and primary sensory dorsal-root ganglion (DRG) neurons from newborn Wistar rats were employed for this study. To mimic neuronal damage, cells were exposed to cytotoxins using either hydrogen peroxide (H2O2) or vincristine.
Results: No direct cytotoxic effects were observed after incubating PC12 cells for 24 hours with increasing concentrations of gabapentin or pregabalin using MTT cytotoxicity assays. Even a 7-day incubation did not cause cellular damage. Furthermore, in preinjured PC12 and DRG neurons, neither gabapentin nor pregabalin prevented or enhanced the cytotoxic effects of H2O2 or vincristine after incubation for 24 hours and 7 days, respectively. Cell morphology and integrity of the cytoskeleton assessed by employing immunostaining of cytoskeletal proteins (α-tubulin, neurofilament L) remained intact and were not altered by gabapentinoids.
Conclusion: Based on these results, gabapentinoids are unlikely to be neurotoxic in cultured autonomic (PC12) and sensory DRG cells, even when cells are preinjured. These results are of high clinical relevance, as it seems unlikely that the morphological changes recently observed in the brains of neuropathic pain patients are caused or worsened by gabapentinoids.
Keywords: gabapentin, pregabalin, cytotoxicity, cytoskeleton, neuropathic pain
Introduction
According to recent estimations, about 7%–8% of the European population suffer from neuropathic pain.1,2 However, the management of this condition remains challenging. Therefore, multimodal approaches consisting of psycho- and physiotherapy, as well as pharmacological management, are usually employed.3 The current mainstay of pharmacological therapy for neuropathic pain is gabapentin and pregabalin (gabapentinoids), which have been effective in several clinical trials.4–7 Nevertheless, despite their frequent and often long-term use, little is known about the consequences these drugs might exert on the structure and integrity of the central nervous system. This is even more important in light of recent evidence indicating morphological alterations in the brains of patients suffering from chronic pain compared to healthy controls.8 Here, particularly cortical neuronal cell bodies (gray matter) seem to be vulnerable to change.9–11 Although gray matter reduction was first described in chronic back pain, similar alterations have subsequently been shown for other chronic-pain entities, including neuropathic pain.8,12,13
However, to this point it remains unclear whether the observed structural changes are a result of the disease processes underlying pain itself or a consequence of the medications applied. The latter notion is supported by clinical experience, as gabapentinoids have been recognized for their ability to worsen the cognitive performance of patients.14 It is thus feasible to hypothesize that gabapentin and pregabalin influence cortical areas in the brain through alteration of neuronal cell density or structure. The present study was hence conducted to examine the effects of gabapentinoids on cell survival, morphology, and cytoskeletal integrity by focusing on the pivotal cytoskeletal proteins α-tubulin and neurofilament L (NFL) in healthy and injured autonomic and sensory neuronal cell models in vitro.
For this purpose, first rat pheochromocytoma line (PC12) cells were used. Since their establishment in 1976, PC12 cells have become one of the most regularly employed systems for neurodegeneration and neuroprotection studies. They resemble sympathetic neurons both functionally and morphologically. For instance, by responding to NGF, they convert from proliferating adrenal chromaffin-like to nondividing sympathetic neuron-like cells that spread out long neuritic processes.15 Additionally, primary cultures of rat dorsal-root ganglion (DRG) neurons have been employed to investigate the effect of gabapentinoids on sensory neuronal architecture and morphology. DRG neurons also differentiate in the presence of NGF, and are characterized by their elaborate neuritic network. They are frequently used to analyze basic mechanisms of the peripheral nervous system, especially sensory physiology and nociception.16–18 Because neuropathic pain often involves changes in both the sensory and autonomic nervous system, the secondary aim of the present study was to investigate whether gabapentinoids equally affected the two different neuronal cell types.
Methods
Ethics statement
The care and treatment of animals were ensured in accordance with the institutional guidelines for animal welfare of the University of Oldenburg, following the standards described by the German animal-protection law (Tierschutzgesetz). The killing of rats for tissue removal is registered with the local authorities (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit) and reported on a regular basis as demanded by law, but needs no further approval if no other treatment is applied before killing.
Chemicals and antibodies
Cell-culture media and B27 supplement were from Thermo Fisher Scientific (Waltham, MA, USA). Collagen type IV, polyethylenimine and cytosine arabinoside were purchased from Sigma-Aldrich (St Louis, MO, USA). Collagenase was obtained from Serva Electrophoresis (Heidelberg, Germany). Nerve growth factor (NGF) was purchased from Alomone Labs (Jerusalem, Israel). Gabapentin, pregabalin, and vincristine were obtained from Sigma-Aldrich.
Cell culture
For this study, PC12 cells and DRG neurons from newborn Wistar rats (Charles River Laboratories, Wilmington, MA, USA) were used. PC12 cells were obtained from ATCC. Cells were maintained in DMEM supplemented with 10% heat-inactivated FBS, 4 mM L-glutamine, 4,500 mg/L D-glucose, 15 mg/L phenol red, 50 U/mL penicillin G, and 50 µg/mL streptomycin at 37°C and 10% CO2 on a collagen type IV-coated surface. Differentiation of PC12 cells was induced by adding NGF at a concentration of 50 ng/mL. During differentiation, PC12 cells were kept in DMEM with 0.5% heat-inactivated FBS. The medium was changed every 2–3 days until cells differentiated from proliferating to nonproliferating cells that resembled sympathetic neurons.19 For morphological observations, cells were cultured in 6 cm culture dishes supplemented with glass coverslips for immunocytochemistry (2×105 cells/6 cm dish). DRG neurons were dissected from newborn Wistar rats.17
MTT cell-viability assay
In a first approach, MTT assays for the measurement of cell viability were performed. Briefly, an MTT assay assesses cell viability by determining metabolic activity. The original substrate, a yellow tetrazole, is reduced to a purple formazan. This reaction is only possible in vital cells with enough nicotinamide adenine dinucleotide phosphate available for oxidoreductase enzymes. First, the absorbance of the supernatant of control cells was detected at a wavelength of 590 nm. Then, the absorbance of the supernatant of treated cells was determined. As absorbance is related to the color (yellow versus purple, and thus indirect to cell metabolism), the ratio of the absorbance of control cells (set as 100% viability) to treated cells can be used to assess cell viability. In our cell model, PC12 cells were grown and differentiated in a 96-multiwell plate at a density of 104/well. After treatment, 10 µL MTT reagent (5 mg/mL solved in PBS) per well containing 100 µl medium was added and cells incubated for a further 2 hours at 37°C/10% CO2. Thereafter, 100 µL detergent reagent (10% sodium dodecyl sulfate in 0.01 M hydrochloric acid) was added and the solution left in the dark for at least 2 hours. The absorbance was detected with a microplate reader (SpectraCount; Packard Bioscience, Meriden, CT, USA) at 590 nm. Data are expressed as percentages of untreated control, and values represent means ± SD. Experiments were carried out 32 times to minimize statistical deviation (n=32).
Cell-injury models
In a next approach, cell injury was induced by exposing PC12 and DRG neurons to either H2O2 as indicated or vincristine (100 nM). H2O2 was added to exert oxidative stress, which is recognized as a major cause of the development of inflammatory and neuropathic pain by altering mitochondrial function.20 The chemotherapeutic agent vincristine was used to model chemotherapy-induced neuropathic pain. Instead of damaging cells by altering mitochondrial function, it likely injures cells by impairing their cytoskeleton, in particular the microtubules.21 Since it is currently unclear whether gabapentinoids protect or damage neuronal cells, the aim of the present study was to generate mild–moderate injury so that either protective or noxious drug effects could be detected. For this purpose, the required concentrations of H2O2 and vincristine in our cell model were determined. To achieve moderate cell injury in PC12 cells, 25 µM H2O2was needed. However, as DRG neurons needed a much higher concentration of H2O2 in our cell model to achieve moderate cell injury, 250 µM was applied. For vincristine in both cells a concentration of 100 nM was needed to achieve a significant cell injury. Cell morphology was monitored by phase-contrast microscopy using inverted microscopy (Olympus, Tokyo, Japan). Each experiment was carried out three times independently.
Incubation with gabapentinoids
To assess potential cytotoxic effects of gabapentin or pregabalin, cells were incubated with either 10 or 100 µM of each drug, respectively. According to a recent study, the lower concentration is equal to a dose of about a third of the daily maximum dose of either gabapentin or pregabalin.22 This is likely the situation in patients undergoing medication with those drugs in a normal clinical setting. The higher concentration reflects a “supratherapeutic” dose, which is unlikely to occur under normal clinical conditions. Nevertheless, we chose this supratherapeutic dose, as cytotoxic effects may only appear in a nontherapeutic range.
Immunocytochemistry
PC12 and DRG neurons were subjected to treatment as indicated. After treatment, cells were washed with PBS, fixed, and permeabilized with ice-cold methanol for 7 minutes. Afterward, cells were washed three times with PBS and incubated with primary antibodies at 4°C overnight (working dilutions given in brackets): mouse monoclonal antibody (mAb) anti α-tubulin (1:250) from Sigma-Aldrich, and rabbit mAb anti-NFL (1:250) from Dr Virginia M Lee. After being washed with PBS, cells were incubated for 1 hour with DyLight 594-conjugated (1:500) goat antimouse secondary antibodies (Thermo Fisher Scientific) and DyLight 488-conjugated (1:500) goat antirabbit secondary antibodies (Thermo Scientific, USA). Nuclei were stained with DAPI (1.5 μg/mL) included in the mounting medium (VectaShield; Burlingame, CA, USA). Fluorescence labeling was studied using epifluorescence microscopy (Carl Zeiss Meditec, Jena, Germany) equipped with a digital camera using a Plan-Neofluar objective. Again, each experiment was carried out three times.
Data analysis
Statistical analysis was done with SPSS 23 (IBM, Armonk, NY, USA) and Prism 5 for Mac (GraphPad Software, La Jolla, Ca, USA). Kruskal–Wallis analysis of variance followed by Dunn’s test for multiple comparisons was employed to compare the effects of different doses of test drugs where appropriate. P<0.05 was considered significant.
Results
Short-term exposure of gabapentinoids does not alter cell survival during H2O2- or vincristine-induced cytotoxicity
To explore the ability of short-term (24-hour) exposure of test drugs and chemicals to induce or augment injury in PC12 cells, MTT assays were performed. Results showed that gabapentin or pregabalin alone did not exert cytotoxic effects at a clinically relevant concentration of 10 µM. Similarly, pregabalin at a concentration of 100 µM did not lead to a significant decrease in cell viability, while a slight cytotoxic effect was observed after treatment with 100 µM gabapentin (5% reduction in cell viability, Figure 1A). Oxidative stress induced by hydrogen peroxide (25–100 µM) reduced cell viability in a dose-dependent manner. Maximum effect was achieved with 100 µM H2O2, leading to a reduction in cell viability of about 28%±12% (Figure 1B). As such, cells were only damaged mildly by H2O2, as intended for subsequent experiments.
To assess whether gabapentin or pregabalin augmented cytotoxic or exerted protective effects against damage caused by oxidative stress, cells were coincubated with 100 µM gabapentin (Figure 1C) or pregabalin (Figure 1D) and H2O2 (25–100 µM). This had no influence on the cytotoxic effects produced by H2O2 alone and was not protective either. Although cell viability in comparison to treatment with H2O2 alone decreased slightly after coincubation with gabapentin or pregabalin at H2O2 doses of 50 and 75 µM, these results were not statistically significant, as additional net-viability reduction was <10% on average.
Conversely, vincristine at a dose of 100 nM led to no reduction in cell viability in this model. Mean viability of cells treated with 100 nM remained at 100%±6%. Also, coincubation with either 100 µM gabapentin or pregabalin did not lead to any injury, keeping cell viability at 99%±8% and 99%±7%, respectively (Figure 1E). Therefore, based on the results of the MTT experiments, short-term exposure of PC12 cells to gabapentinoids is unlikely to produce relevant protective effects or cellular damage, even when cells are exposed at the same time to injury-inducing substances.
Cell morphology and cytoskeleton are not altered by adding gabapentinoids during cytotoxic stress exerted by H2O2 or vincristine
Since MTT assays measure the general viability of cells, they are used to assess the degree of major cellular insults.23 However, minor damage, and in particular, changes in cell morphology, cannot be detected. Therefore, indirect immunofluorescence studies to analyze general cell morphology and cytoskeletal structures were carried out through microscopic inspection of the cells. In addition to PC12 cells, DRG neurons were used, and cells were damaged by treatment with H2O2 or vincristine, which targets the cytoskeleton and was used to simulate chemotherapeutic stress.24
Figure 2 (A–D) demonstrates that short-term (24-hour) exposure of PC12 cells to H2O2 25 µM (Figure 2B) caused changes in neurofilament organization compared to control conditions (Figure 2A). Fluorescence staining using antibodies against NFL showed that staining was prominent in rather dense patches in the cytoplasm near the cell nucleus and not within cellular processes. However, morphological changes in cell membranes, neurites, and cell bodies were not observed. Coincubation with gabapentin (Figure 2C) or pregabalin (Figure 2D) did not improve or worsen this injury pattern.
While H2O2 caused only subtle changes, treatment with vincristine (100 nM) severely affected microtubule organization. In contrast to its negligible effect on cell viability, 100 nM vincristine considerably affected the cytoskeleton of PC12 cells microscopically, hence confirming its injury-inducing properties in the present study (Figure 2, E and F). Vincristine led to a rounding up of cells, depolymerization of α-tubulin, and retraction of neurites. PC12 cells were severely affected and lost a great proportion of their neuritic extensions. Additionally, neurofilaments were disorganized and NFL staining concentrated around the nucleus in a strand-like organization. Coincubation with gabapentin or pregabalin did not alter any of these effects (Figure 2, G and H, respectively).
Next, the effects of both compounds in sensory neuronal cultures were studied. For this purpose, DRG neurons were used. Treatment of DRG neurons with 250 µM H2O2 for 24 hours caused the formation of small protrusions along the outside of neurites (Figure 3B). These protrusions seemed to appear sporadically in control cells, but were observed only very rarely (Figure 3A). Immunocytochemistry further revealed that these protrusions were positively stained with a distinctive signal by antibodies against α-tubulin. Changes in the morphology of cell bodies were not observed. Coincubation with gabapentinoids had no influence on the appearance of the described protrusions along the neurites (Figure 3, C and D).
Treatment of DRG neurons with vincristine (100 nM, 24 hours) revealed that in contrast to PC12 cells, cell morphology was rather preserved; however, as indicated by immunofluorescence staining, α-tubulin was depolymerized and appeared disrupted, in contrast to control conditions (Figure 3, E and F). Similarly to PC12 cells, coincubation with gabapentinoids did not alleviate the α-tubulin-disrupting effects of vincristine or alter the observed injury pattern (Figure 3, G and H). Based on these results, it appears unlikely that short-term application of gabapentin or pregabalin is cytotoxic or has an influence on neuronal changes induced by oxidative or chemical stress.
Extended exposure to gabapentinoids did not affect cell morphology or cytoskeletal proteins
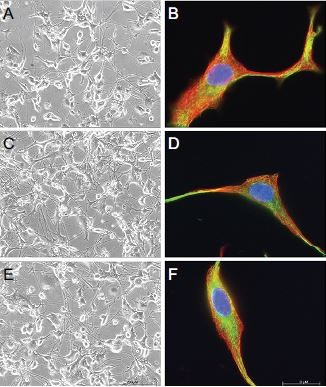
To assess the effects of extended 7-day incubation with either gabapentin or pregabalin on cell morphology and cytoskeletal integrity of PC12 and DRG neurons, additional experiments were conducted. However, neither gabapentin nor pregabalin at doses of 100 µM led to considerable changes in morphology, as analyzed by phase-contrast microscopy or indirect immunofluorescence. Neurites and cell bodies of both cell types incubated for 7 days with gabapentin or pregabalin were similar to those of untreated controls (Figure 4, A, C, and E and Figure 5, A, C, and E).
These findings were consistent with the immunocytochemistry results (Figure 4, B, D, and F and Figure 5, B, D, and F). Here, the pivotal cytoskeletal proteins α-tubulin and NFL were found unchanged in treated cells compared to controls, thus indicating maintained cellular integrity and morphology. Based on these observations, extended exposure to gabapentinoids is unlikely to induce changes in the morphology or cytoskeleton of PC12 and DRG neurons.
Discussion
Increasing evidence suggests brain morphology might change as a result of chronic pain.8,11,13 These changes are possibly clinically relevant, since recent data also indicate chronic pain patients show signs of cognitive impairment.25,26 Despite the underlying mechanisms of both pain-induced morphological and functional changes remaining unknown, it can be hypothesized they are a consequence of the effects of the drugs applied to treat pain. This notion, for instance, is supported by studies showing damaging effects of morphine on fibroblasts and cerebellar Purkinje cells.27,28 Whether gabapentinoids, which are widely used to treat acute and chronic pain, display similar noxious properties on neuronal cells remains unclear. To address this question, 10 µM and 100 µM gabapentin and pregabalin were used in this study. The lower dose was chosen because it is equivalent to the plasma concentration found in patients who received a third of the maximum daily dose of gabapentin (3,600 mg) and pregabalin (600 mg). Conversely, the higher dose represented the plasma concentration of patients who had received triple the maximum daily dose.22 However, results presented here do not support the idea of gabapentinoid-induced neurotoxicity or neuroprotection as neither drug enhanced or improved damage in cultured autonomic (PC12) or sensory (DRG) neuronal cells.
Gabapentinoids have no effect on cell viability
One key finding of this study was that gabapentinoids showed no cytotoxic effect when added to healthy neuronal cells. This was consistent with the limited evidence so far provided by other studies investigating the potential damaging effect of pregabalin in cellular models.29 However, in extension of previous research, here the effects of gabapentinoids on preinjured cells were also examined. Oxidative stress in the nervous system is thought to be a key mechanism in the development of inflammatory and neuropathic pain.20 Experimentally, exposure of cells to hydrogen peroxide is used to activate apoptotic pathways, leading to a reduction in cell viability and ultimately cell death.30 In vitro application of H2O2 has thus become a frequently employed model used (for instance) to assess the potentially cytoprotective properties of drugs and chemicals. The present findings of H2O2 dose dependently reducing viability of PC12 cells were hence in accordance with previous research.31 In addition to the role of H2O2, neuropathic pain is also a common consequence of chemotherapy, especially when vinca alkaloids like vincristine are applied to treat cancer.32 Other than hydrogen peroxide, which produces cell damage at mainly the mitochondrial level, vincristine mostly interacts with the cytoskeleton, predominantly in axons, to cause cellular degeneration.21
Data presented here show that gabapentinoids exerted no effects on cell viability in an in vitro model of mild–moderate cytotoxic stress. This degree of damage was deemed sufficient, because clinically gabapentinoids are given when the nervous system has already been injured. The model hence allowed investigation of whether the drugs enhanced or diminished preexisting damage. However, as coadministration of gabapentinoids with either vincristine or hydrogen peroxide did not reduce or improve cell viability here, short-term exposure to these drugs is thus unlikely to cause or prevent major neuronal injury or death. Although this is different to what has been found in rats with spinal cord injury, where pregabalin was found to be neuroprotective, it is further supported here by the findings that both drugs had only negligible effects on the viability of PC12 cells.33
Further, in this study, 24-hour incubation of PC12 cells with 100 nM vincristine did not produce any change in cell viability. This is likely because of the short incubation chosen at a clinically relevant drug concentration. Results are hence in line with a previous study where 100 nM vincristine could induce major damage in DRG neurons only after exposure for 48 hours.34 Nevertheless, 24-hour incubation with 100 nM vincristine was sufficient here to induce cytoskeletal changes microscopically that were in keeping with mild–moderate cellular damage.
The neuronal cytoskeleton is not altered by gabapentinoids
Exposure (24 hours) of PC12 and DRG neurons to hydrogen peroxide or vincristine induced typical changes in the cytoskeleton. However, coincubation of these toxins with gabapentinoids neither enhanced nor reduced those alterations. Together with the observation that pregabalin and gabapentin alone did not alter the cytoskeleton of either cell line, even after 7-day incubation, these results suggest gabapentinoids are devoid of short-term neurotoxic properties.
Although no other study has yet investigated the influence of gabapentinoids on the cytoskeleton of neuronal cells, these findings are at odds with results obtained in glial cells, where pregabalin induced remodeling of the cytoskeleton.35 The absence of gabapentinoid-induced remodeling in neuronal cells might have at least two implications. First, alterations in the gray matter of patients suffering from neuropathic pain are unlikely to be caused or aggravated by gabapentinoids, thus underlining the safety of those drugs. Secondly, gray matter changes might be the result of mechanisms crucially involving glial cells in neuropathic pain pathways, such as the generation of a “proinflammatory milieu”.36 Nevertheless, the effect of gabapentinoids on the interaction between glial and neuronal cells in neuropathic pain is barely understood, and should be addressed in future research.
Study limitations
One limitation of the present study was that it did not investigate the survival or integrity of the cytoskeleton of neurons after prolonged exposure to gabapentinoids. However, as both drugs showed neither damaging effects on untreated cells nor injury-enhancing activity in preinjured cells, they are likely devoid of any major neurotoxic properties. This finding is of clinical relevance, as gabapentinoids are increasingly used as short-term treatment in acute pain as well.37
Furthermore, toxic effects of gabapentinoids on neurons might only become apparent in more complex models allowing assessment of interactions of different cell types and mediator systems. This notion is supported by studies identifying a variety of different targets for gabapentinoids. For instance, in addition to the well-described interaction with voltage-gated calcium channels,38 gabapentinoids likely also inhibit N-methyl-D-aspartate receptors39,40 and suppress protein kinase C41 and transient receptor-potential A ion channels.42 They might also reduce γ-aminobutyric acidergic activity in the locus coeruleus and induce glutamate release in astrocytes.43,44 Therefore, future research should address this complexity by employing neuronal–glial cocultures, brain slices, or whole animals instead of single-cell models.
Conclusion
The goal of this study was to examine the potentially cytotoxic effects of gabapentinoids on the cytoskeleton and cellular integrity of autonomic and sensory neuronal cultures. Results suggest that both pregabalin and gabapentin are unlikely to induce, enhance, or improve gross neurotoxicity when employed for a short period in clinically relevant doses. Furthermore, both drugs are unlikely to induce changes in the morphology and cytoskeleton of autonomic and sensory neurons either. This study hence supports the idea that the gray matter alterations observed in brains of patients with neuropathic pain are related to the disease process itself, rather than a consequence of the medicines given. However, future research should confirm these findings for prolonged drug applications in more complex cellular models.
Acknowledgments
We would like to thank Irina Fomins for expert technical help. This work was supported financially by the Department of Anesthesiology, Klinikum Oldenburg, Oldenburg, Lower Saxony, Germany.
Disclosure
CB has received consultancy fees from Mundipharma. The authors report no other conflicts of interest in this work.
References
Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin: results from a general population survey. J Pain. 2006;7(4):281–289. | ||
Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. | ||
Baron R. Pharmakologisch nicht interventionelle Therapie chronisch neuropathischer Schmerzen. 2012. Available from: https://www.dgn.org/images/red_leitlinien/LL_2014/PDFs_Download/030-114l_S1_Neuropathischer_Schmerzen_Therapie_2014-verlaengert.pdf. Accessed May 4, 2018. | ||
Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev. 2005;(3):CD005452. | ||
Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31(7):1448–1454. | ||
Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(3):CD007076. | ||
Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–581. | ||
Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. | ||
Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. | ||
Ruscheweyh R, Deppe M, Lohmann H, et al. Pain is associated with regional grey matter reduction in the general population. Pain. 2010;152(4):904–911. | ||
Fritz HC, McAuley JH, Wittfeld K, et al. Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a population-based cohort study. J Pain. 2016;17(1):111–118. | ||
Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007; 27(15):4004–4007. | ||
Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60(4):570–581. | ||
Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia. 2002;43(5):482–490. | ||
Grau CM, Greene LA. Use of PC12 cells and rat superior cervical ganglion sympathetic neurons as models for neuroprotective assays relevant to Parkinson’s disease. Methods Mol Biol. 2012;846:201–211. | ||
Ma W, Zhang Y, Bantel C, Eisenach JC. Medium and large injured dorsal root ganglion cells increase TRPV-1, accompanied by increased α2C-adrenoceptor co-expression and functional inhibition by clonidine. Pain. 2005;113(3):386–394. | ||
Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2(1):152–160. | ||
Calcott G, White JP, Nagy I. Xenon fails to inhibit capsaicin-evoked CGRP release by nociceptors in culture. Neurosci Lett. 2011;499(2):124–126. | ||
Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73(7):2424–2428. | ||
Sui BD, Xu TQ, Liu JW, et al. Understanding the role of mitochondria in the pathogenesis of chronic pain. Postgrad Med J. 2013;89(1058):709–714. | ||
Tanner KD, Levine JD, Topp KS. Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J Comp Neurol. 1998;395(4):481–492. | ||
Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49(10):661–669. | ||
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. | ||
Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents. 2002;2(1):1–17. | ||
Wolrich J, Poots AJ, Kuehler BM, Rice AS, Rahman A, Bantel C. Is number sense impaired in chronic pain patients? Br J Anaesth. 2014;113(6):1024–1031. | ||
Rathbone M, Parkinson W, Rehman Y, Jiang S, Bhandari M, Kumbhare D. Magnitude and variability of effect sizes for the associations between chronic pain and cognitive test performances: a meta-analysis. Br J Pain. 2016;10(3):141–155. | ||
Hauser KF, Gurwell JA, Turbek CS. Morphine inhibits Purkinje cell survival and dendritic differentiation in organotypic cultures of the mouse cerebellum. Exp Neurol. 1994;30(1):95–105. | ||
Aguirre J, Borgeat A, Hasler M, Bühler P, Bonvini JM. Clinical concentrations of morphine are cytotoxic on proliferating humanfibroblasts in vitro. Eur J Anaesthesiol. 2016;33(11):832–839. | ||
Salat K, Librowski T, Nawiesniak B, Gluch-Lutwin M. Evaluation of analgesic, antioxidant, cytotoxic and metabolic effects of pregabalin for the use in neuropathic pain. Neurol Res. 2013;35(9):948–958. | ||
Kim SM, Hwang IK, Yoo DY, et al. Tat-antioxidant 1 protects against stress-induced hippocampal HT-22 cells death and attenuate ischaemic insult in animal model. J Cell Mol Med. 2015;19(6):1333–1345. | ||
Park JB. Isolation and quantification of major chlorogenic acids in three major instant coffee brands and their potential effects on H2O2-induced mitochondrial membrane depolarization and apoptosis in PC-12 cells. Food Funct. 2013;4(11):1632–1638. | ||
Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014;10(12):694–707. | ||
Ha KY, Kim YH, Rhyu KW, Kwon SE. Pregabalin as a neuroprotector after spinal cord injury in rats. Eur Spine J. 2008;17(6):864–872. | ||
Silva A, Wang Q, Wang M, Ravula SK, Glass JD. Evidence for direct axonal toxicity in vincristine neuropathy. J Peripher Nerv Syst. 2006;11(3):211–216. | ||
Park S, Lee J. Proteomic analysis to identify early molecular targets of pregabalin in C6 glial cells. Cell Biol Int. 2010;34(1):27–33. | ||
Suter MR. Microglial role in the development of chronic pain. Curr Opin Anaesthesiol. 2016;29(5):584–589. | ||
Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. 2013;119(5):1215–1221. | ||
Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6(1):108–113. | ||
Hara K, Sata T. Inhibitory effect of gabapentin on N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Acta Anaesthesiol Scand. 2008;51(1):122–128. | ||
Kim YS, Chang HK, Lee JW, et al. Protective effect of gabapentin on N-methyl-D-aspartate-induced excitotoxicity in rat hippocampal CA1 neurons. J Pharmacol Sci. 2009;109(1):144–147. | ||
Yeh CY, Chung SC, Tseng FL, Tsai YC, Liu YC. Biphasic effects of chronic intrathecal gabapentin administration on the expression of protein kinase C gamma in the spinal cord of neuropathic pain rats. Acta Anaesthesiol Taiwan. 2011;49(4):144–148. | ||
Bang S, Yoo S, Hwang SW. Gabapentin attenuates the activation of transient receptor potential A1 by cinnamaldehyde. Exp Neurobiol. 2009;18(1):1–7. | ||
Yoshizumi M, Parker RA, Eisenach JC, Hayashida K. Gabapentin inhibits γ-amino butyric acid release in the locus coeruleus but not in the spinal dorsal horn after peripheral nerve injury in rats. Anesthesiology. 2012;116(6):1347–1353. | ||
Yoshizumi M, Eisenach JC, Hayashida K. Riluzole and gabapentinoids activate glutamate transporters to facilitate glutamate-induced glutamate release from cultured astrocytes. Eur J Pharmacol. 2012;677(1–3):87–92. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






