Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Short-Term Efficacy of Autologous Cellular Micrografts in Male and Female Androgenetic Alopecia: A Retrospective Cohort Study
Authors Zari S
Received 19 August 2021
Accepted for publication 28 October 2021
Published 19 November 2021 Volume 2021:14 Pages 1725—1736
DOI https://doi.org/10.2147/CCID.S334807
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Shadi Zari
Department of Dermatology, Faculty of Medicine, University of Jeddah, Jeddah, Saudi Arabia
Correspondence: Shadi Zari Email [email protected]
Purpose: Autologous cellular micrografts (ACM) is a novel treatment method in hair loss, and few data are available regarding its efficacy. The present study was carried out to assess the short-term clinical efficacy of a single application of ACM in the treatment of male and female androgenetic alopecia (AGA).
Materials and Methods: This was a single-center retrospective study involving 140 consecutive adults with confirmed AGA, who received a single session of ACM (Regenera Activa®). Efficacy was evaluated 1– 6 months after treatment, by analyzing the change of trichometry parameters, which were assessed using TrichoScan digital image analysis.
Results: Depending on the scalp region, there was increase in mean hair density by 4.5– 7.12 hair/cm2, average hair thickness by 0.96– 1.88 μm, % thick hair by 1.74– 3.26%, and mean number of follicular units by 1.30– 2.77, resulting in an increase of cumulative hair thickness by 0.48– 0.56 unit. Additionally, the frontal region showed a significant decrease in % thin hair (− 1.81%, p = 0.037) and yellow dots (− 1.93 N/cm2, p = 0.003). A favorable response was observed in 66.4% of the participants in the frontal region. Further, a gender-specific effect of treatment was observed.
Conclusion: ACM is a promising treatment in AGA with a short-term favorable response observed in up to approximately two-thirds of patients.
Keywords: androgenetic alopecia, pattern hair loss, autologous cellular micrografts, efficacy, regrowth
Introduction
Androgenetic alopecia (AGA) or pattern hair loss (PHL) is the most common cause of hair loss. It affects approximately 50% of men and women by the age of 50, and its prevalence increases with advancing age.1,2 As the name implies, AGA has a clear genetic predisposition mediating an excessive response to androgens.3
The treatments for AGA are limited. Topical minoxidil is the only FDA-approved treatment in females, and topical minoxidil and oral finasteride 1 mg are the only FDA-approved treatments in males. Minoxidil efficacy is limited, and studies suggest that only one-third of the patients experience a cosmetic benefit or moderate terminal hair regrowth after 1 year of use.4–7 Scalp irritation, increased seborrhea, erythema and hypertrichosis are commonly reported after topical minoxidil use.8–10 Oral finasteride 1 mg is FDA-approved for male but not female AGA, due to its teratogenicity. Decreased libido, erectile dysfunction and ejaculatory problems have been reported in around 3–5% of men using finasteride.11–13 In addition, both minoxidil and finasteride require a long-term commitment by the patient on a daily basis, most likely for an individual’s life.
Furthermore, in the advanced stages of PHL, the above treatments are less effective and hair transplantation would be the only option for some of these patients. Hair transplantation also comes with limitations. Many balding males have a limited donor supply, which is not sufficient to cover the bald area. Also, many females with PHL have a weak donor area and are not candidates for hair transplantation.14–16
The previous observations highlight the importance of finding effective new treatments, which ensure better patient compliance and have limited side effects. Since AGA is characterized by defects in and loss of hair progenitor cells, while hair follicle stem cells (HFSCs) remain viable,17 transplantation of multipotent stem cells has become a well-accepted treatment option. Autologous cellular micrografts (ACM) is a method that obtains autologous mature stem cells from scalp biopsies of a patient using a preparation system for mechanical disintegration and filtering of solid tissues.
The advocated mechanisms of action of ACM in AGA include the enhancement of hair follicle regeneration by transplantation of mature multipotent stem cells, besides the reactivation of existing stem cells and progenitor cells of miniaturized follicles, by restoring hair growth signaling via the injection of growth factors.17,18 Yet, these mechanisms have not been established. On the other hand, given the novelty of the treatment method, further data is needed to establish the clinical efficacy in AGA. Yet, the few published reports provide promising results, showing an increase in hair density and thickness in a high percentage of patients.22,37,38 However, these studies comprise limitations that impede the generalizability of the findings, notably due to limited sample size.
In this paper, we report the short-term clinical efficacy of a single application of ACM in the treatment of male and female AGA. We also proposed a pixel-based method to portray the treatment efficacy and cosmetic benefit in hair loss, in a standardized visual fashion.
Materials and Methods
Design and Setting
A retrospective cohort study was carried out at the author’s dermatology clinic in Jeddah, Saudi Arabia, from September 2019 to August 2020.
Population
The study involved 18–65-year-old consecutive males and females with clinically diagnosed and dermoscopy-confirmed AGA, who received a single session of ACM (Regenera Activa®). Only females classified Sinclair 2–4 and males classified Hamilton-Norwood 2–4 were included. Patients using any other topical or systemic medication for hair loss during the 6 months prior to inclusion or during the study period were excluded, as well as those using medical devices such as low-level laser therapy (LLT) or procedures such as platelet-rich plasma (PRP) or micro-needling for hair loss during the same periods. Other exclusion criteria included: cancer within 2 years; pregnancy or breastfeeding; immunosuppression; hemoglobin below 12 mg/dL; presence of other causes of alopecia such as alopecia areata, scarring alopecia or inflammatory scalp disorders; history of hair transplantation; history of scalp tumors; and/or the presence of trichotillomania.
Procedure
Baseline Assessment
As part of our routine practice, patients undergo a structured clinical evaluation to collect demographic and relevant clinical data, followed by dermoscopy to confirm the diagnosis, and global scalp photography following a standardized method and technical settings. Standardized phototrichograms are conducted on all scalps using video-epiluminescence microscopy (FotoFinder Systems, Inc., Columbia, CA, USA) in conjunction with TrichoScan digital image analysis (TrichoScan, Tricolog GmbH, Freiburg, Germany). Trichoscan is a computer‐assisted dermoscopy with dedicated software to diagnose the hair loss and to measure its severity.
Autologous Cellular Micrografts (ACM) Procedure
Under local anesthesia, using anesthesia techniques which were previously described by the author,22 a 2.5 mm punch biopsy was used to extract 3 scalp tissue specimens from the patient’s occiput behind the ear, using Rigeneracons medical device (CE certified class I; Human Brain Wave, Turin, Italy). The collected specimens are placed in Rigeneracons by adding 1.5 mL of sterile physiologic solution to the device. The device then generates a cellular suspension by rotation of Rigeneracons at 80 RPM for 2 minutes. Subsequently, the obtained suspension is diluted with an additional 3 mL sterile physiologic solution. The solution was then injected subdermally into the balding areas of the scalp using a 1mL syringe with a 30-gauge needle; 0.1 mL was injected per point spaced approximately 1 cm apart.
Follow Up and Outcomes
After the intervention, patients were followed up by clinic visits and phone calls for any adverse effects or further concern. The short-term post-treatment assessment including trichometry with TrichoScan digital image analysis was scheduled 1 to 6 months after the intervention, as per the patient’s convenience. Trichometry parameters were divided into positive and negative parameters. Positive parameters correspond to parameters whose increase corresponds to favorable evolution (hair growth) and include hair density (N/cm2), average hair shaft thickness (AHST, μm), percent thick hair (%), cumulative hair thickness (CHT), and number of follicular units (%). Negative parameters correspond to parameters whose decrease corresponds to favorable evolution (hair growth) and include percent thin hair (%), yellow dots (N/cm2), and trichoscopy-derived Sinclair scale (TDSS), which describes the hair midline density calculated from CHT density measured in trichoscopy.23
Ethical Considerations
The study was approved by the institutional review board of King Abdulaziz University Hospital in Jeddah (Reference No 235–21). Waiver of consent has been obtained because of the retrospective nature of the study. The confidentiality of the patients’ data has been protected and identifiable data of patients have been removed in compliance with the Declaration of Helsinki. The baseline assessments, ACM procedure and follow-up methods described above are part of the routine practice in the dermatology clinic, and were not specifically designed for the present study.
Statistical Methods
Data was managed using Microsoft Excel (version 2017, Microsoft) and statistical analysis was performed with the SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL, USA). For all trichometry parameters, absolute and adjusted relative changes were calculated for each participant as follows:
Where,
 : pre- to post-intervention change of the parameter value in the given patient,
: pre- to post-intervention change of the parameter value in the given patient,
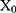 : baseline population mean of the parameter.
: baseline population mean of the parameter.
Paired t-test was used to analyze the absolute changes in the total study population and in males and females separately. The correlation between pre- and post-intervention values was analyzed using Pearson’s correlation.
To visually portray the cosmetic effect of the treatment in the study population, we calculated the area coverage index (ACI) as an estimate of the scalp area, in cm2, that would be completely covered by 1 cm-long hair, with respect of the hair density and thickness. By assuming a one-μm hair thickness, a 1-cm-long hair would visually cover an area of 1cm × 10−4 cm = 10−4 cm2. Thus, by considering the hair density (average number of hairs by cm2) and thickness (AHST), the ACI was calculated using the following formula:ACI (cm2hair/cm2scalp) = HD (hair density,N/cm2)*AHST(μm)* 10-4
Afterwards, a graphical representation was made by generating a 30 × 30 (900-pixel resolution) grid, corresponding to 9cm2 of the scalp (covered by 9*ACI), comprising filled and blank 1mm2 square pixels that correspond to the covered and uncovered scalp areas, respectively. The number of filled pixels is proportional to ACI and is calculated as N = resolution*ACI. Filled pixels are dispersed over the grid using a random generator. The ACI definition, calculation formula and pixel transformation method are illustrated in Figure 1.
For each parameter and scalp region, the percentage of participants who had favorable outcome (defined as the desired change occurring within the given parameter) was calculated along with the respective mean absolute change and mean adjusted relative change.
A p value of <0.05 was considered to reject the null hypothesis.
Results
Baseline Demographic and Clinical Characteristics
A total 140 patients were included, 113 (80.7%) of them were female, and mean (SD) age was 32.1 (10.1) years. The distribution of males according to Hamilton–Norwood classification showed Class II (48.1%), Class III (18.5%) and Class III Vertex (33.3%). The distribution of females according to Sinclair classification showed Grade 2 (32.7%), Grade 3 (56.6%), and Grade 4 or 5 (10.7%). The median follow-up time was 94 days (range = 39–197 days).
Efficacy of Autologous Cellular Micrografts in the Total Population
Absolute Change
There was increase in all positive parameters including hair density (+4.5 to 7.12 hair/cm2), AHST (+0.96 to 1.88 μm), % thick hair (+1.74 to 3.26%), CHT (+0.48 to 0.56 unit), and number of follicular units (+1.30 to 2.77), depending on the scalp region, and the improvements were more remarkable in frontal region where all these changes were statistically significant (p < 0.05). Additionally, negative parameters decreased, notably in frontal region in % thin hair (−1.81%, p = 0.037) and yellow dots (−1.93 N/cm2, p = 0.003). In the majority of parameters, pre- and post-intervention values were moderately to strongly correlated (R = 0.539–0.807) (Table 1).
 |
Table 1 Pre- and Post-Intervention Trichometry Findings by Scalp Region (Intrasubject Analysis) |
Adjusted Relative Change
Depending on scalp region, there was relative increase in hair density (up to 4.1%), AHST (up to +3.6%), % thick hair (up to +6.0%), CHT (up to +6.7%), and number of follicular units (up to +3.8%), with relative decrease in % thin hair (down to −7.6%), as adjusted to the population’s baseline. Besides, a substantial relative decrease in yellow dot density was observed, notably in frontal (−36.0%) and temporal (−42.9%) regions. This was associated with decline in TDSS (Figure 2).
ACI and the Cosmetic Effect
The mean hair-covered scalp area in the total population increased in all three scalp regions including frontal (from 0.85 to 0.91 cm2 hair/cm2 scalp), temporal (0.70 to 0.75 cm2 hair/cm2 scalp) and occipital (0.96 to 1.02 cm2 hair/cm2 scalp), and all results were statistically significant (p < 0.001). Results were depicted in a randomly generated 900-pixel grid representing 9cm2 of the scalp (Figure 3).
Effect Size in Favorable Responders
Two-thirds of patients (63.6–66.4%) had a favorable change in CHT, with a mean absolute change 1.2–1.4 unit depending on the scalp region. Further, more than 50% had favorable change in the other parameters, except yellow dots that decreased in up to 43.6% of the participants depending on the scalp region (Table 2).
 |
Table 2 Change in Hair Growth Indicators Among Favorable Responders |
Efficacy by Gender
In Females
The mean CHT increased by 0.52 to 0.57 units, associated with a significant increase in both AHST (+1.41 to 2.17μm) and % thick hair (+2.64% to 4.16%) in all 3 scalp regions. Increase in hair density was not statistically significant in the frontal region. Further, all negative parameters decreased, but statistical significance was not consistent throughout scalp regions (Table 3). The pre and post treatment scalp photographs of a sample female patient are depicted in Figure 4-Patient 1.
 |
Table 3 Hair Growth Indicators by Gender and Scalp Region in Male and Female Androgenic Alopecia After Treatment with Autologous Cellular Micrografts (ACM) |
In Males
The frontal region showed a statistically significant increase in mean CHT (+0.66 units, p = 0.045) associated with a remarkable increase in mean hair density (+11.2 hair/cm2, p = 0.031) in reference to baseline (Table 3). The pre and post ACM scalp photographs of a sample male patient are depicted in Figure 4-Patient 2.
Discussion
Summary of Findings
The present study provides the largest series of AGA patients treated with ACM to date. Pre- to post-intervention assessment showed favorable changes in all positive and negative trichometry parameters, including growth of an average 5–7 new hairs per cm2 of scalp and an increase in the average hair thickness by 1.6–1.9μm, which is reflected by an increase in the percentage of thick hairs and proportional decrease in the percentage of thin hairs. Additionally, there was considerable reduction of yellow dots by 1–2 per cm2 of scalp combined with increase in the percentage of follicular units by up to 3%. All these changes resulted in the enhancement of the CHT by an average 0.5–0.6 units, representing 5–6 mm2 increment in hair coverage index per cm2 of scalp, which induced a significant cosmetic effect as demonstrated by the visual model developed by the author in this study. Such favorable outcomes were observed in up to two-thirds of patients, depending on the parameter, and the magnitude of changes among positive responders was more remarkable, notably in the frontal region. These findings are very encouraging and indicate multiple effects of ACM in improving the hair of AGA patients in the short term.
Pathophysiological Basis of ACM Mechanisms of Action
Mature HFSCs, located in the hair follicle bulge, are multipotent cells that play a key role in the regeneration of hair follicles and other scalp skin structures. They have the ability to self-regenerate between the telogen and anagen phases of the hair cycle; or migrate down the hair matrix and become progenitor cells, forming the internal hair follicles and hair stem. The activation of bulge-located stem cells is controlled by the surrounding microenvironment “niche” including their daughter cells, as well as by dermal papilla cells (DPCs) signaling pathways, notably the Wnt/β-catenin pathway which is crucial for entry into the anagen phase. DPCs further contribute in regulating hair growth by secreting hair growth-stimulating factors such as insulin-like growth factor 1 (IGF-1), basic fibroblast growth factor, and vascular endothelial growth factor, which respectively have an autocrine effect on the dermal papilla itself and paracrine effect on hair follicle epithelial cells.19,24–30
The most supported pathophysiological mechanism of AGA involves a suppressive effect of systemic or locally produced (by balding DPCs) dihydrotesterone on follicular keratinocyte growth, which leads to catagen. This effect is mediated by the upregulation of transforming growth factor beta (TGF-β) and Wnt antagonist DKK-1 production by DPCs. These alterations result in a number of pathohistological anomalies including impaired HFSC differentiation, oxidative stress and perifollicular fibrosis and inflammation, which altogether lead to the chronic hair miniaturization resulting in smaller dermal papillae and a reduction in CD200+ progenitor cells.31–37 On the other hand, although progenitor cells are damaged in AGA, the HFSCs remain preserved, which explains the reversibility of the condition. These observations constitute the foundation for ACM, which consist of the transplantation of mature multipotent stem cells in the balding areas, thereby enabling hair follicle regeneration.17 Besides unaffected areas of the scalp, ACM may use multipotent stem cells originating from adipose tissue.38 Another plausible mechanism of action of ACM is the reactivation of already existing stem cells and progenitor cells of miniaturized follicles, by reinstating the hair growth signaling via the injection of growth factors.18
Reports on Efficacy of ACM in AGA
The few available reports on ACM efficacy are encouraging. In a small placebo-controlled trial (N = 11), the use of ACM showed ~29% relative increase in hair density in treated scalp areas, approximately 6 months after treatment.19 Another study including 17 patients showed patient-perceived increase in hair thickness (12/17 patients) and reduction in hair fall (8/17), while the remaining cases observed no change for either parameter.20 More recently, Ruiz et al studied the efficacy of ACM at 4, 6 and 12 months, among 100 AGA and showed significant increase in hair density (+33.3/cm2) and percentage of thick hair (+5.6%) at 2 months.21
Efficacy of ACM by Gender
Findings from the present study suggest a gender-specific effect of ACM in AGA. The most remarkable effect in males was the increase in hair density, while in females it was the promotion of hair thickness and reduction of the number of yellow dots. Interestingly, the two patterns resulted in a comparable improvement of CHT across genders. Additionally, although the occipital area was not treated in any of the study patients, women experienced significant improvement in all hair growth indicators in the occipital region with a single ACM session, which could have an implication in improving the donor area in females requiring a hair transplantation.
Pixel-Based Representation of Cosmetic Effect
The author proposes the use of a pixel-based graphical method to portray the cosmetic effects of treatments in hair loss in a standardized fashion. This method provides an intuitive visualization of the change in scalp area coverage after treatment. It can be used in all types of hair loss and treatments (transplantation, topical treatments, etc.) and can have interesting clinical and research applications.
Limitations
The external validity of the present study may be limited by the retrospective and noncontrolled design. Further, the present design does not demonstrate the final clinical improvement due to the short-term endpoint of the study outcomes. A longer follow-up study will be needed in the future.
Conclusion
Two-thirds of patients with AGA would respond favorably to a single treatment session with ACM in the first 6 months following treatment. The pre- to post-ACM trichometry analysis showed significant improvement in hair regrowth, hair thickening, promotion of follicular units and reduction of yellow dots; all combined, these effects result in an increase in the hair area coverage index, representing a noticeable cosmetic change. There is a probable gender-specific effect of ACM in AGA that should be further studied.
Abbreviations
ACI, (Scalp) area coverage index; ACM, autologous cellular micrografts; AGA, androgenetic alopecia; AHST, average hair shaft thickness; ANOVA, analysis of variance; CHT, cumulative hair thickness; DPC, dermal papilla cells; FDA, Food and Drug Administration; HFSC, hair follicle stem cell; LLT, low-level laser therapy; PHL, pattern hair loss; TGF-β, transforming growth factor beta.
Data Sharing Statement
The database that supports the findings of the present study is available upon written request from the author.
Acknowledgment
The author thanks Dr. Mohamed Amine HAIRECHE for his valuable assistance in analyzing the data of the present study.
Funding
The author received no funding for the present study.
Disclosure
The author reports no conflicts of interest for this work and has no financial interests to disclose.
References
1. Bolduc C, Shapiro J. Management of androgenetic alopecia. Am J Clin Dermatol. 2000;1(3):151–158. doi:10.2165/00128071-200001030-00002
2. Price VH. Treatment of hair loss. N Engl J Med. 1999;341(13):964–973.
3. Bienenfeld A, Azarchi S, Lo Sicco K, Marchbein S, Shapiro J, Nagler AR. Androgens in women: androgen-mediated skin disease and patient evaluation. J Am Acad Dermatol. 2019;80(6):1497–1506. doi:10.1016/j.jaad.2018.08.062
4. Villez RL. Topical minoxidil therapy in hereditary androgenetic alopecia. Arch Dermatol. 1985;121(2):197–202. doi:10.1001/archderm.1985.01660020055017
5. Olsen EA, DeLong ER, Weiner MS. Dose-response study of topical minoxidil in male pattern baldness. J Am Acad Dermatol. 1986;15(1):30–37. doi:10.1016/S0190-9622(86)70138-2
6. Katz HI. Topical minoxidil: review of efficacy. Clin Dermatol. 1988;6(4):195–199. doi:10.1016/0738-081X(88)90087-9
7. Olsen EA, Whiting D, Bergfeld W, et al. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2007;57(5):767–774. doi:10.1016/j.jaad.2007.04.012
8. Tsuboi R, Tanaka T, Nishikawa T, et al. A randomized, placebo-controlled trial of 1% topical minoxidil solution in the treatment of androgenetic alopecia in Japanese women. Eur J Dermatol. 2007;17(1):37–44.
9. Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011;65(6):
10. Friedman ES, Friedman PM, Cohen DE, Washenik K. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46(2):309–312. doi:10.1067/mjd.2002.119104
11. Wilton L, Pearce G, Edet E, Freemantle S, Stephens MDB, Mann RD. The safety of finasteride used in benign prostatic hypertrophy: a non-interventional observational cohort study in 14,772 patients. Br J Urol. 1996;78(3):379–384. doi:10.1046/j.1464-410X.1996.00091.x
12. Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011;8(6):1747–1753. doi:10.1111/j.1743-6109.2011.02255.x
13. Ganzer CA, Jacobs AR, Iqbal F. Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. Am J Men’s Health. 2015;9(3):222–228. doi:10.1177/1557988314538445
14. Ekmekci TR, Sakiz D, Koslu A. Occipital involvement in female pattern hair loss: histopathological evidences. J Eur Acad Dermatol Venereol. 2010;24(3):299–301. doi:10.1111/j.1468-3083.2009.03411.x
15. Birch MP, Lalla SC, Messenger AG. Female pattern hair loss. Clin Exp Dermatol. 2002;27(5):383–388. doi:10.1046/j.1365-2230.2002.01085.x
16. Olsen EA. Androgenetic alopecia. In: Olsen EA, editor. Disorders of Hair Growth. McGraw-Hill; 1994:257–284.
17. Owczarczyk-Saczonek A, Krajewska-Włodarczyk M, Kruszewska A, et al. Therapeutic potential of stem cells in follicle regeneration. Stem Cells Int. 2018;2018:1049641. doi:10.1155/2018/1049641
18. Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J Dermatolog Treat. 2018;29(5):431–440. doi:10.1080/09546634.2016.1227419
19. Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58. doi:10.21037/sci.2017.06.04
20. Álvarez X, Valenzuela M, Tuffet J. Clinical and histological evaluation of the Regenera®method for the treatment of androgenetic alopecia. Int Educ Appl Sci Res J. 2018;3(1):2456–5040.
21. Ruiz RG, Rosell JMC, Ceccarelli G, et al. Progenitor-cell-enriched micrografts as a novel option for the management of androgenetic alopecia. J Cell Physiol. 2020;235(5):4587–4593. doi:10.1002/jcp.29335
22. Kohn T, Zari S. Local anesthesia techniques in hair restoration surgery. J Cutan Med Surg. 2016;20(6):610–612. doi:10.1177/1203475416651052
23. Kasprzak M, Sicińska J, Sinclair R. The trichoscopy derived Sinclair scale: enhancing visual assessment through quantitative trichoscopy. Australas J Dermatol. 2019;60(2):134–136. doi:10.1111/ajd.12964
24. Purba TS, Haslam IS, Poblet E, et al. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioessays. 2014;36(5):513–525. doi:10.1002/bies.201300166
25. Turksen K. Tissue-Specific Stem Cell Niche. Springer; 2015.
26. Zhang P, Kling RE, Ravuri SK, et al. A review of adipocyte lineage cells and dermal papilla cells in hair follicle regeneration. J Tissue Eng. 2014;5:2041731414556850. doi:10.1177/2041731414556850
27. Greco V, Chen T, Rendl M, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4(2):155–169. doi:10.1016/j.stem.2008.12.009
28. Rabbani P, Takeo M, Chou W, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145(6):941–955. doi:10.1016/j.cell.2011.05.004
29. Tanimura S, Tadokoro Y, Inomata K, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8(2):177–187. doi:10.1016/j.stem.2010.11.029
30. Lolli F, Pallotti F, Rossi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9–17. doi:10.1007/s12020-017-1280-y
31. Goodarzi HR, Abbasi A, Saffari M, Fazelzadeh Haghighi M, Tabei MB, Noori Daloii MR. Differential expression analysis of balding and nonbalding dermal papilla microRNAs in male pattern baldness with a microRNA amplification profiling method. Br J Dermatol. 2012;166(5):1010–1016. doi:10.1111/j.1365-2133.2011.10675.x
32. Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Identification of androgen-inducible TGF- beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J Invest Dermatol Symp Proc. 2003;8(1):69–71. doi:10.1046/j.1523-1747.2003.12174.x
33. Itami S, Inui S. Role of androgen in mesenchymal epithelial interactions in human hair follicle. J Invest Dermatol Symp Proc. 2005;10(3):209–211. doi:10.1111/j.1087-0024.2005.10107.x
34. Leirós GJ, Ceruti JM, Castellanos ML, Kusinsky AG, Balañá ME. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol Cell Endocrinol. 2017;439:26–34. doi:10.1016/j.mce.2016.10.018
35. Shin H, Yoo HG, Inui S, et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013;46(9):460–464. doi:10.5483/BMBRep.2013.46.9.228
36. Yoo HG, Kim JS, Lee SR, et al. Perifollicular fibrosis: pathogenetic role in androgenetic alopecia. Biol Pharm Bull. 2006;29(6):1246–1250. doi:10.1248/bpb.29.1246
37. Vasserot AP, Geyfman M, Poloso NJ. Androgenetic alopecia: combing the hair follicle signaling pathways for new therapeutic targets and more effective treatment options. Expert Opin Ther Targets. 2019;23(9):755–771. doi:10.1080/14728222.2019.1659779
38. Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose derived stem cells and growth factors applied on hair transplantation. Follow-up of clinical outcome. J Cosmetics Dermatol Sci Appl. 2014;2014. doi:10.4236/JCDSA.2014.44036
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.