Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Short-term efficacy and safety of repaglinide versus glimepiride as augmentation of metformin in treating patients with type 2 diabetes mellitus
Authors Xie J, Li N, Jiang X , Chai L, Chen JJ , Deng W
Received 13 December 2018
Accepted for publication 8 March 2019
Published 17 April 2019 Volume 2019:12 Pages 519—526
DOI https://doi.org/10.2147/DMSO.S198154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Jing Xie,1 Ning Li,1 Xiaoyan Jiang,1 Liyin Chai,1 Jian-Jun Chen,2 Wuquan Deng1
1Department of Endocrinology and Nephrology, Chongqing University Central Hospital, Chongqing Emergency Medical Center, Chongqing 400014, People’s Republic of China; 2Institute of Life Sciences, Chongqing Medical University, Chongqing 400016, People’s Republic of China
Background: Consistent evidence is still lacking on which one, glimepiride plus metformin or repaglinide plus metformin, is better in treating type 2 diabetes mellitus (T2DM). Therefore, this study was conducted to compare the short-term efficacy and safety of these two methods in treating T2DM.
Methods: The literature research dating up to August 2018 was conducted in the electronic databases. The randomized controlled trials (RCTs) comparing the short-term (treatment period ≤12 weeks) efficacy and safety of these two methods in treating patients with T2DM were included. No language limitation was used in this study. The decreased hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), and 2h plasma glucose (2hPG) levels were used as the primary outcome to assess the efficacy, and the adverse events and hypoglycemia were used as the secondary outcome to assess the safety.
Results: In total, 11 RCTs composed of 844 T2DM patients were included. The results showed that there were no significant differences in decreasing HbA1c and FPG levels between the two methods, but the estimated standardized mean differences favored the repaglinide plus metformin. Meanwhile, the repaglinide plus metformin was significantly more effective in decreasing 2hPG levels than glimepiride plus metformin. In addition, fewer patients reported adverse events and experienced hypoglycemia in the repaglinide plus metformin group.
Conclusion: These results indicated that the repaglinide plus metformin might have some advantages over glimepiride plus metformin in the short-term treatment of patients with T2DM, and should be further explored.
Keywords: repaglinide, glimepiride, metformin, diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is an expanding global health problem, which is closely linked to the epidemic of obesity. Obesity and lack of exercise are the two main risk factors of this disease, although some people are more genetically at risk than others. At present, India and China have the first and second largest number of T2DM patients.1 These patients are usually at high risk for both macrovascular complications (such as cardiovascular comorbidities) and microvascular complications (such as retinopathy).2 Meanwhile, the continuous medical care and the huge economic burden usually lead T2DM patients to suffer from mental health problems.3 Generally speaking, most T2DM patients need a strict diet, blood glucose monitoring, hypoglycemic drug, and even moderate physical exercise during the treatment.4
Treatment strategy of T2DM is mainly involved in controlling the blood glucose level, improving the insulin sensitivity and β‑cell function, and reducing the micro and macrovascular complications. In addition, managing the mental health of T2DM patients is also very helpful. Our previous study found that the combined application of antidepressant therapy and hypoglycemic drug could yield a better glycemic control.5 Nowadays, several classes of anti-diabetic medications are available in clinical practice. Metformin is recommended as the first-line treatment for T2DM patients, but should not be used in those with severe liver or kidney problems.6 The mechanism of action of metformin is that it could suppress the hepatic glucose production, and then lead to the reduction in hemoglobin A1c (HbA1c) and fasting plasma-glucose (FPG).7–10 However, the multiple pathogenetic disturbances present in T2DM dictate that the combined application of multiple anti-diabetic medications is needed to maintain normoglycaemia.2
In recent decade, the combination of metformin and sulphonylurea drugs (such as glimepiride and repaglinide) is the most frequently applied. Glimepiride and repaglinide are two relatively new oral hypoglycemic drugs. The mechanism of action of glimepiride is that it could decrease the blood sugar by stimulating pancreatic beta cells to release insulin and by increasing the activity of intracellular insulin receptors.11 The repaglinide could close the ATP-dependent potassium channels in the membrane of beta cells, which results in calcium influx and then induces the insulin secretion.12 Previous study reported that both drugs could effectively decrease blood glucose in newly diagnosed T2DM patients.13 Derosa et al, found that both drugs could improve glycemic control and reduce the levels of other metabolic parameters of interest in T2DM patients.14 However, the consistent evidence is still lacking on which one, glimepiride plus metformin or repaglinide plus metformin, is better in treating T2DM. Therefore, we conducted this meta-analysis to compare the short-term efficacy and safety of glimepiride plus metformin and repaglinide plus metformin in treating T2DM.
Methods
Literature research
The literature research dating up to August 2018 was conducted in the following databases: Cochrane Library, MEDLINE, PubMed, Web of Science, PsycINFO, Embase, CNKI, and CBM-disc. The used search terms in this study included: “diabetes”, “repaglinide”, “glimepiride”, “metformin”, “novonorm”, “prandin”. Language restriction was not used here, for the purpose of mitigating language bias. To avoid omitting potential randomized controlled trials (RCTs), the conference summaries and the references listed in the included studies were also checked.
Inclusion/exclusion criteria
The inclusion criteria of this meta-analysis included: i) using the criteria of American Diabetes Association to diagnose T2DM patients;15 ii) RCT with T2DM patients randomly assigned to either receive glimepiride plus metformin or repaglinide plus metformin; iii) the treatment time was no more than 12 weeks; and iv) all patients provided the written informed consent, and the RCT was approved by the Ethical Committee. Meanwhile, the exclusion criteria of this meta-analysis included: i) retrospective studies, case reports, reviews, and duplicate studies; ii) patients with other forms of diabetes besides of Type II; iii) patients with liver and kidney dysfunction, malignant tumors, and severe physical illness; and iv) patients during the gestation period.
Data extraction
Two authors (JX and JJC) independently checked the potential studies according to the inclusion/exclusion criteria, and conducted the data extraction. The relevant data in the qualified RCTs were extracted and saved according to the Cochrane data extraction template. Any disagreement between these two authors was resolved by group discussion. The following data were obtained from the included RCTs: i) published year, age, sex ration, number of patients, treatment time and medication dose; ii) the decreased HbA1c, FPG and 2h plasma glucose (2hPG) levels after short-term treatment; and iii) adverse events and hypoglycemia. The decreased HbA1c, FPG, and 2hPG levels were used as the primary outcome to assess the efficacy of these two treatment modalities. The adverse events and hypoglycemia were used as the secondary outcome to assess the safety of these two treatment modalities.
Statistical analysis
The meta-analysis was carried out using Review Manager (RevMan 5). The standardized mean difference (SMD) and odds ratio (OR) were calculated in this study for the randomized studies.16 The SMD was used as a summary statistic when the included studies assessed the same outcome. It represented the size of treatment effect in each study relative to the variability observed in that study. The SMDs lower than 0 favor the repaglinide plus metformin, and OR less than 1 also favor the repaglinide plus metformin. The effect size and its corresponding 95% confidence interval (CI) were calculated for each outcome. It was assumed that the randomized studies might have diverse true treatment effects; therefore, we selected the Mantel-Haenszel random-effects model to calculate the effect size.17 Moreover, this method was also much better than the Mantel-Haenszel fixed-effects model, when the heterogeneity was existed.18 Egger’s test was used here to assess the potential presence of publication bias. The sensitivity analysis was conducted when appropriate. This meta-analysis was strictly conducted according to the recommendations of Sacks et al19.
Results
Searching results
The literature research in this study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Figure 1). At first, we obtained 306 potentially relevant studies from the databases. After carefully checking according to the aforementioned inclusion/exclusion criteria, 11 RCTs were included.20–30 The data were extracted from these qualified studies and subsequently analyzed. The exclusion reasons included: i) duplicates (n=40); ii) retrospective studies, case reports, and reviews (n=35); iii) repaglinide or glimepiride as monotherapy in treating T2DM (n=154); iv) the treatment time was more than 12 weeks (n=25); and v) the combination of repaglinide or glimepiride with other medications in treating T2DM (n=41). In total, there were 844 T2DM patients in the 11 RCTs. The average age of patients was approximately 53 years. The treatment time was 12 weeks in 10 RCTs and 8 weeks in one RCT. The detailed information was described in Table 1.
 | Table 1 Clinical characteristics of the patients in the included randomized controlled trials |
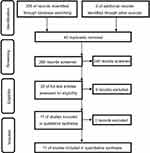 | Figure 1 Workflow of literature research. |
Decreased HbA1c level
Totally, nine studies assessed the decreased HbA1c level in the two groups. The treatment time was 12 weeks in all of these studies. The SMDs of four studies were more than 0, which favored the glimepiride plus metformin. The SMDs of other studies were less than 0, which favored the repaglinide plus metformin. Finally, the pooled SMD was −0.06 (95%CI=−0.27, 0.15) for the random-effects model (Figure 2). These results favored the repaglinide plus metformin in decreasing the HbA1c level. The results of Egger’s test (p=0.39) showed that this conclusion was not influenced by the potential publication bias. Meanwhile, the results of meta-regression analysis demonstrated that the efficacy had a negligible relationship with the baseline HbA1c levels.
 | Figure 2 Meta-analysis of the decreased HbA1c level in the two groups. |
Decreased FPG level
All of the included studies assessed the decreased FPG level in the two groups. The SMDs of five studies were more than 0, which favored the glimepiride plus metformin. The SMDs of four studies were less than 0, which favored the repaglinide plus metformin. The SMDs of two studies were 0, which indicated the same efficacy of these two methods. Finally, the pooled SMD was −0.02 (95%CI=−0.18, 0.13) for the random-effects model (Figure 3). These results favored the repaglinide plus metformin in decreasing the FPG level. The results of Egger’s test (p=0.51) showed that this conclusion was not influenced by the potential publication bias. Meanwhile, the results of meta-regression analysis demonstrated that the efficacy had a negligible relationship with the baseline FPG levels. The sensitivity analysis was conducted after excluding the study with 8 weeks of treatment, and we obtained the similar results (SMD=−0.05, 95%CI=−0.21, 0.11).
 | Figure 3 Meta-analysis of the decreased FPG level in the two groups. |
Decreased 2hPG level
The 11 included studies assessed the decreased 2hPG level in the two groups. The SMD of one study was more than 0, which favored the glimepiride plus metformin. The SMDs of nine studies were less than 0, which favored the repaglinide plus metformin. The SMD of one study was 0, which indicated the same efficacy of these two methods. Finally, the pooled SMD was −0.39 (95%CI=−0.66, −0.12) for the random-effects model (Figure 4). These results favored the repaglinide plus metformin in decreasing the 2hPG level. The results of Egger’s test (p=0.27) showed that this conclusion was not influenced by the potential publication bias. Meanwhile, the results of meta-regression analysis demonstrated that the efficacy had a negligible relationship with the baseline 2hPG levels. The sensitivity analysis was conducted after excluding the study with 8 weeks of treatment, and we obtained similar results (SMD=−0.43, 95%CI=−0.72, −0.14).
 | Figure 4 Meta-analysis of the decreased 2hPG level in the two groups. |
Safety assessment
There were seven studies reporting the adverse events. In these studies, 19 of 232 patients receiving repaglinide plus metformin and 32 of 232 patients receiving glimepiride plus metformin reported adverse events. The adverse events included: hypoglycemia, mild nausea, evanescent eruption, and upper abdominal discomfort. No significant difference in adverse events was observed between the two groups, although fewer patients reported adverse events in the repaglinide plus metformin group. The pooled OR was 0.55 (95%CI=0.26, 1.16) (Figure 5).
 | Figure 5 Meta-analysis of the adverse events in the two groups. |
There were five studies reporting the hypoglycemia. In these studies, 16 of 177 patients receiving repaglinide plus metformin and 27 of 195 patients receiving glimepiride plus metformin experienced hypoglycemia. No significant difference in hypoglycemia was observed between the two groups, although fewer patients experienced hypoglycemia in the repaglinide plus metformin group. The pooled OR was 0.64 (95%CI=0.22, 1.88) (Figure 6).
 | Figure 6 Meta-analysis of the hypoglycemia in the two groups. |
Discussion
This meta-analysis was based on 11 RCTs. The 844 T2DM patients were randomly assigned to either receive repaglinide plus metformin or glimepiride plus metformin. The results showed that the estimated SMDs favored the repaglinide plus metformin in decreasing HbA1c and FPG levels, although no significant difference was observed between the two groups. Meanwhile, the repaglinide plus metformin was significantly more effective in decreasing 2hPG levels than glimepiride plus metformin. In addition, fewer patients reported adverse events and experienced hypoglycemia in the repaglinide plus metformin group. These results indicated that the repaglinide plus metformin might have some advantages over glimepiride plus metformin in the short-term treatment of T2DM, and should be further explored.
Compared to glimepiride, repaglinide has a fast onset and short duration of action, which could effectively enhance the early insulin secretion.31 When combined application with metformin, it could produce a stronger synergistic effect in decreasing the plasma glucose level by improving the islet B-cell function and insulin resistance. With the prolongation of treatment time and the increase of drug concentration, it is difficult to separate the glimepiride and its receptor after binding. Then, the islet B-cells were continuously stimulated until its apoptosis.32 Unlike glimepiride, repaglinide could restore the physiological pattern of insulin secretion. It not only does not continue to stimulate the islet B-cells, but also has a protective effect on the islet B-cells. Previous study found that the metformin has a direct protective effect on the secretory function of the islet B-cells that were exposed to high glucose and high-fat environment for a long time.33 Therefore, the repaglinide plus metformin might be more appropriate for T2DM patients, especially these patients with impaired islet B-cell function.
Limitations of this meta-analysis should be mentioned here: i) many included studies were conducted in China, which might limit the generalisability of these results;34–36 ii) only the short-term efficacy and safety was assessed here; thus, future studies are still needed to compare the long-term efficacy and safety of these two methods; iii) there was heterogeneity that was probably caused by the diverse true treatment effects of the included RCTs; and iv) the dose of repaglinide or glimepiride was not exactly the same in the included studies.
In conclusion, based on the results from the meta-analysis of 11 RCTs, our study firstly compared the short-term efficacy and safety of repaglinide plus metformin versus glimepiride plus metformin in treating T2DM. The results showed that the repaglinide plus metformin was significantly more effective in decreasing 2hPG levels than glimepiride plus metformin. Meanwhile, the repaglinide plus metformin caused fewer adverse events and hypoglycemia during 12 weeks of treatment. Therefore, we thought that the repaglinide plus metformin should be the first choice in treating T2DM patients between these two methods, and should be further explored.
Acknowledgments
The work was supported by the Scientific and Technological Research Program of Chongqing Municipal Education Commission awarded to Wuquan Deng (KJQN201800127). Jian-Jun Chen and Wuquan Deng are co-senior authors in this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Feng D, Zeng Z, Liao F, et al. Analysis on factors influencing anxiety and depression of inpatients with diabetes mellitus type 2. Chin J Health Stat. 2010;27:129–132.
2. DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi:10.1038/nrdp.2015.19
3. Fisher EB, Thorpe CT, Devellis BM, Devellis RF. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ. 2007;33(6):1080–1103. doi:10.1177/0145721707309808
4. Tian L, Xuelian L, Lan Yutao QS. Depression level and relevant factors of the old patients with diabetes mellitus type 2. Chin J Gerontol. 2013;33:2115–2117.
5. Xie J, Deng W. Psychosocial intervention for patients with type 2 diabetes mellitus and comorbid depression: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat. 2017;13:2681–2690. doi:10.2147/NDT.S116465
6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–1596. doi:10.1007/s00125-012-2534-0
7. Cusi K, Consoli A, Defronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81(11):4059–4067. doi:10.1210/jcem.81.11.8923861
8. Weiss R, Fernandez E, Liu Y, Strong R, Salmon AB. Metformin reduces glucose intolerance caused by rapamycin treatment in genetically heterogeneous female mice. Aging (Albany NY). 2018;10(3):386–401. doi:10.18632/aging.v10i3
9. Turner RC, Cull AC, Frighi V, et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281(21):2005–2012.
10. Kim SA, Lam TG, Yook JI, Ahn SG. Antioxidant modifications induced by the new metformin derivative HL156A regulate metabolic reprogramming in SAMP1/kl (-/-) mice. Aging (Albany NY). 2018;10(9):2338–2355. doi:10.18632/aging.101549
11. Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299(13):1561–1573. doi:10.1001/jama.299.13.1561
12. Culy CR, Jarvis B. Repaglinid. Drugs. 2001;61(11):1625–1660. doi:10.2165/00003495-200161110-00008
13. Li Y, Xu L, Shen J, et al. Effects of short-term therapy with different insulin secretagogues on glucose metabolism, lipid parameters and oxidative stress in newly diagnosed type 2 diabetes mellitus. Diabetes Res Clin Pract. 2010;88(1):42–47. doi:10.1016/j.diabres.2009.12.017
14. Derosa G, Mugellini A, Ciccarelli L, Crescenzi G, Fogari R. Comparison between repaglinide and glimepiride in patients with type 2 diabetes mellitus: a one-year, randomized, double-blind assessment of metabolic parameters and cardiovascular risk factors. Clin Ther. 2003;25(2):472–484.
15.
16. Kok JL, Williams A, Zhao L. Psychosocial interventions for people with diabetes and co-morbid depression. A systematic review. Int J Nurs Stud. 2015;52(10):1625–1639. doi:10.1016/j.ijnurstu.pi re 3,j2015.05.012
17. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi:10.1136/bmj.d549
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi:10.1136/bmj.327.7414.557
19. Sacks HS, Berrier J, Reitman D, Ancona-Berk V, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med. 1987;316:450–455. doi:10.1056/NEJM198702193160806
20. Yu D. Comparative analysis of the efficacy of glimepiride or repaglinide in combination with metformin in treating T2DM. Chin J Mod Drug Appl. 2010;4(11):101–102.
21. Ren J, Minjuan G. Efficacy of glimepiride or repaglinide with metformin in treating Type 2 diabetes mellitus. J Clin Med Pract. 2006;12:87–88.
22. Li J, Wang H, Wang L. Clinical observation and nursing intervention of glimepiride in the treatment of type 2 diabetes mellitus in the community. Haixia Med. 2012;24(7):180–181.
23. Kong J. Repaglinide versus glimepiride treatment of type 2 diabetes mellitus. Pract Appl. 2016;20(3):307–308.
24. Li Y. Observation on the therapeutic effect of repaglinide combined with metformin in the treatment of patients with type 2 diabetes mellitus. Chin Commun Doctors. 2016;32(13):41–42.
25. Li Y, Zhu B, Liu Z. Clinical observation of the treatment of T2DM using glimepiride or repaglinide in combination with metformin. J Clin Exp Med. 2009;8(12):75–76.
26. Wang H, Zhang B. Clinical observation of glimepiride or repaglinide in combination with metformin in treating type 2 diabetes. Chin Commun Doctors. 2011;13(274):45.
27. Tian S. Clinical observation of repaglinide with metformin in treating type 2 diabetes. Chin J Clin Res. 2012;25(2):136–137.
28. Cheng Z. Glimepiride and repaglinide together with dimethyldiguanide treat diabetes II. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2006;30(5):490–491.
29. Dimic D, Velojic GM, Antic S, Radenkovic S. Evaluation of the repaglinide efficacy in comparison to the glimepiride in the type 2 diabetes patients poorly regulated by the metmorfine adiminstratino. Bratisl Lek Listy. 2009;110(6):335–339.
30. Zhao H. Therapeutic effect of different drugs combined with metformin on initial type 2 diabetes. Mod J Integr Trad Chin Western Med. 2012;21(25):2785–2787.
31. Liu X, Yan L. A review of the efficacy and safety of glinides. Drugs Clinic. 2015;12(13):16–18.
32. Wang Q, Ji H, Rong H, Sun H, Huang L. Effects of different insulin secretagogues on apoptosis of islet β cells. J Med Res. 2010;39(3):97–101.
33. Wang Y, Xiujun L. Effect of metformin on islet β cell insulin resistance. West China Med J. 2009;24(5):1318–1319.
34. Chen J-J, Xie J, Zeng L, Zhou C-J, Zheng P, Xie P. Urinary metabolite signature in bipolar disorder patients during depressive episode. Aging (Albany NY). 2019;11(3):1008–1018. doi:10.18632/aging.101805
35. Chen J, Bai SJ, Li W, et al. Urinary biomarker panel for diagnosing patients with depression and anxiety disorders. Transl Psychiatry. 2018;8(1):192. doi:10.1038/s41398-018-0245-0
36. Hou L, Wei X, Zhuo Y, et al. GC-MS-based metabolomics approach to diagnose depression in hepatitis B virus-infected patients with middle or old age. Aging (Albany NY). 2018;10(9):2252–2265. doi:10.18632/aging.101535
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
