Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Short-term bisphosphonate treatment reduces serum 25(OH) vitamin D3 and alters values of parathyroid hormone, pentosidine, and bone metabolic markers
Authors Kamimura M, Uchiyama S, Nakamura Y, Ikegami S, Mukaiyama K, Kato H
Received 26 August 2016
Accepted for publication 15 October 2016
Published 13 February 2017 Volume 2017:13 Pages 161—168
DOI https://doi.org/10.2147/TCRM.S120749
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Mikio Kamimura,1 Shigeharu Uchiyama,2 Yukio Nakamura,2,3 Shota Ikegami,2 Keijiro Mukaiyama,2 Hiroyuki Kato2
1Center for Osteoporosis and Spinal Disorders, Kamimura Orthopaedic Clinic, Matsumoto, Japan; 2Department of Orthopaedic Surgery, Shinshu University School of Medicine, Matsumoto, Japan; 3Department of Orthopedic Surgery, Showa-Inan General Hospital, Komagane, Japan
Abstract: This study aimed to clarify the effects of short-term bisphosphonate (BP) administration in Japanese osteoporotic patients retrospectively. Daily minodronate (MIN) at 1 mg/day (MIN group) or weekly risedronate (RIS) at 17.5 mg/week (RIS group) was primarily prescribed for each patient. We analyzed the laboratory data of 35 cases (18 of MIN and 17 of RIS) before the start of treatment and at 4 months afterward. The changes in 25(OH)D3, whole parathyroid hormone (PTH), serum pentosidine, and the bone turnover markers urinary cross-linked N-telopeptide of type I collagen (NTX), serum tartrate-resistant acid phosphatase (TRACP)-5b, bone-specific alkaline phosphatase (BAP), and undercarboxylated osteocalcin were evaluated. Overall, serum 25(OH)D3 was significantly decreased from 21.8 to 18.4 ng/mL at 4 months, with a percent change of –14.7%. Whole PTH increased significantly from 23.4 to 30.0 pg/mL, with a percent change of 32.1%. Serum pentosidine rose from 0.0306 to 0.0337 µg/mL, with a percent change of 15.2%. In group comparisons, 25(OH)D3 and pentosidine showed comparable changes in both groups after 4 months of treatment, whereas whole PTH became significantly more increased in the MIN group. All bone turnover markers were significantly decreased at 4 months in both groups. Compared with the RIS group, the MIN group exhibited significantly larger value changes for urinary NTX, serum TRACP-5b, and BAP at the study end point. This study demonstrated that serum 25(OH)D3 became significantly decreased after only 4 months of BP treatment in Japanese osteoporotic patients and confirmed that MIN more strongly inhibited bone turnover as compared with RIS.
Keywords: minodronate, risedronate, 25(OH) vitamin D3, parathyroid hormone, pentosidine
Introduction
The primary goals of osteoporosis (OP) treatment are the prevention of bone fragility fractures and maintenance of skeletal health. Dietary, exercise, and pharmacological therapies are all prescribed for OP depending on its severity and progress in individual patients.1 There are various drugs available for OP, the mainstay of which are bisphosphonates (BPs), such as alendronate (ALN) and risedronate (RIS).2 These agents were approved in Japan in 2001 and have been prescribed as the core treatments for OP. The currently approved doses of ALN and RIS in Japan are 5 mg/day (35 mg/week) and 2.5 mg/day (17.5 mg/week), respectively, which are often half of those prescribed abroad. Thus, the bone metabolic data of Japanese patients for ALN and RIS may differ from findings worldwide.
Developed and recently approved in Japan in 2009, minodronate (minodronic acid hydrate; MIN) is the strongest inhibitor of bone resorption among commercially available.3 In fact, MIN was the only BP demonstrated to prevent vertebral fractures in Japanese osteoporotic patients in a placebo-controlled Phase III trial.4 We previously compared the early changes in bone turnover markers between MIN and RIS and found MIN to more strongly and immediately inhibit bone turnover.5
The important regulatory hormones in bone metabolism are 1,25(OH)2D3 and parathyroid hormone (PTH). The main form of vitamin D in the serum is 25(OH)D3, which is also its major storage conformation. The concentration of 25(OH)D3 is approximately 1,000 times greater than that of 1,25(OH)2D3. The changes in 25(OH)D3 after BP treatment are controversial since it may remain constant or decrease after short-term BP therapy.6,7 Although adequate levels of serum 25(OH)D3 are important in osteoporotic treatment,8 there have been no detailed reports on the effect of BP treatment on 25(OH)D3 in Japanese patients.
Pentosidine is a well-described advanced glycation end product that has a strong linear correlation with cortical bone pentosidine levels.9,10 As it is also a metabolic product derived from bones, we have speculated that plasma pentosidine decreases with time during short-term BP treatment due to the suppression of bone turnover. However, we lack precise data on the relationship between the immediate effects of BP therapy and serum pentosidine level in the clinical context.
In this study, we analyzed the changes in 25(OH)D3, whole PTH, serum pentosidine, and bone turnover markers after short-term MIN or RIS therapy to uncover novel findings with respect to 25(OH)D3 modulation by BP treatment.
Patients and methods
The subjects were patients who had been newly diagnosed with primary OP between August 2009 and October 2010. MIN or RIS was primarily prescribed for each patient in a randomized fashion using an enveloped method. Subjects were assigned into the group receiving 1 mg/day of MIN (MIN group) or 17.5 mg/week of RIS (RIS group). All diagnoses were made according to the primary OP diagnostic criteria (2000 revision).11 Written informed consent was obtained from all participants prior to this study.
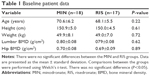
A total of 50 patients with newly diagnosed primary OP had provided consent to participate in a previous study.12 Among these, we analyzed the data of 35 cases (18 of MIN and 17 of RIS) obtained just before treatment and at 4 months afterward. Only female patients were enrolled to avoid gender effects. There were no significant differences between the groups with regard to age, height, weight, lumbar vertebral bone mineral density (BMD), or proximal femoral BMD (Table 1).
Our earlier report evaluated serum bone-specific alkaline phosphatase (BAP) as a marker of bone formation and urinary cross-linked N-telopeptide of type I collagen (NTX) and serum bone tartrate-resistant acid phosphatase (TRACP)-5b as markers of bone resorption.5 Additionally in this study, serum 25(OH)D3 and whole PTH were measured as bone metabolic hormones along with serum undercarboxylated osteocalcin (ucOC).
Serum BAP (reference range in postmenopausal women: 3.8–22.6 μg/L) was determined using a chemiluminescent enzyme immunoassay/antibody radioimmunoassay. Serum TRACP-5b (reference range in women: 120–420 mU/dL) and urinary NTX (reference range in women: 14.3–89.0 nmol BCE/mmol CRE) were measured with an enzyme-linked immunosorbent assay (ELISA; Osteomark, Osteox International, Seattle, WA, USA). We quantified ucOC with an electrochemiluminescence immunoassay (cutoff value: <4.50 ng/mL). Whole serum PTH and 25(OH)D3 (reference range in women: 9–39 pg/mL and 7–41 ng/mL, respectively) were assessed using radioimmunoassays. After overnight fasting, serum and first void urine samples were collected between 8:30 am and 10:00 am. Immunoassays were performed by SRL, Inc. (Tokyo, Japan). Serum pentosidine in microgram per milliliter was measured using ELISA.
BMD was measured using a dual-energy X-ray absorption fan beam bone densitometer (Lunar Prodigy; GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) for the lumbar 1–4 levels of the posteroanterior spine and bilateral hips. Although BMD values were determined for the purpose of diagnosing OP, they were not used to evaluate the effectiveness of BPs in this study.
In both groups, we compared the changes in 25(OH)D3, whole PTH, pentosidine, and bone turnover markers immediately before and 4 months after the start of drug administration using the linear mixed models method for multiple comparisons. Each marker value was individually adopted as a response variable: group (MIN or RIS) and the timing of the measurement were used as fixed effects, and the individuality of the measurement was adopted as a random effect. P-values of <0.05 were considered to be statistically significant. Statistical analyses were performed using the statistical package R, version 3.2.0 (available at http://www.r-project.org).
This study was reviewed by the Institutional Ethics Committee at Showa Inan General Hospital (Protocol No: 2014–2018) and carried out in accordance with the approved guidelines retrospectively.
Results
Both groups had satisfactory drug compliance and exhibited no major adverse events from drug therapy.
Regulatory metabolic hormones and serum pentosidine
The combined data sets of the test groups were analyzed to evaluate the overall effects of short-term BP treatment.
The mean ± standard error serum 25(OH)D3 was significantly decreased at 4 months as compared with the baseline value from 21.8±1.0 to 18.4±1.0 ng/mL (P<0.01), with a percent change of −14.8% (P<0.01). Whole PTH increased significantly over 4 months, from 23.4±1.6 to 30.0±2.7 pg/mL (P<0.01), with a percent change of 32.1% (P<0.01). Serum pentosidine rose markedly from 0.0307±0.0018 to 0.0337±0.0018 μg/mL (P=0.06), with a significant percent change of 15.2% (P<0.01).
Changes in 25(OH)D3
In the MIN and RIS groups, serum 25(OH)D3 values before drug administration were similar and had become significantly decreased at 4 months of BP treatment (both P<0.01; Tables 2 and 3). The percent change of 25(OH)D3 was −15.7% for MIN (P<0.01) and −13.9% for RIS (P<0.01), which were comparable (Figure 1A).
Changes in whole PTH
Whole PTH was significantly increased at 4 months in the MIN group (P<0.01), but had not changed remarkably for RIS (P=0.45; Tables 2 and 3). The percent change of whole PTH was 51.2% for MIN (P<0.01) and 11.8% for RIS (P=0.33), which was significantly different (P<0.05; Figure 1B).
Changes in pentosidine
In both groups, pentosidine increased significantly in a comparable manner (Figure 1C), with a percent change of 16.0% for MIN (P<0.05) and 14.3% for RIS (P=0.09) after 4 months of drug administration (Tables 2 and 3; Figure 1C).
Markers of bone metabolism
Bone resorption markers
Urinary NTX was significantly lower at 4 months of treatment as compared with baseline values in the MIN and RIS groups (both P<0.01). We witnessed a significantly larger decrease in the MIN group (P<0.01; Figure 2A), with a percent change of −62.4% (P<0.01) vs −37.8% in the RIS group (P<0.01; Tables 2 and 3).
Serum TRACP-5b was also significantly lower at 4 months than pretreatment levels in both groups (P<0.01), with a percent change of −50.4% in the MIN group (P<0.01) and −32.1% in the RIS group (P<0.01; Tables 2 and 3). Again, the percent change was significantly larger in the MIN group than in the RIS group (P<0.05; Figure 2B).
Bone formation markers
In both groups, serum BAP was significantly lower at 4 months after the start of drug administration as compared with just prior (P<0.01). The percent change of BAP was −30.7% (P<0.01) in the MIN group and significantly less (−14.7%; P<0.05) in the RIS group (P<0.05) (Tables 2 and 3; Figure 2C).
Other bone turnover markers
Serum ucOC was significantly decreased at the study end point in both groups (P<0.01). The percent changes for this parameter were −38.3% and −29.2% in the MIN (P<0.05) and RIS (P=0.01) groups, respectively (Tables 2 and 3), which were comparable (P=0.54; Figure 2D).
Discussion
The representative major and main storage form of vitamin D in the human body is 25(OH)D3. Although it is generally believed that 25(OH)D3 levels remain stable if nutritional conditions are maintained, this study revealed that serum 25(OH)D3 decreased significantly by short-term BP administration. To the best of our knowledge, this is the first study demonstrating early 25(OH)D3 changes during osteoporotic treatment in Japan.
Olmos et al7 reported that 25(OH)D3 values did not change by monthly ALN treatment for 3 months. In Korean patients, Chung et al6 observed that 25(OH)D3 decreased by 16.9% after 4 months of monthly RIS treatment, which was not significant, but had decreased significantly by 13.6% after weekly RIS treatment. Kim et al13 described that 25(OH)D3 values became significantly reduced after 16 weeks of weekly ALN treatment, with a percent change of approximately −9%. Here, we showed that 25(OH)D3 was significantly decreased by short-term BP treatment. Several BPs may lower 25(OH)D3 values in Asian people. However, no obvious differences have been reported to date, despite administered doses in Korea being double of those in Japan.
The mechanism by which 25(OH)D3 decreases using antiresorptive drugs is currently unknown. Serum calcium (Ca), 1,25(OH)2D3, and PTH are tightly regulated in the human body. Shiraki et al14 and Nakamura et al15 have observed that values of 1,25(OH)2D3, with very a short half-life, were significantly increased by BP and denosumab soon after administration. Thus, we hypothesize that 1) BP treatment decreases serum Ca, which increases serum PTH and 1,25(OH)2D315,16 and 2) a depletion in 25(OH)D3 occurs due to its conversion to 1,25(OH2)D3. In this study, bone inhibitory effects were significantly greater in the MIN group than in the RIS group. These findings using BP doses of half of those elsewhere in Asia14 suggest that bone inhibitory effects alone might not be responsible for the decline in vitamin D values.
This investigation revealed that while the decrease in 25(OH)D3 level in the MIN group was similar to that in the RIS group, PTH was significantly increased at 4 months of treatment in the MIN group only. Okazaki et al16 witnessed that serum PTH values peaked at 1 month by daily MIN administration and displayed significantly high values at 3 months of treatment, but then returned to baseline values at 6 months. In their study of ALN, Shiraki et al14 reported that PTH peaked at 4 weeks and then decreased. They also found that daily RIS administration (2.5 mg) significantly increased PTH until 4 weeks, which fell thereafter. The percent changes of PTH values hovered around 20% at 12–24 weeks. There have been no trials on the changes in PTH by weekly RIS treatment in Japan. We suspect that weekly RIS doses caused smaller changes in serum PTH as compared with daily RIS therapy, and that any increases in PTH by RIS had diminished by 4 months. It was also possible that 25(OH)D3 level was already low as a consequence of 1,25(OH)2D3 formation.
As 1α(OH)-vitamin D3, alfacalcidol (ALF) is a vitamin D analog that is frequently used for OP treatment in Japan.17,18 ALF undergoes 25-hydroxylation in the liver, and the resulting 1,25(OH)2D3 promotes the absorption of Ca and phosphate.17 Orimo et al reported that ALN plus ALF more significantly inhibited vertebral fractures than ALN alone within the first 6 months by an undescribed mechanism. There have been numerous reports that low 25(OH)D3 level is a risk factor for fractures.19 A Korean study concluded that BPs alone decreased 25(OH)D3 readings at 16 weeks, while 25(OH)D3 increased by the combined therapy of native D3 and cholecalciferol.6,13 Thus, it can be presumed that 1) BP alone decreases the values of 25(OH)D3 and thereby augments fracture risk and 2) the combined therapy of BPs with ALF might not affect 25(OH)D3. Such discrepancies may lead to a significant risk of fractures by BP monotherapy rather than combined treatment.
It is unknown why the fracture prevention effects of combined ALN and ALF disappear in the long run, as reported by Orimo et al.19 Nakamura et al20 have shown that serum 25(OH)D3 values increased significantly by long-term BP therapy without vitamin D addition, which suggests that the effects of combined therapy with BPs and vitamin D might negate or decrease the effects caused by the chronic therapy with BPs alone.
Advanced glycation end products including pentosidine might have accumulated and adversely affected the biomechanical properties of the bone. It has been previously shown that treatment with BPs increases bone pentosidine levels or alters bony architecture.21–23 Pentosidine levels were found to be higher in the cortical and trabecular bones of patients with femoral neck fracture than in those of age-matched controls.9,10 However, the direct assessment of pentosidine in bones is not practical in the clinical setting because it involves invasive procedures, such as bone biopsy. An earlier study disclosed that plasma pentosidine content possessed a significant linear correlation with that in cortical bone,24 suggesting its use as a surrogate marker for pentosidine bone content and bone strength. We reported on serum pentosidine levels before and after 3 years of treatment with BPs in different studies, which produced varying results.12,25 This may have been due to differences in the timing of sample testing, which was either right after collection12 or after 3 years of frozen storage.25 The present study showed that serum pentosidine became significantly increased at as early as 4 months of BP administration in patients with OP (percent change: 15.2%, P<0.01). If serum pentosidine were a metabolic bone product following bone resorption, these values would have decreased from bone turnover inhibition by BP treatment. Thus, serum pentosidine appears not to be a metabolic bone product.
Lastly, nitrogen-containing BPs were seen to inhibit farnesyl pyrophosphate synthase (FPPS) in the mevalonate pathway in osteoclasts. This inhibition suppressed the function of osteoclasts and induced their apoptosis, thereby inhibiting osteoclastic activity.26 Both MIN and RIS are nitrogen-containing BPs. However, it is known that the 50% inhibitory concentration against FPPS is considerably lower for MIN at 3 nM than for RIS at 10 nM, indicating a stronger ability of MIN.27 In a recent report on BP binding to FPPS, MIN bound more strongly in pockets for BP side chains and occupied more binding sites as compared with ALN or RIS.28 Here, MIN also exhibited strong effects on bone resorption inhibition, which were particularly apparent in comparisons with RIS, and supported the notion of a stronger binding force with FPPS.
Limitations
The limitations of this study were that 1) it was not strictly randomized or double-blinded, although its principal aim was not direct comparison of the two BPs, 2) 25(OH)D3, whole PTH, and pentosidine were measured only once during the treatment course, and 3) 1,25(OH)2D3 was not evaluated. Thus, the modulation of pentosidine, PTH, 25(OH)D3, and 1,25(OH)2D3 all require further examination in long-term follow-up.
Conclusion
In summary, 25(OH)D3 levels were significantly decreased in both MIN- and RIS-treated groups at 4 months of treatment. PTH and serum pentosidine became significantly increased in the MIN group only, which confirmed the significantly stronger inhibition of bone resorption by this drug.
Author contributions
YN and MK directed this study; MK and YN collected the patients’ data and prepared all the figures; SI performed statistical analyses; MK, SU, and YN wrote the main manuscript text; KM, SU, and HK gave advice on this study. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Guideline Committee for Prevention and Treatment of Osteoporosis. Japanese guidelines for prevention and treatment of osteoporosis. 2011 ed. Tokyo: Life Science Publishing; 2011. | ||
Peters ML, Leonard M, Licata AA. Role of alendronate and risedronate in preventing and treating osteoporosis. Cleve Clin J Med. 2001;68:945–951. | ||
Mori H, Kayasuga R, Tanaka M, et al. Inhibitory effects of minodronic acid hydrate on bone resorption: comparison of risedronate and alendronate. Clin Pharmacol. 2008;18(Suppl 1):S19–S32. | ||
Matsumoto T, Hagino H, Shiraki M, et al. Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int. 2009;20:1429–1437. | ||
Kamimura M, Ikegami S, Hirabayashi H, Mukaiyama K, Uchiyama S, Kato H. The comparison of clinical effectiveness between MIN and RIS in primary Japanese osteoporotic patients. J New Rem Clin. 2012;61:493–500. Japanese. | ||
Chung HY, Chin SO, Kang MI, et al. Efficacy of risedronate with cholecalciferol on 25-hydroxyvitamin D level and bone turnover in Korean patients with osteoporosis. Clin Endocrinol (Oxf). 2011;74:699–704. | ||
Olmos JM, Hernández JL, Llorca J, Nan D, Valero C, González-Macías J. Effects of 25-hydroxyvitamin D3 therapy on bone turnover markers and PTH levels in postmenopausal osteoporotic women treated with alendronate. J Clin Endocrinol Metab. 2012;97:4491–4497. | ||
Kumar R. Metabolism of 1,25-dihydroxyvitamin D3. Physiol Rev. 1984;64:478–504. | ||
Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17:986–995. | ||
Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int. 2006;79:160–168. | ||
Orimo H, Hayashi Y, Fukunaga M; Osteoporosis Diagnostic Criteria Review Committee; Japanese Society for Bone and Mineral Research. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–337. | ||
Uchiyama S, Ikegami S, Kamimura M, et al. The skeletal muscle cross sectional area in long-term bisphosphonate users is smaller than that of bone mineral density-matched controls with increased serum pentosidine concentrations. Bone. 2015;75:84–87. | ||
Kim KJ, Min YK, Koh JM, et al; VALUE study group. Efficacy and safety of weekly alendronate plus vitamin D3 5600 IU versus weekly alendronate alone in Korean osteoporotic women: 16-week randomized trial. Yonsei Med J. 2014;55:715–724. | ||
Shiraki M, Fukunaga M, Kushida K, et al. A double-blind dose-ranging study of risedronate in Japanese patients with osteoporosis (a study by the Risedronate Late Phase II Research Group). Osteoporos Int. 2003;14:225–234. | ||
Nakamura Y, Kamimura M, Ikegami S, et al. Changes in serum vitamin D and PTH values using denosumab with or without bisphosphonate pre-treatment in osteoporotic patients: a short-term study. BMC Endocrine Disord. 2015;15:81. | ||
Okazaki R, Hagino H, Ito M, et al. Efficacy and safety of monthly oral minodronate in patients with involutional osteoporosis. Osteoporos Int. 2012;23:1737–1745. | ||
Mukaiyama K, Uchiyama S, Nakamura Y, et al. Eldecalcitol, in combination with bisphosphonate, is effective for treatment of Japanese osteoporotic patients. Tohoku J Exp Med. 2015;237:339–343. | ||
Orimo H, Schacht E. The D-hormone analog alfacalcidol: the pioneer beyond the horizon of osteoporosis treatment. J Rheumatol Suppl. 2005;76:4–10. | ||
Orimo H, Nakamura T, Fukunaga M, et al; A-TOP (Adequate Treatment of Osteoporosis) research group. Effects of alendronate plus alfacalcidol in osteoporosis patients with a high risk of fracture: the Japanese Osteoporosis Intervention Trial (JOINT) – 02. Curr Med Res Opin. 2011;27:1273–1284. | ||
Nakamura Y, Uchiyama S, Kamimura M, Ikegami S, Komatsu M, Kato H. Long-term changes in serum native vitamin D values by bisphosphonate monotherapy in Japanese osteoporotic patients. unpublished data. | ||
Saito M, Mori S, Mashiba T, Komatsubara S, Marumo K. Collagen maturity, glycation induced-pentosidine, and mineralization are increased following 3-year treatment with incadronate in dogs. Osteoporos Int. 2008;19:1343–1354. | ||
Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–337. | ||
Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–894. | ||
Odetti P, Rossi S, Monacelli F, et al. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710–717. | ||
Hashidate H, Kamimura M, Ikegami S, et al. Serum pentosidine levels after 3 years of bisphosphonate treatment in post-menopausal osteoporotic women. Endocr Res. 2015;40:172–176. | ||
Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. | ||
Fleisch H. Bisphosphonates in Bone Disease, From the Laboratory to the Patient. 4th ed. San Diego: Academic Press; 2000. | ||
Ohno K, Mori K, Orita M, Takeuchi M. Computational insights into binding of bisphosphates to farnesyl pyrophosphate synthase. Curr Med Chem. 2011;18:220–233. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.