Back to Journals » Psychology Research and Behavior Management » Volume 12
Sexual dimorphism of oxytocin and vasopressin in social cognition and behavior
Authors Lu Q, Lai J, Du Y , Huang T, Prukpitikul P , Xu Y , Hu S
Received 31 October 2018
Accepted for publication 15 March 2019
Published 17 May 2019 Volume 2019:12 Pages 337—349
DOI https://doi.org/10.2147/PRBM.S192951
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Mei-Chun Cheung
Qiaoqiao Lu,1,2 Jianbo Lai,1,3–4 Yanli Du,2 Tingting Huang,2 Pornkanok Prukpitikul,2 Yi Xu,1,3–4 Shaohua Hu1,3–4
1Department of Psychiatry, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, 310003, People’s Republic of China; 2Department of Clinical Medicine, College of Medicine, Zhejiang University, Hangzhou 310003, People’s Republic of China; 3Brain Research Institute of Zhejiang University, Hangzhou 310003, People’s Republic of China; 4Key Laboratory of Mental Disorder Management in Zhejiang Province, Hangzhou, 310003, People’s Republic of China
Abstract: The neuropeptides oxytocin (OT) and vasopressin (VP) are hormones that are known to mediate social behavior and cognition, but their influence may be sex-dependent. This paper aims to provide a comprehensive review of the sex-related influence of OT and VP on social cognition, focusing on partner preference and sexual orientation, trust and relevant behaviors, memory modulation, and emotion regulation. Most studies have suggested that OT facilitates familiar-partner preference in both sexes, with females being more significant, increased trust in others, especially for male, enhanced memory in either sex, and reduced anxious emotion in males. However, VP-regulated social cognition has been less studied. Other relevant studies have indicated that VP facilitated familiar-partner preference, improved memory, induced empathy formation, increased positive-emotion recognition, and induced anxiety without any sex difference. However, there was a male preponderance among studies, and results were often too complex to draw firm conclusions. Clarifying the interplay between OT/VP and sex hormones in the regulation of social cognition is necessary for further applications.
Keywords: oxytocin, vasopressin, sexual difference, social cognition, social behavior
Introduction
Oxytocin (OT) and vasopressin (VP) are nonpeptide hormones. They differ by two amino acids, the third and the eighth amino acids, which are isoleucine and leucine for VP, and phenylalanine and arginine for OT, respectively. However, they have major physiological differences when acting on the central nervous system (CNS) and peripheral tissue. In peripheral tissue, OT facilitates lactation and uterine contraction during the perinatal period,1 while VP plays an antidiuretic role.2 In the CNS, OT and VP shape sex-based social cognition depending on their concentration and the variable expression of receptors in different brain regions. Several aspects of sex-based social cognition have been explored, including partner preference, trust and relevant behaviors, memory modulation, and emotion regulation. Some have shown salient sex-based differences, while others have not. In regard to the different aspects of social cognition, OT and VP have variable effects. Both OT and VP are archaic and highly conserved peptides across placental mammals, and their homologues can even be found in invertebrates.3
This review first introduces the conception of social cognition, OT, VP, and their receptor systems, then summarizes the animal models, intervention methods of OT and VP, and interplay between OT, VP, and sex hormones in different studies, and finally focuses on the role of OT and VP in the regulation of social cognition.
Concept of social cognition
Social cognition is the ability that people or social animal process, store, and apply information about conspecifics, other specifics, and social stimuli. Assessing social cognition is a key to clarifying social psychology. The major concerns of social cognition include the sensing, coding, integration, and memory of social stimuli, affective effects on information processing, and behavioral outcomes of cognitive processes.4 While social cognition is usually influenced by one’s cultural upbringing,5 at an individual level social cognition is also closely related to neurobiological homeostasis. Changing this homeostasis may affect various aspects of cognitive performance. Anatomic injuries of brain tissue,6 novel activation of neuronal circuits, and disruption of neurotransmitters can also influence cognition. It is well known that OT and VP are brain neuropeptides that modulate social cognition.
OT, VP, and their receptor systems
OT is produced by the magnocellular neurosecretory cells of the supraoptic nucleus (SON) and paraventricular nucleus (PVN) of the hypothalamus and stored in the posterior pituitary.7 OT is released into blood as a hormone from the posterior pituitary. In the PVN, other neurons project to the spinal cord and other parts of the brain.8 OT binds both peripherally and centrally to the OT receptors (OTRs). In the peripheral nervous system, OT promotes lactation and uterine contraction during parturition. In the CNS, OTRs are expressed throughout the brain.9 Hypothalamic OT neurons project axons to other brain regions and deliver OT for specific brain activities.10 OTRs are not expressed evenly across the whole brain and show distinct differences across species, populations, life stages, and sexes,11–15 which contributes to distinct social behaviors.16
In addition to magnocellular neurosecretory cells of the PVN and SON, VP is also synthesized by parvocellular neurosecretory neurons of the PVN. VP produced by magnocellular neurosecretory cells is stored in the posterior pituitary and later released into blood, where it acts as an antidiuretic hormone. VP produced by parvocellular neurosecretory neurons of the PVN is transported finally to the anterior pituitary, and is involved in the hypothalamic–pituitary–adrenal axis. In addition, the bed nucleus of the stria terminalis (BNST) and the medial amygdala are extrahypothalamic sources of VP.17 VP receptors are classified into three subtypes: V1a receptors (V1aRs), V1bRs, and V2Rs.17 The majority of VP receptors in the brain are V1aRs, while the second-most common are V1bRs. However, V2Rs are primarily expressed in the renal collecting ducts, and have scarce expression in the brain.17 As such, VP regulates social cognition, mainly through V1aR and V1bR.
Animal models
The prairie vole (Microtus ochrogaster) is a socially monogamous rodent, and is the premier rodent model for pair bonding.18 Monogamous relationships are expressed as pair bonding, and belong to the category of social cognition. Monogamous animals usually form and maintain partner preference, which is considered one index of pair bonding.19 The mandarin vole is another monogamous rodent,20 and is also chosen as a primary animal model for pair-bonding studies. This kind of pair bonding usually refers to a relationship between opposite-sex pairs. To study the effect of OT on same-sex partner preference, the meadow vole is the preferred choice. The meadow vole is socially promiscuous, and its gonadal hormones are less secreted during winter, which is appropriate for nonreproductive social behavior research. In a winter day–length laboratory, female meadow voles have smaller uteri than those in summer day–length environments. The females can form partner preference for either same- or opposite-sex cage mates.21–23 Laboratory rats usually include the Wistar rat, Long–Evans rat, Sprague Dawley rat, biobreeding rat, Brattleboro rat, hairless rat, Lewis rat, Royal College of Surgeons rat, shaking rat Kawasaki, Zucker rat, and knockout rat. This review includes studies using Wistar and Sprague Dawley rats as animal models, since they are used in OT-related memory-modulation tests. Other included animal models that are less used in studies, such as marmosets and macaques, are also listed in Table 1(OT) and Table 2(VP).
 | Table 1 Animal models and species, doses, routes, and effects of OT administration |
 | Table 2 Animal models and species, routes, doses, and effects of VP administration |
Intervention methods of OT and VP
Methods of intervention covered by this review (see Tables 1 and 2) include intracranial injection (intracerebroventricular injection), intranasal spray, subcutaneous injection, and intraperitoneal injection for rodents and nasal administration for marmosets and humans. Of note, increased OT concentration in the CNS can also be observed after intranasal administration of OT.24,25
Interplay between OT/VP and sex hormones
Androgen and estrogen exist in both males and females. These CNS sex hormones indirectly modulate sexual dimorphism in social cognition through influencing OT, VP, and their receptors through discrepant gene expression. For example, the estrogen and androgen metabolites 3β-diol upregulate OT gene expression in the PVN via ERβ.26 Estrogen regulates OTR gene expression in the medial amygdala through ERα27 and increases OT expression in PVN.28 OT mediates social cognition in both male and female, but is more prominent in females. However, the expression of VP seems to be mainly dependent on androgen, which is more abundant in the brains of males than in females.29 As a result, VP seems mainly to mediate social cognition in males.29
Sexually dimorphic influence of OT and VP on social cognition
Partner preference and sexual orientation
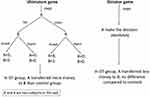
In monogamous animals, OT and VP are the only neuropeptides that have been found to mediate partner preference. The partner-preference performance regulated by OT and VP in monogamous animals reflects the mechanism of sexual orientation in humans and underlies the basis of fidelity. The effects of OT and VP on males and females are discussed separately herein (Figure 1). The previously mentioned monogamous rodents — prairie voles and mandarin voles — and marmosets were used in the following studies. Animal models, intervention means, OT/VP dosage, and assessment methods in this field are listed in Tables 1 and 2.
 | Figure 1 Influence of oxytocin (OT) and vasopressin (VP) on partner preference. |
Rodents or humans under temporary treatment with OT show a significant preference for familiar mates or objects in both sexes. This effect is not dependent on the intervention means and is more significant in females. Facing opposite-sex cage mates, prairie voles exhibit familiar-partner preference in both males30–32 and females19,33,34with OT injections intracranially. Injecting the selective OTR antagonist d(CH)(Tyr[Me]Thr4,Try-NHi into the left lateral ventricle blocks this effect.19,34 Similar effects of OT can be found in male and female mandarin voles,20 although this effect tended to be more prominent in females.35,36 A study of prairie voles showed similar results in females, but not males, when OT was injected subcutaneously.35 With marmosets as a model, intranasal OT resulted in females spending more time in close contact with partners and males spending less time with both partners and strangers.37 The results were consistent with other methods to increase OT in the CNS substantially. For example, with viral vector gene transfer to overexpress OTRs in the nucleus accumbens of female prairie voles during prepuberty, they showed accelerated partner-preference formation in adulthood.38 When the choice was between two same-sex cage mates, both male and female voles chose the familiar partner,39,40 but this was more critical for females. For instance, following a 21-day continuous intervention of low-dosage (0.08 IU/kg) and medium-dosage (0.8 IU/kg) OT, male prairie voles showed significant impairment of same-sex partner preference, while high dosage (8 IU/kg)–treated males and all females were unaffected.41 This indicated that the familiar-partner preference is maybe dose dependent for males. Interestingly, for monogamous rodents, this effect not only occurred in conspecifics but was also seen in object preference. Centrally OT-treated female rats preferred a familiar object to a novel one.42 According to these results, OT accelerates familiar-partner preference regardless of partner sex, although this was more obvious in females. The brain regions involvedare possibly different in females and males: nucleus accumbens in females,31,38,43 and laterodorsal thalamic nucleus, lateral septum, posterior cingulate nucleus, ventroposterior thalamic nucleus,44 and dorsomedial prefrontal cortex45 in males.
VP enhances partner preference in both sexes (Figure 1), primarily by activating V1aR in the ventral pallidum.46,47 Overexpression of V1aR in the ventral pallidum facilitates partner preference not only in monogamous male voles48,49 but also in socially promiscuous male voles.48 When using a V1aR antagonist or downregulating the activity of V1aR, there is weakened partner preference in adult voles of either sex.50–52 High-dose VP (80 IU) intervention through nasal mucosa for adult male monkeys led to frequent partner contact.53 The partner-preference tests mentioned thus far were all performed between opposite-sex cage mates. As previously reported in other studies, VP appears to be more critical for male social cognition regulation.36
Both OT and VP are involved in the regulation of partner preference and facilitate the preference for familiar ones, although OT appears more important. More interestingly, this familiarity preference occurs not just between opposite-sex conspecifics but also between same-sex conspecifics and preference for objects. This phenomenon may be implicated in homosexuality. Future research into OT, VP, OTRs, and V1aR gene-expression levels in postmortem homosexual brains may be useful.
Memory modulation
Animal and human studies on the relationship between OT/VP and memory have mainly been done in males, although several studies have included nonpregnant females. Females experience large hormonal fluctuations during the perinatal period, so studies on memory impairment in pregnant women are usually excluded.
OT has positive effects on social memory in either sex, but is more significant in males. Acute OT treatment increases working memory in male macaques, but not females,54 and improves stress-induced memory impairment in both male and female Wistar rats.55 Another study found that OT prevented stress-induced hippocampal memory and plasticity impairment in male Sprague Dawley rats.56 Human studies have found that intranasal OT helps encoding and retrieval of recognition memory of negative social stimuli57 and recall of social affiliation memories in men.58 However, there have also been contrary results, eg, intranasally OT-treated men performed worse than a placebo group in visual object recall.59 It should be noted that there is variable study design in this field, and future series-designed studies should aim to clarify the phenomena, functional/histological changes in the CNS and molecular mechanisms of how OT influences memory in either sex.
Studies have shown that VP improves memory, mainly via V1aR.60–63 VP acts on different brain areas, including the lateral septum62 and hippocampus.64 Other studies have shown that VP4–9,65 the main active metabolite of VP1–9, and its derivative (VP4–8),66 have effects on memory modulation. Most studies have been performed only in males, and those that included both sexes did not find any significant sex difference. Memory evaluated in these studies included object-recognition memory,60 social recognition memory,67 short-term memory, long-term memory,66 and spatial memory.63,68,69 In general, studies found that men have better spatial sensibility than women, such as better direction-sense and geometric mathematical scores. This may well contribute to the spatial memory enhancement effect of VP. As previously mentioned, VP is more critical in males in the regulation of social cognition. To obtain more conclusive evidence, normatively designed experiments are needed in future.
Emotion regulation
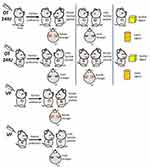
OT and VP are also involved in the regulation of emotion, including anxiety, happiness, and anger (Figure 2).
 | Figure 2 Influence of oxytocin (OT) and vasopressin (VP) on emotion in both sexes. |
OT reduces anxious emotion, which appears more prominent in males, although studies have used male rodents more frequently than females. CNS injection of OT into the hypothalamic PVN and periamygdala regions was found to decrease anxiety and amygdala activity in males.70,71 When OT was infused into prelimbic region of the medial prefrontal cortex intracerebroventricularly, anxious mood in females also remitted.72,73 Of note, dosage of OT and treatment course modulated the study outcome. Chronic high-dose (10 ng/h for 15 days) intervention led to the development of an anxiogenic phenotype, while a low dose (1 ng/h for 19 days) prevented hyperanxiety in male mice.71 The results of human studies using variable intervention methods are listed in Table 1. In general, high OT levels in the CNS are associated with lower anxiety in men, but not in women.
Studies of OT modulation of happiness and anger in humans are listed in detail in Table 1.
VP also appears to facilitate empathy formation, positive-emotion recognition, and anxiety (Figure 2) without any sex differences. It should be noted that V1aR,74,75 V1bR,76,77 and many other brain regions are involved in emotion regulation. Relevant studies and results are listed in Table 2.
In general, OT reduces anxious mood, which is more significant in males. OT also reduces perception of anger and increases perception of happiness in males, but facilitates the perception of anger in women. On the other hand, VP increases anxious feelings in both sexes, promotes the formation of empathy, and enhances the perception of positive emotions in either sex. However, its relationship with anger/happiness perception remains unclear (Figure 2). Men with high OT levels in the CNS appear more relaxed, insensitive, and optimistic, while pregnant women during their perinatal period seem to be more sensitive to others’ emotions, especially anger. This change in women may be related to the sudden release of large amounts of intrapartum OT, and may be involved in the onset of postpartum depression.78
Implications for human studies
Human studies in this section explore how partner preference and trust are regulated by OT and VP. Human studies involve OT and VP being given by intranasal spray.
Partner preference
OT promotes human familiar-partner preference in ways analogous to monogamous rodents, although the preferred partner is not limited to the opposite sex.79 After nasal administration of OT, men in a monogamous relationship preferred keeping a greater distance from an attractive female encounter,80 while keeping a closer contact with a male friend.45 To date, only one trial has been performed on homosexual men, where intranasal OT was associated with being easily attracted by men’s faces, but not by facial expressions.81 No study on homosexual women was found.
Trust and related behaviors
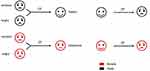
High OT levels in the CNS help to increase trust among people. Means of reflecting trust in these trials include the ultimatum game, dictator game, prisoner’s dilemma, envelope task, and monetary game.
A study on the ultimatum and dictator games included only male adults. In the ultimatum game, the OT-treated decision-maker split more money to the other group member than controls. In the dictator game, the OT-treated decision-maker transferred less money to the other member compared to the ultimatum game, and no significant difference was found in comparison with controls.82 OT may help to maintain an optimal balance between loss and benefit. The decision-maker seemed to be more generous in the ultimatum game. OT appears to regulate trust-related behaviors in a context-dependent way. In the ultimatum game, the two members kept a gambling relationship, while in the dictator game the decision-maker owned full discretion. Differences between the ultimatum game and dictator game are displayed in Figure 3.
There have been several studies using the prisoner’s dilemma, envelope task (Figure 4),83 and monetary game (Figure 5)84 to research trust-related behaviors. The study designs and OT-detection means were similar, and validity criteria were controversial. Overall, OT contributes to increased feelings of trustworthiness in men. Few studies on women and on how VP influences trust-related behaviors have been done.
Discussion
The results of this review support the notion that OT can facilitate familiar-partner preference, increase trust, enhance memory, and reduce anxious emotion. There were sex differences on the effects of OT on partner preference, trust, memory, and anxious emotion, with the first more significant in females and the latter three more obvious in males. On the other hand, VP can facilitate familiar-partner preference, improve memory, induce empathy formation, increase positive-emotion recognition, and induce anxiety without any sex difference. In addition, as verified by many trials, OT regulates a series of social cognitions. However, there is significant heterogeneity across studies in terms of the type of animal model and behavioral paradigm used.
Centrally increased OT-facilitated partner-preference formation is achieved by increasing the contact time between OT-treated rodents and their same- or opposite-sex partner during cohabitation. Cntrally increased OT in neonatal rodents reduced Fos expression in the SON, PVN, lateral septal nucleus, medial preoptic area, BNST, ventromedial nucleus of the hypothalamus, mediodorsal thalamic nucleus, the central amygdaloid nucleus, and medial amygdaloid nucleus (MeA) in females, but decreased in the PVN, BNST, and mediodorsal thalamic nucleus and increased in the medial preoptic area, central amygdaloid nucleus, and lateral septal nucleus in males.20 Fos in these studies was used as a marker of neural responses to social stimuli. These results indicated that neonatal manipulations of central OT changed neural activity of specific brain regions. OTRs mediated neural activity. Activation of OTRs in anteromedial BNST of female mice resulted in vigilance responses which promotes avoidance of unfamiliar social stimuli.85 In gonadectomized male mice, exogenous estrogen significantly increased OT mRNA transcription and decreased VP mRNA transcription in the PVN of wild-type mice, while in ERβ-knockout mice, these effects disappeared completely.28 In ovariectomized female rats, acute estradiol administration significantly decreased VP immunoreactivity in the SON and PVN after 24 hours.86 Fetal exposure to higher testosterone resulted in a decrease in OT mRNA expression in the SON and PVN and an increase of VP mRNA expression in the SON and suprachiasmatic nuclei in adult rats, without sex differences.87 Under physiological conditions, females secrete higher levels of estrogen than males, while males secrete more testosterone than females. Therefore, we infer that OT is more expressed and VP less expressed in females than in males in the PVN. OT is less synthesized and VP more synthesized in males. This demonstrates why it was more significant for females that OT facilitated familiar-partner preference than for males. Interactions between OT/VP and sex hormones are potential mechanisms involved in homosexuality.
OT-expression levels in females fluctuate with plasma-estrogen concentration across the estrous cycle.88 This could be the reason that human subjects or animals included in the studies of memory were males. The majority of study results found that both OT and VP improved memory. As mentioned, estrogen increased OT levels more significantly in female brains, including in the MeA. Studies also found that gene expression of OTRs in the MeA region was essential to maintain normal social recognition in females.89 Therefore, it is likely that OT improves memory in nonpregnant females. Gonadotropin-releasing hormone receptors were also expressed in brain regions such as the hippocampus and amygdala.90 Chronic gonadotropin-releasing hormone–agonist application in male sheep was shown to facilitate long-term spatial memory retention.90 However, for older men who had low testosterone levels and memory impairment due to aging, treatment of poor memory with testosterone for 1 year was ineffective.91 As previously mentioned, testosterone increases VP expression in male brain regions, and our review concludes that VP may improve memory in males. However, various types of memory should be researched separately.
Estrogen deficiency led to anxiety- and depression-like behavior and inflammation in hippocampi in mice through NLRP3 signaling.92 In effect, estrogen exerted its effects on anxiety in discrepant ways, which depended on the ER subtypes: ERα was generally anxiogenic, ERβ was generally anxiolytic, and GPR30 was found both to decrease and increase anxious behaviors.93 OT-expression levels are higher in females, due to the higher level of estrogen; however, our review found that the anxiolytic effect was more significant in males. This may well be due to the discrepant expression of ER subtypes between males and females. Previous studies on how testosterone influences anxiety were mixed, with some not finding an anyanxiolytic effect,94 while others did.95 Our review suggests that VP may induce anxiety in both sexes. While VP-expression level was positively correlated with testosterone, the current findings cannot explain why VP enhances anxiety in both sexes from the testosterone-expression viewpoint.
In summary, OT and VP appear to modulate social cognition via different mechanisms, many of which have interplay with sex hormones. These findings can inform future research on psychological and clinical applications.
Acknowledgments
We acknowledge Professor Chee H Ng from the Department of Psychiatry, University of Melbourne for English-language editing. This work was supported by grants from the Public Welfare Project of the Science Technology Department of Zhejiang Province (2015C33133), National Natural Science Foundation of China (81671357), National Clinical Research Center for Mental Health Disorders (2015BAI13B02), Key Research Project of Zhejiang Province (2015C03040), and National Key Research and Development Program (2016YFC1307100).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Higuchi T, Okere CO. Role of the supraoptic nucleus in regulation of parturition and milk ejection revisited. Microsc Res Tech. 2002;56(2):113–121.
2. Ivanova LN. [Vasopressin: molecular mechanisms of antidiuretic effect]. Rossiiskii fiziologicheskii zhurnal imeni IM Sechenova. 2011;97(3):235–262.
3. Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science (New York, NY). 2008;322(5903):900–904. doi:10.1126/science.1158668
4. Ebert A, Brune M. Oxytocin and social cognition. Curr Top Behav Neurosci. 2018;35:375–388. doi:10.1007/7854_2017_21
5. Nisbett RE, Peng K, Choi I, Norenzayan A. Culture and systems of thought: holistic versus analytic cognition. Psychol Rev. 2001;108(2):291–310.
6. Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34(4):247–261.
7. Eisenberg Y, Murad S, Casagrande A, et al. Oxytocin alterations and neurocognitive domains in patients with hypopituitarism. Pituitary. 2019. doi:10.1007/s11102-019-00936-0
8. Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3–4):150–176. doi:10.1016/j.yfrne.2004.05.001
9. Busnelli M, Chini B. Molecular basis of oxytocin receptor signalling in the brain: what we know and what we need to know. Curr Top Behav Neurosci. 2018;35:3–29. doi:10.1007/7854_2017_6
10. Knobloch HS, Charlet A, Hoffmann LC, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi:10.1016/j.neuron.2011.11.030
11. Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct. 2017;222(2):981–1006. doi:10.1007/s00429-016-1260-7
12. Vaidyanathan R, Hammock EA. Oxytocin receptor dynamics in the brain across development and species. Dev Neurobiol. 2017;77(2):143–157. doi:10.1002/dneu.22403
13. Olazabal DE. Comparative analysis of oxytocin receptor density in the nucleus accumbens: an adaptation for female and male alloparental care? J Physiol Paris. 2014;108(2–3):213–220. doi:10.1016/j.jphysparis.2014.10.002
14. Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi:10.1016/S0079-6123(08)00401-9
15. Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12(12):1145–1148.
16. Mooney SJ, Coen CW, Holmes MM, Beery AK. Region-specific associations between sex, social status, and oxytocin receptor density in the brains of eusocial rodents. Neuroscience. 2015;303:261–269. doi:10.1016/j.neuroscience.2015.06.043
17. Frank E, Landgraf R. The vasopressin system–from antidiuresis to psychopathology. Eur J Pharmacol. 2008;583(2–3):226–242. doi:10.1016/j.ejphar.2007.11.063
18. Carter CS, Getz LL. Monogamy and the prairie vole. Sci Am. 1993;268(6):100–106.
19. Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J Neuroendocrinol. 1994;6(3):247–250.
20. Jia R, Tai F, An S, Broders H, Sun R. Neonatal manipulation of oxytocin influences the partner preference in mandarin voles (Microtus mandarinus). Neuropeptides. 2008;42(5–6):525–533. doi:10.1016/j.npep.2008.06.001
21. Beery AK, Loo TJ, Zucker I. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Horm Behav. 2008;54(1):153–159. doi:10.1016/j.yhbeh.2008.02.007
22. Parker KJ, Lee TM. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J Comp Psychol (Washington, DC: 1983). 2003;117(3):283–289. doi:10.1037/0735-7036.117.3.283
23. Beery AK, Routman DM, Zucker I. Same-sex social behavior in meadow voles: multiple and rapid formation of attachments. Physiol Behav. 2009;97(1):52–57. doi:10.1016/j.physbeh.2009.01.020
24. Dal Monte O, Noble PL, Turchi J, Cummins A, Bb A. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One. 2014;9(8):e103677. doi:10.1371/journal.pone.0103677
25. Pietrowsky R, Struben C, Molle M, Fehm HL, Born J. Brain potential changes after intranasal vs. intravenous administration of vasopressin: evidence for a direct nose-brain pathway for peptide effects in humans. Biol Psychiatry. 1996;39(5):332–340. doi:10.1016/0006-3223(95)00180-8
26. Hiroi R, Lacagnina AF, Hinds LR, Carbone DG, Uht RM, Handa RJ. The androgen metabolite, 5α-androstane-3β,17β-diol (3β-diol), activates the oxytocin promoter through an estrogen receptor-β pathway. Endocrinology. 2013;154(5):1802–1812. doi:10.1210/en.2012-2253
27. Choleris E, Ogawa S, Kavaliers M, et al. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5(7):528–539. doi:10.1111/j.1601-183X.2006.00203.x
28. Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;109(1–2):84–94.
29. Bluthe RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18(4):323–335.
30. Johnson ZV, Walum H, Jamal YA, et al. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav. 2016;79:8–17. doi:10.1016/j.yhbeh.2015.11.011
31. Duclot F, Wang H, Youssef C, Liu Y, Wang Z, Trichostatin KM. A (TSA) facilitates formation of partner preference in male prairie voles (Microtus ochrogaster). Horm Behav. 2016;81:68–73. doi:10.1016/j.yhbeh.2016.04.001
32. Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster). Behav Neurosci. 2003;117(4):854–859.
33. Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci. 1992;652:487–489.
34. Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109(4):782–789.
35. Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37(1):49–56. doi:10.1006/hbeh.1999.1558
36. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi:10.1038/nn1327
37. Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology. 2014;49:1–10. doi:10.1016/j.psyneuen.2014.06.020
38. Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60(5):498–504. doi:10.1016/j.yhbeh.2011.07.018
39. Triana-Del Rio R, Tecamachaltzi-Silvaran MB, Diaz-Estrada VX, et al. Conditioned same-sex partner preference in male rats is facilitated by oxytocin and dopamine: effect on sexually dimorphic brain nuclei. Behav Brain Res. 2015;283:69–77. doi:10.1016/j.bbr.2015.01.019
40. Beery AK, Zucker I. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience. 2010;169(2):665–673. doi:10.1016/j.neuroscience.2010.05.023
41. Kl B, Am P, Og C, et al. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74(3):180–188. doi:10.1016/j.biopsych.2012.08.025
42. Madularu D, Athanassiou M, Yee JR, Mumby DG. Centrally-administered oxytocin promotes preference for familiar objects at a short delay in ovariectomized female rats. Behav Brain Res. 2014;274:164–167. doi:10.1016/j.bbr.2014.08.015
43. Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–138. doi:10.1006/hbeh.2001.1691
44. Cushing BS, Martin JO, Young LJ, Carter CS. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Horm Behav. 2001;39(1):48–58. doi:10.1006/hbeh.2000.1633
45. Cohen D, Perry A, Gilam G, et al. The role of oxytocin in modulating interpersonal space: a pharmacological fMRI study. Psychoneuroendocrinology. 2017;76:77–83. doi:10.1016/j.psyneuen.2016.10.021
46. Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125(1):35–45. doi:10.1016/j.neuroscience.2003.12.008
47. Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi:10.1038/365545a0
48. Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429(6993):754–757. doi:10.1038/nature02539
49. Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21(18):7392–7396.
50. Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci. 1999;113(5):1071–1079.
51. Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav. 2013;63(3):518–526. doi:10.1016/j.yhbeh.2013.01.005
52. Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124(1):159–163. doi:10.1037/a0018094
53. Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 2011;10(3):375–383. doi:10.1111/j.1601-183X.2010.00677.x
54. Simpson EA, Paukner A, Sclafani V, Kaburu SS, Suomi SJ, Ferrari PF. Acute oxytocin improves memory and gaze following in male but not female nursery-reared infant macaques. Psychopharmacology. 2017;234(3):497–506. doi:10.1007/s00213-016-4480-x
55. Dayi A, Cetin F, Sisman AR, et al. The effects of oxytocin on cognitive defect caused by chronic restraint stress applied to adolescent rats and on hippocampal VEGF and BDNF levels. Med sci monit. 2015;21:69–75. doi:10.12659/MSM.893159
56. Lee SY, Park SH, Chung C, Kim JJ, Choi SY, Han JS. Oxytocin protects hippocampal memory and plasticity from uncontrollable stress. Sci Rep. 2015;5:18540. doi:10.1038/srep18540
57. Weigand A, Feeser M, Gartner M, et al. Effects of intranasal oxytocin prior to encoding and retrieval on recognition memory. Psychopharmacology. 2013;227(2):321–329. doi:10.1007/s00213-012-2962-z
58. Cardoso C, Orlando MA, Brown CA, Ellenbogen MA. Oxytocin and enhancement of the positive valence of social affiliation memories: an autobiographical memory study. Soc Neurosci. 2014;9(2):186–195. doi:10.1080/17470919.2013.873079
59. Herzmann G, Young B, Bird CW, Curran T. Oxytocin can impair memory for social and non-social visual objects: a within-subject investigation of oxytocin‘s effects on human memory. Brain Res. 2012;1451:65–73. doi:10.1016/j.brainres.2012.02.049
60. Barsegyan A, Atsak P, Hornberger WB, Jacobson PB, van Gaalen MM, Roozendaal B. The vasopressin 1b receptor antagonist A-988315 blocks stress effects on the retrieval of object-recognition memory. Neuropsychopharmacol. 2015;40(8):1979–1989. doi:10.1038/npp.2015.48
61. Hicks C, Ramos L, Reekie TA, Narlawar R, Kassiou M, McGregor IS. WAY 267,464, a non-peptide oxytocin receptor agonist, impairs social recognition memory in rats through a vasopressin 1A receptor antagonist action. Psychopharmacology. 2015;232(15):2659–2667. doi:10.1007/s00213-015-3902-5
62. Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav. 2012;61(1):50–56. doi:10.1016/j.yhbeh.2011.10.002
63. Mishima K, Tsukikawa H, Inada K, et al. Ameliorative effect of vasopressin-(4-9) through vasopressin V(1A) receptor on scopolamine-induced impairments of rat spatial memory in the eight-arm radial maze. Eur J Pharmacol. 2001;427(1):43–52.
64. Alescio-Lautier B, Paban V, Soumireu-Mourat B. Neuromodulation of memory in the hippocampus by vasopressin. Eur J Pharmacol. 2000;405(1–3):63–72.
65. Dietrich A, Allen JD. Vasopressin and memory. I. The vasopressin analogue AVP4-9 enhances working memory as well as reference memory in the radial arm maze. Behav Brain Res. 1997;87(2):195–200.
66. Vawter MP, De Wied D, Van Ree JM. Vasopressin fragment, AVP-(4-8), improves long-term and short-term memory in the hole board search task. Neuropeptides. 1997;31(5):489–494.
67. Shahar-Gold H, Gur R, Wagner S. Rapid and reversible impairments of short- and long-term social recognition memory are caused by acute isolation of adult rats via distinct mechanisms. PLoS One. 2013;8(5):e65085. doi:10.1371/journal.pone.0065085
68. Pan YF, Chen XR, Wu MN, Ma CG, Qi JS. Arginine vasopressin prevents against Abeta(25-35)-induced impairment of spatial learning and memory in rats. Horm Behav. 2010;57(4–5):448–454. doi:10.1016/j.yhbeh.2010.01.015
69. Aarde SM, Jentsch JD. Haploinsufficiency of the arginine-vasopressin gene is associated with poor spatial working memory performance in rats. Horm Behav. 2006;49(4):501–508. doi:10.1016/j.yhbeh.2005.11.002
70. Blume A, Bosch OJ, Miklos S, et al. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27(8):1947–1956. doi:10.1111/j.1460-9568.2008.06184.x
71. Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. doi:10.1016/j.psyneuen.2014.01.021
72. Sabihi S, Durosko NE, Dong SM, Leuner B. Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology. 2014;45:31–42. doi:10.1016/j.psyneuen.2014.03.009
73. de Jong TR, Beiderbeck DI, Neumann ID, Kalueff AV. Measuring virgin female aggression in the female intruder test (FIT): effects of oxytocin, estrous cycle, and anxiety. PLoS One. 2014;9(3):e91701. doi:10.1371/journal.pone.0091701
74. Uzefovsky F, Shalev I, Israel S, et al. Oxytocin receptor and vasopressin receptor 1a genes are respectively associated with emotional and cognitive empathy. Horm Behav. 2015;67:60–65. doi:10.1016/j.yhbeh.2014.11.007
75. Bayerl DS, Honig JN, Bosch OJ. Vasopressin V1a, but not V1b, receptors within the PVN of lactating rats mediate maternal care and anxiety-related behaviour. Behav Brain Res. 2016;305:18–22. doi:10.1016/j.bbr.2016.02.020
76. Wu N, Shang S, Su Y. The arginine vasopressin V1b receptor gene and prosociality: mediation role of emotional empathy. PsyCh J. 2015;4(3):160–165. doi:10.1002/pchj.102
77. Hodgson RA, Mullins D, Lu SX, et al. Characterization of a novel vasopressin V1b receptor antagonist, V1B-30N, in animal models of anxiety-like and depression-like behavior. Eur J Pharmacol. 2014;730:157–163. doi:10.1016/j.ejphar.2014.02.027
78. Gu V, Feeley N, Gold I, et al. Intrapartum synthetic oxytocin and its effects on maternal well-being at 2 months postpartum. Birth (Berkeley, Calif). 2016;43(1):28–35. doi:10.1111/birt.12198
79. Liu JC, Guastella AJ, Dadds MR. Exploring the role of intra-nasal oxytocin on the partner preference effect in humans. Psychoneuroendocrinology. 2013;38(4):587–591. doi:10.1016/j.psyneuen.2012.07.009
80. Scheele D, Striepens N, Gunturkun O, et al. Oxytocin modulates social distance between males and females. J Neurosci. 2012;32(46):16074–16079. doi:10.1523/JNEUROSCI.2755-12.2012
81. Thienel M, Heinrichs M, Fischer S, Ott V, Born J, Hallschmid M. Oxytocin‘s impact on social face processing is stronger in homosexual than heterosexual men. Psychoneuroendocrinology. 2014;39:194–203. doi:10.1016/j.psyneuen.2013.09.013
82. Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2(11):e1128. doi:10.1371/journal.pone.0001128
83. Mikolajczak M, Pinon N, Lane A, de Timary P, Luminet O. Oxytocin not only increases trust when money is at stake, but also when confidential information is in the balance. Biol Psychol. 2010;85(1):182–184. doi:10.1016/j.biopsycho.2010.05.010
84. Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi:10.1038/nature03701
85. Duque-Wilckens N, Steinman MQ, Busnelli M, et al. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol Psychiatry. 2018;83(3):203–213. doi:10.1016/j.biopsych.2017.08.024
86. Lagunas N, Marraudino M, de Amorim M, et al. Estrogen receptor beta and G protein-coupled estrogen receptor 1 are involved in the acute estrogenic regulation of arginine-vasopressin immunoreactive levels in the supraoptic and paraventricular hypothalamic nuclei of female rats. Brain Res. 2019. doi:10.1016/j.brainres.2019.02.002
87. Dzirbikova Z, Talarovicova A, Stefanik P, Olexova L, Krskova L. Testosterone enhancement during pregnancy influences social coping and gene expression of oxytocin and vasopressin in the brain of adult rats. Acta Neurobiol Exp (Wars). 2018;78(3):264–270.
88. Sarkar DK, Frautschy SA, Mitsugi N. Pituitary portal plasma levels of oxytocin during the estrous cycle, lactation, and hyperprolactinemia. Ann N Y Acad Sci. 1992;652:397–410.
89. Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci U S A. 2007;104(11):4670–4675. doi:10.1073/pnas.0700670104
90. Hough D, Bellingham M, Haraldsen IRH, et al. Spatial memory is impaired by peripubertal GnRH agonist treatment and testosterone replacement in sheep. Psychoneuroendocrinology. 2017;75:173–182. doi:10.1016/j.psyneuen.2016.10.016
91. Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. Jama. 2017;317(7):717–727. doi:10.1001/jama.2016.21044
92. Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 2016;56:175–186. doi:10.1016/j.bbi.2016.02.022
93. Borrow AP, Handa RJ. Estrogen receptors modulation of anxiety-like behavior. Vitam Horm. 2017;103:27–52. doi:10.1016/bs.vh.2016.08.004
94. Filova B, Malinova M, Babickova J, et al. Effects of testosterone and estradiol on anxiety and depressive-like behavior via a non-genomic pathway. Neurosci Bull. 2015;31(3):288–296. doi:10.1007/s12264-014-1510-8
95. McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. 2014;35(1):42–57. doi:10.1016/j.yfrne.2013.09.001
96. Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76(4):281–288. doi:10.1016/j.biopsych.2013.09.017
97. Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2013;18(9):958–960. doi:10.1038/mp.2012.156
98. Chen X, Hackett PD, DeMarco AC, et al. Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging Behav. 2016;10(2):581–593. doi:10.1007/s11682-015-9411-7
99. Domes G, Steiner A, Porges SW, Heinrichs M. Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology. 2013;38(7):1198–1202. doi:10.1016/j.psyneuen.2012.10.002
100. Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: oxytocin effects on response to social stress in men and women. Biol Psychol. 2012;90(1):1–9. doi:10.1016/j.biopsycho.2012.02.010
101. Domes G, Normann C, Heinrichs M. The effect of oxytocin on attention to angry and happy faces in chronic depression. BMC Psychiatry. 2016;16:92. doi:10.1186/s12888-016-0794-9
102. Evans S, Shergill SS, Averbeck BB. Oxytocin decreases aversion to angry faces in an associative learning task. Neuropsychopharmacol. 2010;35(13):2502–2509. doi:10.1038/npp.2010.110
103. Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M. Intranasal oxytocin increases covert attention to positive social cues. Psychol Med. 2013;43(8):1747–1753. doi:10.1017/S0033291712002565
104. Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry. 2010;67(12):1220–1222. doi:10.1016/j.biopsych.2010.03.014
105. Uzefovsky F, Shalev I, Israel S, Knafo A, Rp E. Vasopressin selectively impairs emotion recognition in men. Psychoneuroendocrinology. 2012;37(4):576–580. doi:10.1016/j.psyneuen.2011.07.018
106. Brunnlieb C, Munte TF, Tempelmann C, Heldmann M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013;1499:29–42. doi:10.1016/j.brainres.2013.01.009
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.