Back to Journals » Clinical Interventions in Aging » Volume 11
Sex-specific predictors of left ventricular diastolic dysfunction in untreated hypertension
Authors Jaroch J, Vriz O , Bociąga Z, Driussi C, Łoboz-Rudnicka M, Rzyczkowska B, Łoboz-Grudzień K
Received 4 June 2016
Accepted for publication 12 August 2016
Published 25 October 2016 Volume 2016:11 Pages 1495—1504
DOI https://doi.org/10.2147/CIA.S114337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Joanna Jaroch,1 Olga Vriz,2 Zbigniew Bociąga,1 Caterina Driussi,2 Maria Łoboz-Rudnicka,1 Barbara Rzyczkowska,1 Krystyna Łoboz-Grudzień1,3
1Department of Cardiology, T Marciniak Hospital, Wroclaw, Poland; 2Division of Cardiology, San Antonio Hospital, San Daniele del Friuli, Udine, Italy; 3Health Science Faculty, Wroclaw Medical University, Wroclaw, Poland
Background: Little is known about the sex-specific differences in left ventricular (LV) diastolic dysfunction (DD) predictors. We hypothesized that arterial stiffness (AS) may play a different role in the etiology of LV DD in hypertensive men and postmenopausal women, acting independently from other established predictors of this condition, such as age, obesity, diabetes mellitus, LV remodeling, and systolic function.
Objectives: The aim of the study was to analyze the sex-specific differences in AS and other predictors of LV DD in men and postmenopausal women with untreated hypertension (HTN).
Patients and methods: The study included 144 patients (63 postmenopausal women and 81 men, mean age 62.7±6.7 years) with previously untreated HTN and no history of cardiovascular diseases. All patients were subjected to detailed echocardiography, vascular ultrasound, and high-resolution echotracking (eTracking) of carotid arteries.
Results: In the multivariate analysis, concomitant diabetes mellitus turned out to be an independent predictor of LV DD in women (P=0.02). In turn, two independent predictors of LV DD have been identified in men: S'-tissue Doppler-derived peak LV longitudinal systolic shortening velocity (P=0.001) and β, beta stiffness index (P=0.004).
Conclusion: There are sex differences in the predictors of LV DD in untreated HTN. In postmenopausal women, LV DD is mostly determined by diabetes, while in men, it is determined by S', reflecting LV systolic longitudinal function, and β, a parameter of AS.
Keywords: sex differences, left ventricular diastolic dysfunction, hypertension
Introduction
Hypertension is one of the major risk factors for left ventricular (LV) diastolic dysfunction (DD) and heart failure with preserved ejection fraction (HFpEF).1 Some previously documented sex-specific differences in structural and functional parameters of the heart and arteries may influence LV diastolic function in men and women.2 However, still little is known on the sex-specific differences in LV DD predictors. Arterial stiffness (AS) has been recently considered as one of the new paradigms in LV DD and HFpEF development.1,3–6 The incidence of HFpEF increases in women after menopause, although the underlying mechanisms of this phenomenon are unclear; one postulated cause is an increase in AS.7,8
We hypothesized that AS may play a different role in the etiology of LV DD in hypertensive men and postmenopausal women, acting independently from other established predictors of this condition, such as age, obesity, diabetes mellitus, and LV remodeling.
The aim of the study was to analyze the sex-specific differences in AS and other determinants of LV DD as the predictors of LV DD in postmenopausal women and men with untreated hypertension.
Patients and methods
A total of 198 consecutive patients with previously untreated hypertension and no history of cardiovascular diseases were enrolled in the study. After excluding 54 subjects due to inadequate quality of echocardiographic or echotracking data, eventually, we included 144 patients (63 postmenopausal women and 81 men, mean age 62.7±6.7 years) who were recruited from two centers (Department of Cardiology, T Marciniak Hospital, Wroclaw, Poland and Division of Cardiology, San Antonio Hospital, San Daniele del Friuli, Udine, Italy).
Hypertensives were defined as the subjects with a history of hypertension (mean duration 2.9 years) in whom sustained elevation of blood pressure (systolic pressure >140 mmHg and/or diastolic pressure >90 mmHg) was observed during at least three independent measurements taken on different days. All patients presented with grade I hypertension according to the European Society of Cardiology/European Society of Hypertension9 and had no history of previous antihypertensive treatment. Individuals with concomitant diabetes (n=52) were treated with statins (simvastatin and atorvastatin). All participants were examined by a cardiologist and were subjected to electrocardiography and echocardiography. Only individuals with normal LV systolic function, that is, ejection fraction (EF) >55%, and no evidence of cardiomyopathy, pericardial disease, or valve dysfunctions were enrolled. Subjects with evidence of ischemic heart disease (history of angina and/or myocardial infarction, Q waves on electrocardiography, and regional wall motion abnormalities on echocardiography) were not eligible for the study.
All patients were subjected to detailed echocardiography, vascular ultrasound, and high-resolution eTracking of carotid arteries.This study was approved by the Ethics Commitee of Wroclaw University of Medicine and written informed consent was sought from each participant.
Echocardiography
Detailed two-dimensional Doppler and tissue Doppler echocardiographic recordings (alpha 10; ALOKA, Tokyo, Japan) were obtained from each patient. Ventricular volumes were calculated with Teichholz method.10 LV end-diastolic volume (EDV) normalized for body surface area (BSA) served as preload index (EDV/BSA), and LV stroke volume (SV) normalized for pulse pressure (PP) was considered as a measure of total arterial compliance (SV/PP). Cardiac output (CO) was calculated using the formula: CO = SV × HR, where SV is the stroke volume and HR is the heart rate. The M-mode measurements of end-diastolic wall thickness (interventricular septum [IVS] and posterior wall [PW]) and cavity diameter (LV end-diastolic diameter [EDD]) were used to calculate LV mass (LVM) from the formula proposed by Devereux et al11 and normalized for BSA to obtain LV mass index (LVMI). Relative wall thickness (RWT) was calculated from the formula: RWT = 2PW/EDD. Left ventricular hypertrophy (LVH) was diagnosed whenever LVMI was >110 g/m2 for women or >125 g/m2 for men.
Assessment of LV systolic and diastolic function
LV systolic function was assessed on the basis of EF estimated with Teichholtz method, midwall fractional shortening obtained according to de Simone et al,12 and tissue Doppler-derived peak longitudinal systolic shortening velocity (S′) determined in apical four-chamber view at the lateral and septal mitral annulus and averaged.13 LV diastolic function was evaluated based on conventional Doppler mitral inflow and tissue Doppler of mitral annulus, in line with the recently published recommendations.14 Early (E) and late (A) velocities, E/A ratio, and E wave deceleration time (DTE) were determined on the basis of transmitral pulse wave Doppler. Tissue Doppler parameters, that is, early (e′) and late (a′) diastolic mitral annular velocities and the ratio thereof (e′/a′), were measured at septal and lateral sides of the mitral annulus and averaged. E/e′ was calculated as an index of LV filling pressure. Based on the following diagnostic criteria, patients were assigned one out of the three grades of LV dysfunction: 1) grade I, that is, mild DD, impaired relaxation: mitral E/A ratio <0.8, DTE >200 ms, e′ septal <8 cm/s, e′ lateral <10 cm/s, averaged E/e′ <8; 2) grade II, that is, moderate DD, impaired relaxation with mild to moderate elevation of LV filling pressure: mitral E/A ratio 0.8–1.5 (pseudonormal) with more than 50% decrease during the Valsalva maneuver, DTE 160–200 ms, e′ septal <8 cm/s, e′ lateral <10 cm/s, averaged E/e′ 9–12; or 3) grade III, that is, severe DD, restrictive LV filling: E/A ≥2, DTE <160 ms, averaged E/e′ >13.
Integrated assessment of arterial function
Vascular ultrasonography of the right common carotid artery was performed with an alpha 10 ALOKA device equipped with an integrated and automated ultrasonographic, Doppler, and high-resolution echotracking system. After clear visualization of the intima–media complex of both anterior and posterior arterial walls in its longitudinal axis with maximal internal diameter, an echotracking sample was positioned at the end of the intima, with 1 kHz sampling rate for continuous detection of carotid diameter changes. In experimental studies, diameter changes closely follow changes in intravascular pressure, which enables an automatic conversion of carotid diameter waveform changes into arterial pressure waveforms by calibrating peak and minimal values to systolic and diastolic brachial blood pressures.15 The data for three to five beats were averaged to obtain a representative waveform. The following AS parameters were evaluated online:5,6
- β (beta stiffness index), calculated as a ratio of the natural logarithm of systolic/diastolic blood pressure to the relative change in the arterial diameter: β = ln (Ps/Pd)/[(Ds − Dd)/Dd], where ln is the natural logarithm, Ps the systolic blood pressure, Pd the diastolic blood pressure, Ds the arterial systolic diameter, and Dd is the arterial diastolic diameter.
- Epsilon (Ep): Young’s modulus, also referred to the pressure–strain elasticity modulus: Ep = (Ps − Pd)/[(Ds − Dd)/Dd].
- Arterial compliance (AC): AC, determined from the arterial cross-sectional area and blood pressure: AC = π(Ds × Ds − Dd × Dd)/[4 × (Ps − Pd)].
- Pulse wave velocity (PWV)-β: One-point PWV, calculated from the time delay between two adjacent distension waveforms, based on the water hammer equation and using the β:
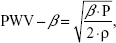
where P is the diastolic blood pressure and ρ is the blood density (1,050 kg/m3). - Augmentation index (AI): AI as a wave reflection parameter was calculated from the formula:
AI = ΔP/PP,
where P1 is the first systolic peak, P2 the second systolic peak, and ΔP = P2 − P1, and PP is the pulse pressure. AI was normalized to equal to 75 beats per minute (AI/75).
The reproducibility of the measurements mentioned earlier has been reported elsewhere.16
Intima–media thickness was determined in line with the established standards.
In addition, serum concentrations of creatinine, glucose, and lipids were determined in all the study subjects.
Statistical analysis
Mean and standard deviations were calculated for quantitative variables and percentages for qualitative variables. All variables were not normally distributed, and therefore, differences between groups were tested by Mann–Whitney U-test for quantitative variables and by chi-square test for percentages of qualitative variables. The statistical significance was set at P<0.05 (two-sided tests), and for multiple testing, we used a statistical significance of P<0.01. Univariate and multivariable logistic regression analyses were conducted considering as dependent variables of the occurrence of DD. All the variables presenting a significant value <0.25 in univariate analysis were included in the model. The stepwise method with backward elimination was used, and odds ratios with 95% confidence intervals were calculated. The model was evaluated using the Hosmer–Lemeshow test.
Results
Clinical characteristics
The clinical characteristics of the patients are presented in Table 1.
Male and female patients did not differ significantly in terms of their age, body mass index (BMI), heart rate, systolic blood pressure, glucose and cholesterol concentrations, and cigarette smoking. Concomitant diabetes mellitus was more frequent in women than in men (47.6% vs 27.2%, P=0.02).
Women presented with significantly lower mean values of “LV dimensions and volumes”, such as EDD (46.8 vs 51.2 mm, P<0.001), end-systolic diameter (27.8 vs 31.0 mm, P=0.001), EDV (95.2 vs 110.8 mL, P=0.001), end-systolic volume (30.2 vs 37.7 mL, P=0.001), and SV (64.9 vs 74.4 mL, P=0.003) than men (Table 2).
Mean value of LVMI turned out to be higher in men than in women (109 vs 97.6 g/m2, P=0.02) (Table 2). Based on their LVMI and RWT (with 0.42 considered as the cutoff value), hypertensives were diagnosed with one out of the four patterns of LVH geometry: 1) normal geometry (N, n=60), both LVMI and RWT within normal limits; 2) concentric remodeling (CR, n=22), normal LVMI with increased RWT (>0.42); 3) concentric hypertrophy (CH, n=24), both LVMI and RWT increased; or 4) eccentric geometry (EH, n=36), increased LVMI, normal RWT (≤0.42). Women presented with CR (19.7% vs 12.3%) as well as with EH (31.7% vs 21%) more often than men, but the differences were not statistically significant (P=0.2). No significant sex-specific differences were observed in terms of the distribution of LVH patterns.
Similarly, no significant intergroup differences were found in terms of “LV systolic function indices” (EF, fractional shortening), midwall function (midwall fractional shortening), and longitudinal function (S′).
Male and female patients did not differ significantly in the prevalence of grade I–III LV DD.
Mean values of e′ (7.8 vs 8.78 cm/s, P=0.003) and e′/a′ ratio (0.707 vs 0.802, P=0.02) were significantly lower in men than in women (Table 2); no sex-specific differences in E/e′ were observed.
Men and women did not differ significantly in terms of their “mean values of AS (eTracking [eT]) markers”. A measure of total compliance (SV/PP) was significantly lower in women than in men (1.046 vs 1.216, P=0.02), similar to intima–media thickness (0.66 vs 0.74 mm, P=0.009) (Table 2).
Linear correlations
The following statistically significant correlations were found in female patients: E/A ratio with age (r=−0.28), E/A ratio with diabetes mellitus (r=−0.3), E/A ratio with AI (r=0.3), and e′ with S′ (r=0.97). In men, isovolumic relaxation time and e′ correlated significantly with age (r=0.3 and r=−0.3, respectively). Furthermore, e′ correlated significantly with diabetes mellitus (r=−0.3), LVH parameters (PW [r=−0.2], IVS [r=−0.3], and RWT [r=−0.3]), S′ (r=0.4) (Figure 1) and AS indices (β [r=−0.3] (Figure 2), Ep [r=−0.2], and PWV-β [r=−0.2]). Moreover, significant correlations were found between e′/a′ and β (r=−0.2), as well as between a′ and S′ (r=0.5) in male patients. Finally, e/e′ was demonstrated to correlate significantly with S′ (r=−0.2), diabetes mellitus (r=0.4), and IVS (r=0.2).
  | Figure 1 The correlation of e′ and S′ in men. |
  | Figure 2 The correlation of e′ and β in men. |
Univariate analysis of the overall study population is presented in Table 3. Univariate analysis in separate sex subgroups (Tables 4 and 5) identified BMI (P=0.016), LVH indices (PW [P=0.01], IVS [P=0.02], and RWT [P=0.02]), CH pattern (P=0.05), and the measure of longitudinal systolic function (S′) (P=0.001) as significant predictors of LV DD in men, but not in women. In contrast, EDV (P=0.03), SV (P=0.02), CO (P=0.02), and preload (P=0.02) turned out to be significant predictors of LV DD in women, but not in men. Irrespective of patient sex, the occurrence of LV DD was predicted by concomitant diabetes mellitus (P=0.002 for women and P=0.03 for men), as well as by the parameters of AS, that is, β (P=0.02 and P=0.01, respectively) and PWV-β (P=0.05 and P=0.02, respectively).
Multivariate analysis
The results of multivariate analysis are presented in Tables 3–5.
Age (P=0.03), BMI (P=0.03), diabetes (P=0.003), S′ (P=0.001), and β (P=0.01) were proved to be significant determinants of LV DD in overall population. Concomitant diabetes mellitus turned out to be an independent predictor of LV DD in women (P=0.02) (Table 3). In turn, two independent predictors of LV DD, S′ (P=0.001) and β (P=0.004) (Table 4), have been identified in men.
To exclude the influence of the greater prevalence of diabetes in females on the results of multivariate analysis, we have provided the power analysis for the study with β parameter value =0.813. We have also demonstrated statistically significant inter-relationship of diabetes and LV DD occurrence in women (Figure 3A and B).
  | Figure 3 The significant inter-relationship of diabetes and LV DD in women. |
Discussion
Prevalence of LV DD in men and women
Some discrepancies exist regarding the prevalence of HFpEF determined on the basis of clinical criteria and echocardiographic evidence of LV DD in men and women. As far as the clinical criteria are considered, HFpEF seems to be more prevalent in women than in men. However, no evident sex-specific differences were documented with regard to the echocardiographic evidence of LV DD17 and some authors even observed higher prevalence of echocardiographic diastolic abnormalities in men than in women.18,19
In our study including untreated hypertensives with no symptoms of HF, men and women did not differ significantly in the prevalence of grade I–III LV DD. However, relaxation abnormalities tended to be more prevalent in men, as shown by significantly lower values of e′ and e′/a′ ratio in male than in female patients (P=0.003 and P=0.02, respectively) (Table 2).
Predictors of LV DD in men and women
Well-established predictors of LV DD in hypertension include age, obesity, diabetes mellitus, blood pressure, heart rate, LV remodeling, and systolic function.20 Recently, the role of AS also as a novel predictor of LV DD has been documented.3–6 However, still little is known about the sex-specific differences in the predictors of LV DD in hypertensive subjects.
According to some authors, “aging” may worsen LV DD to a larger extent in women than in men.2,17 In our previous study, age turned out to be a potent independent determinant of LV DD in untreated hypertensive men and women.6 However, in the present study, although age was identified as a significant predictor of LV DD in both women and men in the multivariate analysis of overall population (Table 3), analysis provided in separate sex subgroups showed age as a significant determinant of LV DD only in men and only on univariate analysis (Table 5).
In the PARAMOUNT trial, “obesity” was shown to be associated with worse LV remodeling and greater impairment of LV diastolic function in women.2 In contrast, in our study, multivariate analysis of the overall population revealed BMI as a significant determinant of LV DD in both women and men (Table 3) and univariate analysis performed in separate sex subgroups identified BMI as a predictor of LV DD in men, but not in women (Tables 4 and 5). This discrepancy may stem from the fact that Gori et al2 examined symptomatic HF patients, mostly obese women, whereas our study included hypertensives with no signs of heart failure.
Diabetes mellitus as a sex-specific predictor of LV DD
Diabetes mellitus was previously shown to exert a relatively greater detrimental effect on diastolic function in women than in men.21 In a Pakistani study of diabetic patients without concomitant hypertension and ischemic heart disease,22 LV DD was more common and more severe in women than in men. In our study, women presented with concomitant diabetes mellitus significantly more often than men (47.6% vs 27.2%, P=0.02) (Table 1). Although this comorbidity was identified as a significant predictor of LV DD in both women and men on multivariate analysis of overall population (Table 3) as well as on univariate analysis of the sex subgroups, it turned out to be an independent predictive factor in female patients only on multivariate separate analyses for the sex subgroups (Tables 4 and 5).
Also, Peterson et al21 observed an interaction between diabetes and sex in the prediction of LV relaxation, with the latter being much worse in diabetic women than in men. However, according to Araz et al,23 the unfavorable effect of concomitant diabetes on LV diastolic function occurs independently of patient sex. Potential mechanisms explaining the effects of sex and type 2 diabetes mellitus on LV diastolic function have been a subject of a recently published review paper.24 Some sex-specific differences in the prevalence of diabetes mellitus may result from different profiles of metabolic and lusitropic changes in men and women. Sex is known to modulate the effect of insulin resistance on cardiac structure. Ha et al25 demonstrated that during physical exercise, diabetic women present with worse LV elastance, a measure of LV stiffness, than any other group, and suggested that it is the smaller ventricle size in female patients which makes diabetes-related LV stiffening more pronounced.
LV remodeling and hypertrophy as sex-specific predictors of LV DD
Available evidence suggests that the effect of hypertension on left ventricle remodeling is sex specific.26 Women are more likely to present with CR in response to pressure overload, whereas chamber dilation is more common in men.27 However, in our study, both CR and EH patterns were more frequently found in women than in men (19.7% vs 12.3% and 31.7% vs 21%, respectively), but the relationship did not reach the threshold of statistical significance. According to literature, women with LV DD and HFpEF present with smaller LV chamber volume and lower SV than the male patients with these conditions.27 Our findings are consistent with these data, as hypertensive women with no signs of HF presented with lower mean values of LV dimensions and volumes, namely, EDD, end-systolic diameter, EDV, end-systolic volume, and SV (Table 2). Furthermore, on univariate analysis in separate sex subgroups, EDV, SV, CO, and preload turned out to be significant predictors of echocardiographic LV DD in women, but not in men (Tables 4 and 5).
One of the established sex-specific cardiovascular factors is LVMI, which is usually greater in men.27 However, women usually develop a greater degree of LVH in response to hypertension and obesity26 and experience a steeper age-related increase in LVM.28 In our study, male patients presented with higher values of LVMI (P=0.02) (Table 2), and univariate analysis in separate sex subgroups (Tables 4 and 5) revealed that some LVH indices, namely, PW (P=0.01), IVS (P=0.02), and RWT (P=0.02), as well as the CH pattern (P=0.04) were significant predictors of LV DD in men, but not in women. In contrast, in the previously mentioned PARAMOUNT trial, a concentric phenotype (remodeling or hypertrophy) was associated with female sex and there was a tendency toward more abnormal LV geometry in women with more severe LV DD.2 This discrepancy may result from the different exclusion criteria used in both studies: while Gori et al2 examined HF patients, of whom the majority were obese women, our series included hypertensive subjects with no clinical evidence of heart failure.
Longitudinal LV systolic function and LV DD in men and women
“Pure” DD has been shown to be associated with long-axis systolic dysfunction in various populations.29 Impaired systolic dysfunction of LV longitudinal fibers is an established sign of early hypertensive cardiomyopathy.30 Systolic myocardial velocity (S′) at the lateral mitral annulus is a measure of longitudinal systolic function.13 Jorge et al31 observed a reduced contractility in the longitudinal axis in patients with suspected HFpEF, and found a linear correlation between the systolic subendocardial dysfunction (S′) and the diastolic abnormalities (E/e′, e′). In the PARAMOUNT trial, mitral S′ velocities were lower in women than in men, but no sex-specific differences were found in deformation imaging parameters. However, the authors of this study did not explore S′ and strain parameters as sex-specific predictors of LV DD and HFpEF.2
In the study presented hereby, however, S′ was identified in the multivariate analysis of the overall study population as a significant determinant of LV DD in both women and men; in the multivariate analysis performed in the separate sex subgroups, it turned out to be an independent predictor of LV DD in men, but not in women (Tables 3–5). To the best of our knowledge, this is the first study in which LV long-axis systolic dysfunction was identified as a sex-specific predictor of LV DD in untreated hypertension. Consequently, the question arises why S′ influenced the occurrence of LV DD in men to a larger extent than in women. One potential reason is higher prevalence of silent ischemia in men. Also, limitations of tissue doppler imaging versus strain assessments of LV subendocardial systolic function should be considered. Nevertheless, the influence of LV long-axis longitudinal dysfunction as a determinant of LV DD in men and women should be a subject of further research.
AS and LV DD in men and women
LV DD is not solely a diastolic disorder, but is also characterized by ventricular and arterial stiffening with adverse coupling between both these phenomena.3,4,17 It has been proved that AS increase is responsible for diminished exercise tolerance in subjects with LV diastolic dysfunction.4 Although a few previous studies demonstrated an association between AS and LV DD in various populations,5,6 still little is known about the sex-specific differences in AS as a predictor of LV DD.
Differences in AS in men and women
In a large cohort Anglo-Cardiff Collaborative Trial study,32 healthy men and women did not differ significantly in terms of their AS indices determined by means of applanation tonometry. However, in the PARAMOUNT trial, women with HFpEF presented with greater AS (lesser arterial elastance) than men with this condition, although this difference was no longer significant after adjusting for body height. The same study revealed no sex-specific differences in arterial–ventricular coupling.2
In our present study, hypertensive male and female patients without HFpEF did not differ in terms of their mean values of eT AS parameters, but the total compliance index (SV/PP) was lower in women than in men (P=0.02) (Table 2). According to Coutinho et al, women present with lower values of total arterial compliance and greater proximal aortic stiffness on applanation tonometry.8 In the Bogalusa Heart Study, the difference between central and peripheral arterial pressure was greater in women than in men.33 Furthermore, women were reported to present with greater pulsatile arterial loading (expressed as arterial elastance), as well as with higher values of aortic AI and central PP.17,34 Altogether, these findings suggest that due to increased vascular stiffening, women may display greater load-related DD and are more prone to develop HFpEF.17,35 Also, in our study, women showed higher mean values of PP and AI, which points to greater pulsatile load; but none of these differences were statistically significant.
AS as a sex-specific predictor of LV DD
Based on available evidence,7,8 we hypothesized that the association between AS and LV DD may be stronger in postmenopausal women than in men. In a cohort study of subjects with multiple risk factors that was conducted by Coutinho et al,8 greater proximal aortic stiffness and lower total arterial compliance were identified as independent predictors of DD only in women, and irrespective of sex, no significant association was found between PP amplification and LV DD. In another study conducted by Shim et al,7 central hemodynamics reflecting AS correlated significantly with LV diastolic function only in women; however, these authors did not report sex-specific differences in associations between PWV and mitral annular diastolic velocity (Em). In our study, a wave reflection parameter (AI) in female patients correlated significantly solely with E/A ratio. While no correlations between e′ (reflecting relaxation abnormalities) and carotid AS parameters were found in women, this parameter correlated significantly with β, PWV-β, and Ep values in male patients.
However, multivariate analysis of the overall present study population (Table 3) and univariate analysis of separate sex subgroups (Tables 4 and 5) demonstrated that β (beta stiffness index) was a significant determinant of LV DD in both women and men. On multivariate analysis performed in separate sex subgroups, β was identified as an independent predictor of LV DD solely in men; however, this parameter tended to reach statistical significance with P=0.09 in women also (Table 5).
Definitely, more research is needed with regard to AS as a potential sex-specific predictor of LV DD.
Clinical implications
This paper demonstrates the necessity of separate analyses for the sex subgroups in investigating the predictive factors of LV DD. The identification of different LV DD determinants in men and women may have significant implications for developing LV DD and HFpEF sex-specific prevention strategies. This issue requires further research.
Study strengths
The present study was conducted in a population of untreated hypertensives. This is one of the few studies that investigated sex-specific predictors of LV DD occurrence, including AS as one of the novel LV DD determinants in untreated hypertension. To the best of our knowledge, this is the first study to document LV long-axis systolic dysfunction as a sex-specific predictor of LV DD in untreated hypertension.
Study limitations
This two-center, observational, cross-sectional cohort study included solely Caucasian individuals. Blood pressure values used to calculate carotid echotracking AS indices were measured on the brachial artery, which tends to overestimate the carotid pressure due to central to peripheral blood pressure amplification. While this might be an important issue in younger subjects, it likely did not confound the results obtained in our group of relatively older patients (mean age 62.7±6.7 years). All patients presented with grade I hypertension according to the European Society of Cardiology/European Society of Hypertension,9 with quite low baseline blood pressure values; the subjects had no history of previous antihypertensive treatment, which was established based on patients’ statements. We believe that the possibility of including hypertensives with medication or high normal blood pressure population as well as with longer history of occult hypertension is very low. One should also consider some limitations inherent to echocardiography itself. The longitudinal systolic function was assessed by S′ measure, which is angle dependent and cannot discriminate between active and passive movements due to wall or heart movement as a whole; the longitudinal function was not measured by other techniques such as strain.
Statin treatment per se affects the LV diastolic function. In our study, subjects with concomitant diabetes – both women and men – were treated with statins, at a similar rate (90% of women, n=27; 91.3% of men, n=21), which might have influenced the conclusion of the study.
Conclusion
There are sex differences in the predictors of LV DD in untreated hypertension. In postmenopausal women, LV DD is mostly determined by diabetes, while in men, it is determined by S′, reflecting LV systolic longitudinal function, and β, a parameter of AS.
Author contributions
JJ and KŁG conceived the idea for the study. JJ, KŁG, and OV contributed to the design of the research. All authors were involved in data collection. JJ and KŁG analyzed the data. All authors edited and approved the final version of the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis and treatment. Eur Heart J. 2011;32(6):670–679. | ||
Gori M, Lam CSP, Gupta DK, et al; Paramount Investigators. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(5):S35–S42. | ||
Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4(1):23–36. | ||
Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–720. | ||
Vriz O, Bossone E, Bettio M, Pavan D, Carerj S, Antonini-Canterin F. Carotid artery stiffness and diastolic function in subjects without known cardiovascular disease. J Am Soc Echocardiogr. 2011;24(8):915–921. | ||
Jaroch J, Łoboz Grudzień K, Bociąga Z, et al. The relationship of carotid arterial stiffness to left ventricular diastolic dysfunction in untreated hypertension. Kardiol Pol. 2012;70(3):223–231. | ||
Shim C-Y, Park S, Choi D, et al. Sex diffrences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57(10):226–233. | ||
Coutinho T, Borlaug B, Pelikka P, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61(1):96–103. | ||
Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–1187. | ||
Lang RM, Bierig M, Devereux RB, et al; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108. | ||
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458. | ||
de Simone G, Devereux RB, Roman MJ, et al. Assessment of left ventricular function by the midwall farctional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23(6):1444–1451. | ||
Ho C, Solomon SD. A clinician’s guide to tissue Doppler imaging. Circulation. 2006;113(10):e396–e398. | ||
Nagueh SF, Appleton ChP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165–193. | ||
Van Bortel LM, Balkestein EJ, van der Heijden-Spek JJ, Vanmolkot FH, Staessen JA, Kragten JA. Non-invasive assessment of local arterial pulse pressure: comparison of applanation tonometry and echo-tracking. J Hypertens. 2001;19(6):1037–1044. | ||
Magda SL, Ciobanu AO, Florescu M, Vinereanu D. Comparative reproducibility of the noninvasive ultrasound methods for the assessment of vascular function. Heart Vessels. 2013;28(2):143–150. | ||
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112(15):2254–2262. | ||
Fischer M, Baessler A, Hense HW, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24(4):320–328. | ||
Hart CY, Redfield MM. Diastolic heart failure in the community. Curr Cardiol Rep. 2000;2(5):461–469. | ||
Łoboz-Grudzień K, Jaroch J, Kowalska A. Factors influencing left ventricular diastolic function in hypertension. Kardiol Pol. 2000;53(12):495–499. | ||
Peterson LR, Saeed IM, McGill JB, et al. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring). 2012;20(4):802–810. | ||
Hameedullah, Khan SB, Khan SS, Khan ZA, Shah I, Hafizullah M. Gender differences in left ventricular diastolic dysfunction in normotensive type 2 diabetic patients. Pak Heart J. 2012;45(2):74–80. | ||
Araz M, Bayrac A, Ciftci H. The impact of diabetes on left ventricular diastolic function in patients with arterial hypertension. North Clin Istanbul. 2015;2:177–181. | ||
Miller TM, Gilligan S, Herlache LL, Regensteiner JG. Sex differences in cardiovascular disease risk and exercise in type 2 diabetes. J Investig Med. 2012;60(4):664–670. | ||
Ha JW, Lee HC, Park S, et al. Gender-related difference in left ventricular diastolic elastance during exercise in patients with diabetes mellitus. Circ J. 2008;72(9):1443–1448. | ||
Krumholtz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72(3):310–313. | ||
Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55(11):1057–1065. | ||
Lieb W, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the Framingham Heart Study. Circulation. 2009;119(24):3085–3092. | ||
Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG. “Pure” diastolic dysfunction is associated with long-axis systolic dysfunction: implications for the diagnosis and classification of heart failure. Eur J Heart Fail. 2000;7(5):820–828. | ||
Kolouris SN, Kostopoulos KG, Triantafyllou KA, et al. Impaired systolic dysfunction of left ventricular longitudinal fibers: a sign of early hypertensive cardiomyopathy. Clin Cardiol. 2005;28(6):282–286. | ||
Jorge AJ, Silva EN, Fernandes LC, Ribeiro ML, Mesquita ET, Licio FV. Evaluation of systolic longitudinal function in heart failure with normal ejection fraction. Arq Bras Cardiol. 2010;94(6):799–805. | ||
McEniery CM, Yasmin, Hall IR, Quasem A, Wilkinson IB, Cockroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: he Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46(9):1753–1760. | ||
Chester R, Sander G, Fernandez C, Chen W, Berenson G, Giles T. Women have significantly greater difference between central and peripheral arterial pressure compared with men: the Bogalusa Heart Study. J Am Soc Hypertens. 2013;7(5):379–385. | ||
Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30(7):1863–1871. | ||
Borlaug BA. Sex, load and relaxation. Are women more susceptible to load-dependent diastolic dysfunction? J Am Coll Cardiol. 2011;57(10):1234–1236. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





