Back to Journals » International Journal of General Medicine » Volume 15
Sex-Specific Differences in the Clinical Profile Among Patients with Tracheobronchial Tuberculosis: A Hospital-Based Cross-Sectional Study in Shenzhen, China
Authors Fu J, Li J, Liu Z , Zheng S, Li X, Ning X, Wang J , Gao W, Li G
Received 19 March 2022
Accepted for publication 1 June 2022
Published 21 June 2022 Volume 2022:15 Pages 5741—5750
DOI https://doi.org/10.2147/IJGM.S367070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jiapeng Fu,1– 3,* Jian Li,1– 3,* Zhi Liu,1– 3 Shasha Zheng,1– 3 Xue Li,1– 3 Xianjia Ning,4,5 Jinghua Wang,4,5 Wenying Gao,1– 3 Guobao Li1– 3
1Department of the Third Pulmonary Disease, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China; 2The Second Affiliated Hospital of Southern University of Science and Technology, Shenzhen, Guangdong, People’s Republic of China; 3National Clinical Research Center for Infectious Diseases, Shenzhen, Guangdong, People’s Republic of China; 4Center of Clinical Epidemiology, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China; 5Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, 300052, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guobao Li; Wenying Gao, Department of the Third Pulmonary Disease, The Third People’s Hospital of Shenzhen, 29 Bulan Road, Longgang District, Shenzhen, Guangdong Province, 518112, People’s Republic of China, Tel +86-755-61238942, Fax +86-755-61238928, Email [email protected]; [email protected]
Purpose: Tracheobronchial tuberculosis (TBTB) has been proposed to occur more commonly in female patients. However, to date, studies that systematically delineate differences between female and male patients with TB infection are lacking. We aimed to comprehensively assess the sex-specific differences in clinical manifestation, bronchoscopy performance, bacteriological examination, and imaging of TBTB in Shenzhen, China.
Methods: All patients with diagnosed TBTB from August 1, 2018 to July 31, 2021 at The Third People’s Hospital of Shenzhen were enrolled in the present study. Demographic information, clinical manifestations, blood tests, chest computed tomography, and bronchoscopic findings were collected, and assessed their sex-specific differences.
Results: Of these 331 patients, 238 patients (71.9%) were female, and 93 patients (28.1%) were male, with an overall average age of 37.3 years. The average age of male patients with TBTB was more than 5 years older than that of female patients. The prevalence of lymph fistula and diabetes mellitus was significantly higher in male patients than female patients (8.6% vs 1.7%, P = 0.005; 17.2% vs 2.1%, P < 0.001). The positive proportion of sputum smear was higher in male patients (27.9%) than in female patients (16.7%, P = 0.026). Moreover, the mean monocyte-to-lymphocyte ratio, serum CRP, and IL-6 levels were significantly higher in male patients than in female patients (P < 0.05).
Conclusion: In summary, in patients with TBTB diagnosis, male sex was associated with a high prevalence of diabetes mellitus, lymph fistula, and smear-positive ratio, as well as high inflammation levels. The management of young female and male patients with diabetes mellitus and high inflammation levels should be strengthened. Furthermore, to reduce the burden of TBTB, we must pay attention to the risk of TBTB in past tuberculosis patients, especially male patients under 45 years old and female patients over 45 years old.
Keywords: sex difference, tracheobronchial tuberculosis, inflammation
Introduction
Pulmonary tuberculosis (TB), caused by Mycobacterium tuberculosis complex, is the ninth leading cause of death worldwide and remains a major global public health priority.1 According to the World Health Organization, there are approximately 10.0 million people with TB disease, and 1.4 million deaths from TB occurred in 2019.2 Tracheobronchial TB (TBTB) is a special type of TB infecting the tracheobronchial tree that could affect any part and any layer of the tracheobronchial wall.3,4 TBTB is present in approximately 4.1–54.3% of patients with active TB.5 Moreover, more than two-thirds of patients develop severe bronchial stenosis and stricture formation despite adequate medical treatment.6–8 Refractory tracheobronchial stenosis may eventually lead to a decline in pulmonary function, respiratory failure, or death.9
In previous studies, TBTB was more common in female than male adult patients.5,10–12 Moreover, a large-scale, multi-center, prospective investigation revealed the risk of TBTB in female patients is 1.53 higher than in male patients in southern China. In addition, another prospective study in a tertiary referral hospital in Korea found female sex is an important risk factor for both TBTB and severe bronchostenosis.5 A possible explanation might be that the bronchial lumen size is smaller in female patients than in patients, and thus female patients would not actively expectorate to the same degree as male patients.11–13 Furthermore, long-term exposure to sputum may make female patients more vulnerable to tubercle bacilli infection. Accordingly, different anatomy, living habits, and sociocultural and esthetic factors might lead to sex-specific differences in susceptibility to TBTB, including the clinical manifestations and prognosis. However, there is a paucity of studies systematically examining the differences between female patients and male patients with TB infection.
Therefore, this study aimed to comprehensively assess the sex-specific differences in the clinical manifestation, bronchoscopy performance, bacteriological examination, and imaging of TBTB in Shenzhen, China, from 2018 to 2021.
Methods
Study Population
All patients with diagnosed TBTB from August 1, 2018 to July 31, 2021 at The Third People’s Hospital of Shenzhen were enrolled in the present study. The Third People’s Hospital of Shenzhen is the largest specialized TB hospital in Shenzhen and undertakes the prevention, diagnosis, and treatment of TB infection in Shenzhen.
The following patients were excluded: (1) those younger than 18 years, (2) those with serious cardiopulmonary diseases, (3) those unable to tolerate bronchoscopy, and (4) bronchoscopy and pathological examination showed nontuberculous tracheobronchial lesions.
The present study was approved by the Ethics Committee of The Third People’s Hospital of Shenzhen and conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from all patients.
Diagnostic Criteria for TBTB
The TBTB diagnosis and treatment guidelines in China were adopted;14 TBTB was diagnosed based on visible tracheobronchial lesions under bronchoscopy and either (1) pathological diagnosis of TB; (2) positive acid-fast bacilli (AFB) in a sputum smear, brushing smear, or bronchial alveolar lavage fluid; (3) positive M. tuberculosis culture; or (4) typical TB pathologic changes on bronchoscopy biopsy. All patients in this study underwent bronchoscopy and pathological examination to ensure the accuracy of diagnosis.
Physical Examinations
All data were obtained from the hospital medical record system, including demographic information (including name, sex, date of birth, and educational level), individual medical history (including the presence of hypertension, diabetes mellitus, coronary artery disease, and previous TB), and lifestyle factors (including smoking and drinking). The levels of C-reactive protein (CRP), procalcitonin (PCT) and interleukin-6 (IL-6) were tested in the central laboratory of the Third People’s Hospital of Shenzhen. The criteria for TB drug resistance classification was referred to the World Health Organization Criteria in 2013.15
Imaging Analysis
All patients underwent chest computed tomography examination. The cavity, as defined by the Fleischner Society, is a gas-filled space, seen as a lucency or low-attenuation area, within a nodule, mass, or area of parenchymal consolidation.16
Bronchoscopic Examination
Bronchoscopy was performed in all patients. According to the Chinese guidelines for the classification of TBTB,14 TBTB was classified into six subtypes: inflammatory infiltration, ulceration necrosis, granulation hyperplasia, cicatrices stricture, tracheobronchial malacia, and lymph fistula. Each bronchoscopic examination was reviewed and classified by two pulmonary specialists.
Definitions
Hypertension was defined as a history of hypertension or taking antihypertensive drugs. Diabetes was defined as a fasting blood glucose ≥7.0 mmol/L, taking medication for diabetes, or a self-reported history of diabetes. Smoking was defined as smoking ≥1 cigarette daily for more than 1 year. Drinking was defined as drinking >50 mL of alcohol at least once per week for more than 6 months. Moreover, the participants were categorized into three educational groups according to the length of formal education (0–11, 12–15, and ≥16 years). According to the age classification of the World Health Organization, this study defined the group of <45 years old as the youth group, and the patients over 45 years old as the middle-aged and elderly group.
Statistical Analysis
Continuous variables were summarized by means and standard deviations; Student’s t-test was used to compare between-group differences. Categorical variables were summarized as numbers with frequencies, and the chi-squared test was performed to compare the differences between the two groups. All statistical analyses were performed using SPSS version 25.0 statistical software (SPSS Inc., Chicago, IL), and two-sided P < 0.05 was considered statistically significant.
Results
Demographic Characteristics of All Participants
A total of 331 patients diagnosed with TBTB were analyzed in this study. Of these 331 patients, 238 patients (71.9%) were female, and 93 (28.1%) were male, with an average overall age of 37.3 years. The mean age of the male patients was higher than that of the female patients (41.10 years vs 35.85 years P= 0.005). The prevalence of diabetes mellitus was 6.3% overall, 17.2% in male patients, and 2.1% in female patients (P < 0.001). Male patients had a higher prevalence of smoking and drinking compared to that of female patients (all P < 0.05) (Table 1).
 |
Table 1 Demographic Characteristics for All Patients with Tracheobronchial Tuberculosis |
Clinical Characteristics for Patients with TBTB Between Sex Groups
The frequency of lymph fistula was 3.6% overall and was significantly higher in male patients than in female patients (8.6% vs 1.7%; P = 0.005). The positive proportion of sputum smear was higher in male patients than in female patients (27.9% vs 16.7%, P = 0.026). Moreover, the mean monocyte-to-lymphocyte ratio and serum CRP level were significantly higher in men than in women (P < 0.05; Table 2).
 |
Table 2 Clinical Characteristics for Patients with Tracheobronchial Tuberculosis Between Sex Groups |
Clinical Characteristics Between Sex Groups Among Patients with TBTB Younger Than 45 Years
Among all 234 patients aged <45 years old, male patients accounted for 22.6% (n = 53). Compared to female patients, male patients were more likely to have higher frequency of diabetes (5.7% vs 0; P = 0.011), smoking (17% vs 0.6%; P < 0.001), pulmonary TB history (26.4% vs 10.5%, P = 0.003), and lymph fistula (7.5% vs 1.1%; P = 0.025). Moreover, there was no statistical difference in inflammatory indicators between the sex groups (all P > 0.05; Table 3).
 |
Table 3 Demographic and Clinical Characteristics Between Sex Groups Among Patients with Tracheobronchial Tuberculosis Younger Than 45 Years Old |
Clinical Characteristics Between Sex Groups Among Patients with TBTB 45 Years or Older
Among patients aged 45 years old and over, male patients were more likely than female patients to have a higher frequency of diabetes (32.5% vs 8.8; P = 0.003), smoking (32.5% vs 0; P < 0.001), and positive proportion of sputum smear (40.5% vs 16.7%, P = 0.011). But the frequency of expectoration (82.5% vs 57.5%; P = 0.007), and pulmonary TB history (22.8% vs 7.5%, P = 0.046) was higher in female patients than in male patients. Moreover, the mean monocyte-to-lymphocyte ratio, serum CRP, and IL6 levels were significantly higher in male patients than in female patients (all P < 0.05; Table 4).
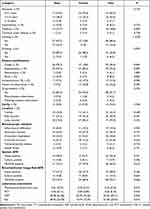 |
Table 4 Demographic and Clinical Characteristics Between Sex Groups Among Patients with Tracheobronchial Tuberculosis Older Than 45 Years Old |
Discussion
In this study, the epidemiology and clinical manifestations of TBTB in different genders were analyzed by the combined method of bronchoscopy and pathology. In the present study, TBTB occurred mainly in young female patients. Male TBTB patients were more complicated with diabetes and lymphatic fistula, and the positive ratio of sputum smear, CRP, and IL-6 were significantly higher than those of female patients. Notably, the prevalence of prior TB history was significantly higher in men than in women patients younger than 45 years; however, women were significantly higher than men in patients 45 years and older.
The size of the bronchial lumen of women is smaller than that of men, and sputum retention may make the bronchial lumen more susceptible to infection by Mycobacterium tuberculosis.11,17 Therefore, TBTB is more common in women.5 Previous studies have showed that female younger than 50 years old is an independent risk factor for TBTB.18 In addition, studies have shown that smoking may be a protective factor for TBTB, making it difficult for Mycobacterium tuberculosis to invade the trachea and bronchi.19 Since the smoking rate of men is significantly higher than that of women, this may be the reason why the prevalence of TBTB in men is lower than that in women in this study. The results of this study found that male tuberculosis patients younger than 45 years old and female tuberculosis patients older than 45 years old are more prone to relapse from TBTB. This study supplements the results of previous studies. We must pay attention to the risk of TBTB in past tuberculosis patients, especially male patients under 45 years old and female patients over 45 years old.
Moreover, diabetes is the main risk factor for the development of active and latent tuberculosis.20,21 People with diabetes are approximately three times more likely to develop tuberculosis than non-diabetics.22 An Iranian study showed that the majority of DM-TB patients are over 50 years old, and women account for more than half. The prevalence of DM-TB in women is significantly higher than that in men.23 However, in contrast to the results of this study, the prevalence of DM-TB in men is higher. This study is a single-center study, and the subjects are all hospitalized patients, which is different from the previous population, so there may be differences in the results of the study.
TBTB is difficult to diagnose because of the nonspecific clinical manifestations, chest radiographs, and low incidence of positive acid-fast bacilli staining. In addition, the clinical diagnosis of TBTB mainly relies on bronchoscopic examination,8 and it is classified into six types based on bronchoscopic findings, including inflammatory infiltration (type I), ulceration necrosis (type II), granulation hyperplasia (type III), cicatrices stricture (type IV), tracheobronchial malacia (type V), and lymph fistula (type VI).24 Lymph fistula type is a very rare type among the Chinese population.25 A retrospective study in Wuhan demonstrated that the incidence of VI type was higher in young adults and in male patients, where the prevalence of lymph fistula was significantly higher in male patients than in female patients.26 Consistent with previous studies, in the present study, the prevalence of lymph fistula in male patients was 5-fold greater than that in female patients.
M. tuberculosis usually invades the respiratory tract via aerosol delivery and interacts with alveolar macrophages, dendritic cells, neutrophils, type II pneumocytes, and epithelial cells in the microenvironment, resulting in the release of a wide array of cytokines, chemokines, and other molecules by different immune cells.27 A large cross-sectional study demonstrated that serum CRP levels in patients with active TB disease are substantially increased.28 Moreover, CRP level is associated with severe lung tissue damage.29 The CRP +1444C/T polymorphism is associated with increased TB risk among men, while no such connection was found in women.30 Moreover, IL-6 plays a crucial role in the pathogenesis of TB infection, mediating numerous chronic inflammatory and immune processes. The link between IL-6 polymorphisms and TB risk has been investigated.31 Serum IL-6 level increased significantly in patients with active TB and was correlated with TB-related inflammation.32,33 In addition, IL-6 is a useful inflammatory marker for unfavorable TB outcomes.34,35 In this study, the serum CRP level and IL-6 levels in male patients were significantly higher than that in female patients. This may indicate that males have stronger immune responses than females when they are infected with Mycobacterium tuberculosis.
The current data further suggest that the sex-specific immune response might be due to sex hormones. Both estrogens and androgens affect the immune response.36 Female patients exhibit a higher degree of immunocompetence than do male patients, as indicated by a higher incidence of autoimmune disease. Male sex is associated with high inflammation levels and a less effective immune response; this may be caused by androgens, which can suppress immune reactivity and inflammation reactions, thus resulting in a greater number of infections and more severe infections in male patients.36,37
Moreover, the rate of TB history was higher in male patients than in female patients with TBTB aged less than 45 years. The prevalence of lymph fistula and pulmonary TB history was significantly higher in male patients than in female patients with TBTB aged more than 45 years. This conclusion is difficult to explain based on existing knowledge, and it is hoped that further research will be conducted into this phenomenon.
There are certain limitations to this study. First, the investigation was performed at a single site in southern China, so its representation and generalizability are limited. Second, some potential risk factors, including symptom duration and severity of TBTB, were not assessed. Third, the subjects in this study were all diagnosed TBTB patients and did not expand to tuberculosis. Fourth, the information related to radiological findings in this study is limited. Further expanded study is needed. Finally, this was a cross-sectional study, so causal relationships could not be determined.
Perspectives and Significance
It is crucial to monitor the bronchoscopy to reduce the incidence of TBTB for men aged <45 years and women aged ≥45 years.
Conclusion
In summary, in patients diagnosed with TBTB, male sex was associated with a high prevalence of diabetes mellitus, lymph fistula, and smear-positive ratio and high inflammation levels. Although TBTB is more common in female patients, the high inflammation levels in male patients demand attention. To reduce the burden of TBTB, management of young women, men with diabetes mellitus, and high inflammation levels should be strengthened. We must pay attention to the risk of TBTB in past tuberculosis patients, especially male patients under 45 years old and female patients over 45 years old.
Ethics approval and informed consent
The present study was approved by the Ethics Committee of The Third People’s Hospital of Shenzhen and conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from all patients.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
We would like to thank all patients for supporting this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research work was partly supported by the National Key Research and Development Plan (No.2021YFA1300902).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Weil D, Marinkovic K, Kasaeva T. Back to the future, again: greater leadership, collaboration and accountability to accelerate progress to end TB. BMC Med. 2018;16(1):172. doi:10.1186/s12916-018-1165-9
2. World Health Organization. Global tuberculosis report 2020; 2020. Available from: https://www.who.int/publications/i/item/global-tuberculosis-report-2020.
3. Pathak V, Shepherd RW, Shojaee S. Tracheobronchial tuberculosis. J Thorac Dis. 2016;8(12):3818–3825. doi:10.21037/jtd.2016.12.75
4. Shim YS. Endobronchial tuberculosis. Respirology. 1996;1(2):95–106. doi:10.1111/j.1440-1843.1996.tb00017.x
5. Jung SS, Park HS, Kim JO, Kim SY. Incidence and clinical predictors of endobronchial tuberculosis in patients with pulmonary tuberculosis. Respirology. 2015;20(3):488–495. doi:10.1111/resp.12474
6. Shahzad T, Irfan M. Endobronchial tuberculosis-A review. J Thorac Dis. 2016;8(12):3797–3802. doi:10.21037/jtd.2016.12.73
7. Siow WT, Lee P. Tracheobronchial tuberculosis: a clinical review. J Thorac Dis. 2017;9(1):E71–77. doi:10.21037/jtd.2017.01.49
8. Faisal M, Harun H, Hassan TM, Ban A, Chotirmall SH, Rahaman JA. Treatment of multiple-level tracheobronchial stenosis secondary to endobronchial tuberculosis using bronchoscopic balloon dilatation with topical mitomycin-C. BMC Pulm Med. 2016;16(1):53. doi:10.1186/s12890-016-0209-1
9. Kashyap S, Solanki A. Challenges in endobronchial tuberculosis: from diagnosis to management. Pulm Med. 2014;2014:594806. doi:10.1155/2014/594806
10. Peng AZ, Yang A, Li SJ, Qiu Q, Yang S, Chen Y. Incidence, laboratory diagnosis and predictors of tracheobronchial tuberculosis in patients with pulmonary tuberculosis in Chongqing, China. Exp Ther Med. 2020;20(6):174. doi:10.3892/etm.2020.9304
11. Lee JH, Park SS, Lee DH, Shin DH, Yang SC, Yoo BM. Endobronchial tuberculosis. Clinical and bronchoscopic features in 121 cases. Chest. 1992;102(4):990–994. doi:10.1378/chest.102.4.990
12. Lee JH, Lee DH, Park SS. Endobronchial tuberculosis: clinical and bronchofiberscopic features. Korean J Intern Med. 1986;1(2):229–232. doi:10.3904/kjim.1986.1.2.229
13. Carlen R. Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis. 1991;143(4 Pt 1):895–896. doi:10.1164/ajrccm/143.4_Pt_1.895
14. Chinese Medical Association. Diagnosis and treatment guideline for tracheobronchial tuberculosis. Chin J Tuberc Respir Dis. 2012;35:581–587.
15. World Health Organization. Definitions and Reporting Framework for Tuberculosis-2013 Revision. Geneva: World Health Organization; 2013.
16. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi:10.1148/radiol.2462070712
17. Lee P. Endobronchial tuberculosis. Indian J Tuberc. 2015;62(1):7–12. doi:10.1016/j.ijtb.2015.02.002
18. Su Z, Cheng Y, Wu Z, et al. Incidence and predictors of tracheobronchial tuberculosis in pulmonary tuberculosis: a multicentre, large-scale and prospective study in Southern China. Respiration. 2019;97(2):153–159. doi:10.1159/000492335
19. Rikimaru T, Kinosita M, Yano H, et al. Diagnostic features and therapeutic outcome of erosive and ulcerous endobronchial tuberculosis. Int J Tuberc Lung Dis. 1998;2(7):558–562.
20. Cheng J, Zhang H, Zhao YL, Wang LX, Chen MT. Mutual impact of diabetes mellitus and tuberculosis in China. Biomed Environ Sci. 2017;30(5):384–389. doi:10.3967/bes2017.051
21. Ayelign B, Negash M, Genetu M, Wondmagegn T, Shibabaw T. Immunological impacts of diabetes on the susceptibility of mycobacterium tuberculosis. J Immunol Res. 2019;2019:6196532. doi:10.1155/2019/6196532
22. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. doi:10.1371/journal.pmed.0050152
23. Ozma MA, Rashedi J, Poor BM, et al. Tuberculosis and diabetes mellitus in Northwest of Iran. Infect Disord Drug Targets. 2020;20(5):667–671. doi:10.2174/1871526519666190715142100
24. Sahin F, Yıldız P. Characteristics of endobronchial tuberculosis patients with negative sputum acid-fast bacillus. J Thorac Dis. 2013;5(6):764–770. doi:10.3978/j.issn.2072-1439.2013.12.15
25. Donath J, Khan FA. Tuberculous and posttuberculous bronchopleural fistula. Ten year clinical experience. Chest. 1984;86(5):697–703. doi:10.1378/chest.86.5.697
26. Jia L, Chen H, Huang H, Shen L. Clinical analysis and early diagnosis on 32 tracheobronchial tuberculosis induced by lymphatic fistula. J Clin Pulm Med. 2017;22(12):2248–2252.
27. O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi:10.1146/annurev-immunol-032712-095939
28. Chegou NN, Sutherland JS, Malherbe S, et al. Diagnostic performance of a seven-marker serum protein biosignature for the diagnosis of active TB disease in African primary healthcare clinic attendees with signs and symptoms suggestive of TB. Thorax. 2016;71(9):785–794. doi:10.1136/thoraxjnl-2015-207999
29. Shapiro AE, Hong T, Govere S, et al. C-reactive protein as a screening test for HIV-associated pulmonary tuberculosis prior to antiretroviral therapy in South Africa. AIDS. 2018;32(13):1811–1820. doi:10.1097/QAD.0000000000001902
30. Xu Y, Cheng M, Wang X. C-reactive protein +1444C/T polymorphism is associated with the susceptibility to pulmonary tuberculosis. Biomed Res Int. 2020;2020:6634879. doi:10.1155/2020/6634879
31. Sun W, Jiao L, Liu T, et al. No significant effects of IL-6 and IL-13 gene variants on tuberculosis susceptibility in the Chinese population. DNA Cell Biol. 2020;39(7):1356–1367. doi:10.1089/dna.2020.5404
32. Masuda K, Ripley B, Nishimura R, et al. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci U S A. 2013;110(23):9409–9414. doi:10.1073/pnas.1307419110
33. Shah JA, Musvosvi M, Shey M, et al. A functional toll-interacting protein variant is associated with Bacillus Calmette-Guérin-specific immune responses and tuberculosis. Am J Respir Crit Care Med. 2017;196(4):502–511. doi:10.1164/rccm.201611-2346OC
34. Gupte AN, Kumar P, Araújo-Pereira M, et al. Baseline IL-6 is a biomarker for unfavorable tuberculosis treatment outcomes: a multi-site discovery and validation study. Eur Respir J. 2021:2100905. Doi:10.1183/13993003.00905-2021
35. Manabe YC, Andrade BB, Gupte N, et al. A parsimonious host inflammatory biomarker signature predicts incident tb and mortality in advanced HIV. Clin Infect Dis. 2020;71(10):2645–2654. doi:10.1093/cid/ciz1147
36. Taneja V. Sex hormones determine immune response. Front Immunol. 2018;9:1931. doi:10.3389/fimmu.2018.01931
37. Gubbels Bupp MR, Jorgensen TN. Androgen-induced munosuppression. Front Immunol. 2018;9:794. doi:10.3389/fimmu.2018.00794
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
