Back to Journals » Infection and Drug Resistance » Volume 15
Severe Thrombocytopenic Purpura Associated with COVID-19 in a Pediatric Patient
Authors Marinescu AR, Lazureanu VE, Musta VF, Nicolescu ND, Mocanu A , Cut TG , Muresan CO, Tudoran C , Licker M , Laza R
Received 23 February 2022
Accepted for publication 21 June 2022
Published 30 June 2022 Volume 2022:15 Pages 3405—3415
DOI https://doi.org/10.2147/IDR.S363716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Adelina Raluca Marinescu,1– 3 Voichita Elena Lazureanu,1,2 Virgil Filaret Musta,1,2 Narcisa Daniela Nicolescu,1,2 Alexandra Mocanu,1– 3 Talida Georgiana Cut,1– 4 Camelia Oana Muresan,4,5 Cristina Tudoran,6,7 Monica Licker,8,9 Ruxandra Laza1,2
1Discipline of Infectious Diseases, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 2Victor Babes Clinical Hospital of Infectious Diseases and Pneumophtisiology, Timisoara, Romania; 3Doctoral School, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 4Center for Ethics in Human Genetic Identifications, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 5Discipline of Forensic Medicine, Bioethics, Deontology and Medical Law, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 6Discipline of Internal Medicine II, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 7Center of Molecular Research in Nephrology and Vascular Disease, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 8Discipline of Microbiology, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania; 9Multidisciplinary Research Centre on Antimicrobial Resistance, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania
Correspondence: Talida Georgiana Cut, Discipline of Infectious Diseases, Victor Babes University of Medicine and Pharmacy Timisoara, Timisoara, Romania, Tel +4 0755690250, Email [email protected]
Purpose: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is known to cause a diverse spectrum of clinical manifestations ranging from mild, flu-like symptoms to severe progressive pneumonia, acute respiratory distress syndrome with or without other extrapulmonary impairment. Hematological changes such as lymphopenia, neutrophilia, and anemia as the disease progresses, are frequently found in COVID-19. Thrombocytopenia may be drug-induced or can occur secondary to sepsis, disseminated intravascular coagulation or bone marrow suppression. Immune thrombocytopenic purpura (ITP) is frequently observed in children aged 2– 5 years and in 60% of cases may proceed an upper respiratory tract infection. The present paper aimed to raise awareness of ITP as a possible pediatric presentation of coronavirus disease.
Patients and Methods: We present the case of previously healthy, eight-year-old female patient, who developed an immune thrombocytopenia flare, also known as immune thrombocytopenic purpura (ITP), in the context of COVID-19, with diffuse petechiae and ecchymosis on her body, face and oral mucosa, and a nadir platelet count of 0× 103/μL.
Results: Platelet count recovery was observed after seven days of combined treatment with intravenous immunoglobulin (IVIG) and corticosteroids.
Conclusion: The growing body of literature regarding the clinical and laboratory manifestations of COVID-19 infection in children, has reported thrombocytopenia in relation to unfavorable disease progression or multisystem inflammatory syndrome (MIS-C). Clinicians must be aware that ITP may appear both in mild and severe COVID-19, at any time during its course, and can be associated with a higher bleeding risk, thus its diagnostic may be critical.
Keywords: SARS-CoV-2 infection, immune thrombocytopenia, bleeding risk, intravenous immunoglobulin
Introduction
Immune thrombocytopenia (ITP) is a common acquired acute autoimmune bleeding disorder during childhood, characterized by isolated thrombocytopenia of <100×103/μL, petechial rash, bruising and bleeding in an otherwise healthy child.1,2 It affects approximately 1.9–6.4/100,000 children per year and is due to a loss of immunological tolerance to platelet membrane antigens, resulting into an increased platelet destruction coupled on occasion with impaired/inadequate platelet production.1,3 Although the pathophysiology may not yet be entirely understood, it appears that autoantibodies related to commonly immunoglobulin G (IgG), are directed against platelet membrane antigens such as glycoprotein IIb/IIIa complex, glycoprotein Ib/IX, glycoprotein Ia/IIa and glycoprotein VI.4 ITP has been linked to vaccines, antiviral drugs but can also be triggered by silent or overt viral pathogens such as human immunodeficiency virus (HIV), cytomegalovirus, Epstein–Barr virus, parvovirus B19, adenovirus, varicella-zoster virus, hepatitis C and seasonal influenza.5–7 Unlike the common form of ITP found in adults, ITP in children generally recovered with or without treatment within 6 to 12 months after diagnosis.8
Since being firstly documented at the end of 2019, in Wuhan (China), the corona-virus disease 2019 (COVID-19), a zoonotic infection caused by the enveloped, positive-sense RNA single-stranded SARS-CoV-2, a novel member of the β-coronavirus genus, has spread rampantly, raising major public health concerns, and applying continuous strain on the global medical infrastructure.9,10 SARS-CoV-2, initially thought to be limited to the respiratory tract, is now known to cause a diverse spectrum of clinical manifestations.11,12 As more reports emerge in literature, it appears that glycoprotein spikes on the surface of SARS-CoV-2 engage angiotensin-converting enzyme-2 (ACE-2) receptors on respiratory epithelium, macrophages, and cardiac myocytes.13 Receptor engagement leads to virus entry, replication and cell lysis, initiating release of pro-inflammatory cytokines, including interleukins, various glycoproteins and acute phase reactants such as C-reactive protein, fibrinogen and procalcitonin.14 Hematological changes such as lymphopenia, neutrophilia and anemia are commonly found in COVID-19. The incidence of thrombocytopenia in patients with COVID-19 has been variable across scientific reports. According to Guan et al, mild thrombocytopenia has been observed in up to one-third of these patients and a higher rate has been reported in patients with severe disease in 2020.15 Thrombotic consumption of platelets in microvasculature, direct infection of megakaryocytes and autoimmune destruction of platelets may cause a new presentation of ITP or could trigger relapse in an existing patient.16
Children infected with SARS-CoV-2 usually have mild symptoms such as fever, cough, headaches, and sore throat.17,18 A small proportion of acutely infected children, develop severe respiratory symptoms requiring hospitalization or admission to the pediatric intensive care unit.19 Cutaneous findings reported in children and young adults, include maculopapular, urticarial, vesicular eruptions and painful purple and reddish papules on distal extremities of the upper and lower limbs, commonly known as COVID toes.20 Thrombocytopenia has been reported in relation to severe disease or to the multisystem inflammatory syndrome (MIS-C), a hyperinflammatory syndrome characterized by fever, inflammation and organ dysfunction in the setting of recent SARS-CoV-2 infection.21–23 Possible pathogenetic mechanisms in ITP secondary to SARS-CoV-2 infection are similar to other postinfectious and postvaccination ITP.24 The pathogenetic mechanisms depend on the phase of COVID‐19 infection, and include increased platelet destruction via molecular mimicry, reduced platelet production due to destruction of the bone marrow progenitor cells by the cytokine storm or due to reduced thrombopoietin caused by direct liver damage.25–27 The median time between the onset of COVID-19 symptoms and the diagnosis of SARS-CoV-2-induced ITP has been reported to be 13 days, with the majority of cases occurring after one month of the onset of COVID-19 symptoms.28 The variable time delay between COVID-19 symptoms and the occurrence of ITP in some cases may call into question the causal nature of SARS-CoV-2 with ITP.29
Although the emergence of ITP in pediatric patients with COVID-19 is not a novel subject, we present a case of a child with ITP and SARS-CoV-2 infection, with a nadir platelet count of 0×103/μL and complete response to IVIG and corticosteroids in order to raise awareness.
Case Report
A previously healthy eight-year-old girl, with no family and personal history of hematologic or autoimmune disorders, medication, or previous drug reactions, presented to the emergency department with petechiae and ecchymoses in several parts of her body. Disease onset was in the previous 24 h with low-grade fever. Because the patient’s father had confirmed diagnosis of COVID-19, reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2 from nasal swabs was performed. The patient tested positive for COVID-19 and was referred to our department of infectious diseases. Upon admission, the patient was hemodynamically stable (body temperature: 36.4°C, heart rate: 90 beats/minute, blood pressure: 115/70 mmHg, respiratory rate: 19 breaths/minute, oxygen saturation 100% on room air), with unremarkable physical examination apart from multiple petechiae and ecchymoses on torso, limbs, face, and oral mucosa with no active mucosal bleeding. The complete blood count showed severe thrombocytopenia (0×103μ/L) and leukopenia (white blood cells [WBC]=2500/μL, N: 59.2%, L: 32.1%, M: 7.5%). Renal and liver function and coagulation tests were normal (creatinine: 0.44 mg/dL, urinalysis within limits, ALT: 14 U/L, AST: 23 U/L, D-dimers: 0.22 μg/mL). The chest X-ray, abdominal ultrasound (including portohepatic system Doppler sonography) and echocardiography were otherwise unremarkable with no radiological signs of viral pneumonia, organomegaly, lymphadenopathy or features of myocardial dysfunction, pericarditis, or coronary abnormalities.
In the first 48 h from admission, the patient maintained the severe thrombocytopenia (platelets=0×103/μL) and developed acute functional renal failure (creatinine: 2.37 mg/dL) and hepatic cytolysis syndrome (ALT: 233.3 U/L, AST: 108.7 U/L), with laboratory evidence of inflammation including an elevated C-reactive protein, ferritin, and elevated D-dimers (as presented in Table 1). Serology for viruses commonly associated with ITP (Epstein–Barr virus, seasonal influenza, adenovirus, hepatitis A, B, C, varicella-zoster virus, cytomegalovirus, HIV, herpes simplex virus, parvovirus B19) was negative. The antinuclear factor was nonreactive and no other obvious bacterial cause (Escherichia coli, Bordetella pertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae, staphylococcal or streptococcal infection) was detected. The peripheral blood smear, as presented in Figure 1, showed normal red blood cells (RBC) morphology, decreased number of leukocytes and absent platelets.
 |
Table 1 Laboratory Findings Before Treatment with IVIG |
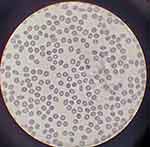 |
Figure 1 Peripheral blood smear with normal RBC morphology and absent platelets. |
Upon clinical examination, the patient maintained a good clinical condition (hemodynamically stable, absent signs of shock or hypotension) but with enlarged wet purpura on oral mucosa, petechias and ecchymoses as presented in Figures 2 and 3.
 |
Figure 2 Patient with wet purpura and petechias in the first 48 h since hospital admission. |
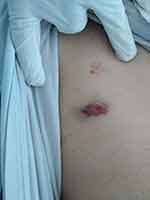 |
Figure 3 Ecchymosis in the first 48 h since hospital admission. |
Complement-mediated thrombotic microangiopathy (TMA) can be diagnosed based on the following seven criteria adapted from Gloude et al: elevated lactate dehydrogenase (LDH) levels; schistocytes on blood smear; new thrombocytopenia below the normal range for age; new anemia; evidence of proteinuria; blood pressure >99th percentile for age, sex, and height; elevated soluble C5b9 membrane attack complex.30 Because our patient presented normal lactate dehydrogenase (LDH) levels upon admission and hospital stay, normal RBC morphology on blood smear, normal hemoglobin levels for age group (11.3–13.7g/dL), normal blood pressure and absent proteinuria on urinalysis, she did not meet the criteria for TMA (at least five out of the seven criteria).31,32
Considering ITP as the most likely diagnosis a bone marrow examination was performed. The bone marrow aspirate showed megakaryocytic hyperplasia, consistent with a peripheral destruction of the platelets, and normocellularity of the granulocytic and erythrocytic series, thus confirming the diagnosis of ITP. Overall bleeding severity was assessed using the modified Buchanan and Adix bleeding score.25 Given the patient’s severe thrombocytopenia (platelet count <20×103/μL) and Buchanan and Adix moderate grade score with high risk of bleeding, treatment with intravenous methylprednisolone 1 mg/kg/day and platelet concentrate was administrated. After four doses of high-dose corticoid therapy, the hepatic and renal parameters returned within normal range, but the platelet count maintained the value of 0×103/μL, thus therapy with IVIG at a programmed dose of 1 g/kg/day for two days was administered, with immediate clinical and laboratory improvement (Figures 4 and 5, Table 2).
 |
Table 2 Laboratory Findings After Initiating Treatment with IVIG |
 |
Figure 4 Patient on D-8 of hospital admission, with clinical improvement of wet purpura. |
 |
Figure 5 Clinical improvement of ecchymosis. |
Twenty-four hours after the administration of the second dose of IVIG, the platelet count was 133×103/μL. Seventy-two hours after the administration of the second dose of IVIG (11 days from symptoms’ onset), the platelet count normalized (293×103/μL) and the nasopharyngeal swab for SARS-CoV-2 resulted negative, therefore no further therapies were administered. The patient was discharged the following day with regressed petechiae and ecchymosis on her body and oral mucosa. During 12 weeks of follow-up, her platelet count gradually increased to 440×103/μL and remained in optimal clinical condition at 12 months follow-up.
Throughout the hospital admission period, the patient remained hemodynamically stable with a good clinical condition. In light of these considerations, we hypothesize that the characteristic immune mechanism of ITP may be secondary to SARS-CoV-2 infection.
Discussion
Infections have been known as the most environmental trigger in the complex pathophysiology of autoimmune diseases and taking into consideration that viruses can induce type II and IV hypersensitivity reactions beside their specific cytopathic effect, COVID-19-mediated autoimmunity might be rationalized.33,34 The most common hematological abnormalities in SARS-CoV-2 infection are lymphopenia, cited in literature in up to 83.2% of patients and thrombocytopenia in 5–21% of cases.35 The potential mechanisms of thrombocytopenia in COVID-19 involve antiretroviral drugs, megakaryocyte suppression, hepatic or splenic sequestration, platelet clearance due to increased endothelial damage or due to platelet autoantibody formation.36
ITP is defined as immunological destruction of platelets that result in a low number of circulating platelets and possible thrombocytopenia pathogenic mechanisms such as molecular mimicry, cryptic antigen expression or epitope spreading depend on the phase of COVID-19.37
Platelets are critical for maintaining vascular integrity. They provide the surface for coagulation proteins and adhere to the vessel wall at sites of endothelial injury. Platelet-type bleeding in ITP manifests as spontaneous skin bruises or petechiae (“dry purpura”); mucous membrane purpura, typically visible in the mouth (“wet purpura”); and frank bleeding from mucosal surfaces causing vaginal, gastrointestinal, or intracranial hemorrhage.3 A systematic review of 7780 pediatric patients with COVID-19 by Hoang et al, did not find disease manifestations such as petechiae, ecchymosis or thrombocytopenia, therefore our patient presented a clinical condition different from the usual pattern, reporting dry and wet purpura as the initial symptoms.38 Soares et al reported the case of a 2-year-old patient who developed thrombocytopenia at two different times: during the acute infection and later, ITP, with complete response to IVIG as opposed to our patient who developed ITP in the first week since symptoms’ onset.39 According to Chen et al, thrombocytopenia in the late stage of the disease, 14 days after the onset of symptoms, is less frequent (11.8%) and significantly associated with higher mortality rate.40
Xu et al proposed three mechanisms by which thrombocytopenia can occur in COVID-19: (1) increased consumption of platelets due mainly to lung injury, generating microthrombi in the circulation and thus increasing their consumption; (2) reduction of platelet production by medullary invasion of the virus, inhibiting hematopoiesis, as well as by the reduction of hematopoietic precursors secondary to the cytokine storm; and (3) increased platelet destruction due to autoantibodies and immune complexes.41 In a study on 57 cases of ITP and COVID-19, Alonso-Beato et al reported a median nadir platelet count of 8×103/μL, similar to the findings of Bhattacharjee et al, who reported a median nadir platelet count of 5×103/μL in a review of 45 cases.28,42 Platelets counts were significantly lower in our patient compared with other ITP series.
A systemic approach is necessary to diagnose COVID-19 related ITP, especially in new-onset cases, since excluding other causes of thrombocytopenia, as well as looking for secondary causes is mandatory during the diagnostic process of ITP.42
TMA results from endothelial cell damage to small blood vessels, leading to hemolytic anemia, thrombocytopenia, and in some cases, organ damage.43 Complement dysregulation results in unregulated formation of the C5b9 membrane attack complex, leading to the clinical manifestations of TMA.32 In order to be categorized as having TMA, the patients had to meet at least five of the seven criteria adapted from Gloude et al: elevated LDH levels greater than the upper limit of normal for age; schistocytes on blood smear; new thrombocytopenia; new anemia; evidence of proteinuria on urinalysis; blood pressure >99th percentile for age, sex, and height; and elevated soluble C5b9.30 Because our patient presented normal lactate dehydrogenase (LDH) levels upon admission and hospital stay, normal RBC morphology on blood smear, normal hemoglobin levels for age group (11.3–13.7 g/dL), normal blood pressure, she did not meet the criteria for TMA despite having renal dysfunction and alanine aminotransferase or aspartate aminotransferase levels >3 times the upper limit of normal.13,30,44 The etiology of acute kidney disease (AKI) in SARS-CoV-2 infection is diverse and multifactorial.44 A review by Chen et al, proposed several mechanisms to justify the renal involvement in COVID-19 patients, including multiple organ dysfunction syndrome, direct renal infection by SARS-CoV-2, AKI after acute respiratory distress syndrome, and cytokine storm syndrome.45 In a retrospective study on 89 pediatric patients, by Kari et al, AKI occurred in one-fifth of children with SARS-CoV-2 infection requiring hospital admission, with one-third of those requiring pediatric ICU.46 Although our patient presented on the second admission day an increase in serum creatinine three times greater that the baseline level (AKI stage III), she did not develop hypervolemia, oliguria or required renal replacement therapy.
In cases like ours, it can be challenging to assess and differentiate between ITP and MIS-C, in which its manifestations mimic Kawasaki disease and demonstrate both type II and IV hypersensitivity characteristics.47
The most common presentations of MIS-C are fever >3 days, acute gastrointestinal symptoms such as diarrhea, vomiting, and abdominal pain, mucocutaneous inflammation signs on oral mucosa, hands or feet accompanied by rash or bilateral non-purulent conjunctivitis and periorbital edema, features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities, headaches or respiratory manifestations (including sore throat).48 Associated laboratory findings are elevated inflammation markers (C-reactive protein, ferritin, interleukin 6), neutrophilia, thrombocytopenia, lymphopenia, elevated troponin, D-dimers, procalcitonin and fibrinogen in the absence of obvious microbial cause of inflammation (including bacterial sepsis, staphylococcal or streptococcal shock syndrome).49 The disease course is typically severe (hypotension, shock and de novo coronary aneurysms), with high rates of intensive care unit (ICU) admissions, mechanical ventilation, and death.50 Even though our patient presented a history of low grade fever, elevated D-dimers, ferritin, C-reactive protein, hepatic transaminase, and creatinine, it should be noted that she maintained a clinically good condition throughout the admission period with no signs or symptoms of shock, viral pneumonia, gastrointestinal manifestations, organomegaly, or features of myocardial dysfunction, pericarditis, or coronary abnormalities. Given the WHO defining criteria for MIS-C and the degree of thrombocytopenia, peripheral blood smear, and lack of other physical examination findings, our patient presentation raised the concern for acute ITP rather than MIS-C.51 According to a consensus report by Provan et al, bone marrow aspiration is not required to make a diagnosis of ITP, but the procedure was performed on our patient in order to assess platelet production and to rule out any lymphoproliferative disorders.52
Our goal of treatment for the patient was to reduce the bleeding risk by a rapid increase in platelet count thus we selected methylprednisolone as a first-line treatment because corticosteroids have been shown to reduce platelet autoantibody production and increase platelet production.53
We added to the corticosteroid therapy platelet transfusions to prevent bleeding because her platelet count was below 10×103/μL; however, transfusion is not usually recommended in ITP patients who are not bleeding irrespective of the platelet count.54 Despite our efforts, after five days of combined treatment, platelet count remained 0×103/μL. In light of this event, we decided to administer a high-dose of IVIG (1 g/kg daily for two days) in association with corticosteroid therapy. IVIG has many complex mechanisms of action in decreasing inflammation including suppression or elimination of platelet autoantibodies, thus a rapid increase in platelet count was observed in our patient after the first dose of IVIG.53 The International Working Group defines complete response to ITP treatment as platelets count >100×103/μL and the absence of bleeding.55 A complete response in up to 12 months, is the natural evolution of ITP in children, as it was in our presented case.52
Conclusion
There is a growing body of literature regarding the clinical and laboratory manifestations of COVID-19 infection in children and is a well-known fact that ITP pathogenesis involves infection. ITP is characterized by isolated thrombocytopenia and platelet-type bleeding in ITP manifests as dry purpura, wet purpura and frank bleeding from mucosal surfaces. Possible pathogenetic mechanisms for secondary ITP depend on the phase of COVID-19 infection and include reduced platelet production due to destruction of the bone marrow progenitor cells or due to reduced thrombopoietin caused by direct liver damage and increased platelet destruction via production of cross-reacting autoantibodies. Considering that the natural evolution of ITP in children is remission in up to 12 months, the management of children with newly diagnosed ITP consists of careful observation, regardless of the platelet count an therapy with IVIG and/or corticosteroids. During the SARS-CoV-2 pandemic, it is important to remember the association between isolated thrombocytopenia and COVID-19 and to include the RT-PCR for SARS-CoV-2 in the laboratorial investigation and follow-up, as ITP can develop.
Ethics and Consent Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Hospital for Infectious Diseases “Victor Babes” Timisoara, No. 4536/14.05.2021. Written informed consent has been obtained from the patient’s legal guardian to publish this paper.
Funding
The authors received no specific funding for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ceglie G, De Ioris MA, Mercadante S, et al. Immune thrombocytopenia in a child with COVID‐19: is it the calm after the (cytokine) storm? Pediatr Blood Cancer. 2022;69(1). doi:10.1002/pbc.29326
2. Rand ML, Fraser WJ. Virus-associated idiopathic thrombocytopenic purpura. Transfus Sci. 1998;19(3):253–259. doi:10.1016/S0955-3886(98)00039-3
3. Kuhne T, Berchtold W, Michaels LA, et al. Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica. 2011;96(12):1831–1837. doi:10.3324/haematol.2011.050799
4. Aydin S, Altinkaynak GG, Kocabaş BA. A pediatric case report with immune thrombocytopenic purpura associated with COVID-19. Arch Pediatr. 2021;6(1):196.
5. Hamada M, Yasumoto S, Furue M. A case of varicella-associated idiopathic thrombocytopenic purpura in adulthood. J Dermatol. 2004;31(6):477–479. doi:10.1111/j.1346-8138.2004.tb00536.x
6. Malleson PN, Mackinnon MJ, Sailer-Hoeck M, Spencer CH. Review for the generalist: the antinuclear antibody test in children - when to use it and what to do with a positive titer. Pediatr Rheumatol. 2010;8(1):27. doi:10.1186/1546-0096-8-27
7. McCrae K. Immune thrombocytopenia: no longer ‘idiopathic. CCJM. 2011;78(6):358–373. doi:10.3949/ccjm.78gr.10005
8. Lim JH, Kim YK, Min SH, Kim SW, Lee YH, Lee JM. Epidemiology and viral etiology of pediatric immune thrombocytopenia through Korean Public Health Data Analysis. JCM. 2021;10(7):1356. doi:10.3390/jcm10071356
9. Enache A, Ciocan V, Muresan CO, et al. Postmortem documentation of SARS-CoV-2 in utero and postpartum transmission, through amniotic fluid, placental, and pulmonary tissue RT-PCR. Appl Sci. 2021;11(20):9505. doi:10.3390/app11209505
10. Marinescu AR, Laza R, Musta VF, et al. Clostridium difficile and COVID-19: general data, ribotype, clinical form, treatment-our experience from the largest infectious diseases hospital in Western Romania. Medicina. 2021;57(10):1099. doi:10.3390/medicina57101099
11. Tudoran M, Tudoran C, Lazureanu V, et al. Alterations of left ventricular function persisting during post-acute COVID-19 in subjects without previously diagnosed cardiovascular pathology. JPM. 2021;11(3):225. doi:10.3390/jpm11030225
12. Dumache R, Daescu E, Ciocan V, et al. Molecular testing of SARS-CoV-2 infection from blood samples in disseminated intravascular coagulation (DIC) and elevated D-dimer levels. Clin Lab. 2021;67. doi:10.7754/Clin.Lab.2020.200704
13. Tiwari NR, Phatak S, Sharma VR, Agarwal SK. COVID-19 and thrombotic microangiopathies. Thromb Res. 2021;202:191–198. doi:10.1016/j.thromres.2021.04.012
14. Cao W, Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30(5):367–369. doi:10.1038/s41422-020-0327-4
15. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
16. Hashemieh M, Tabatabaee S, Radfar M, Fahim P. COVID-19 associated thrombocytopenia in children: an emerging issue. Int J Pediatr. 2020. doi:10.22038/ijp.2020.52364.4162
17. Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020;16(3):240–246. doi:10.1007/s12519-020-00345-5
18. Alsohime F, Temsah MH, Al-Nemri AM, Somily AM, Al-Subaie S. COVID-19 infection prevalence in pediatric population: etiology, clinical presentation, and outcome. J Infect Public Health. 2020;13(12):1791–1796. doi:10.1016/j.jiph.2020.10.008
19. Castagnoli R, Votto M, Licari A, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882. doi:10.1001/jamapediatrics.2020.1467
20. Laza R, Musta VF, Nicolescu ND, et al. Cutaneous manifestations in SARS-CoV-2 infection—a series of cases from the largest infectious diseases hospital in Western Romania. Healthcare. 2021;9(7):800. doi:10.3390/healthcare9070800
21. Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4):968–981.e7. doi:10.1016/j.cell.2020.09.016
22. Patel PA, Chandrakasan S, Mickells GE, Yildirim I, Kao CM, Bennett CM. Severe pediatric COVID-19 presenting with respiratory failure and severe thrombocytopenia. Pediatrics. 2020;146(1):e20201437. doi:10.1542/peds.2020-1437
23. Kok EY, Srivaths L, Grimes AB, Vogel TP, Sexson Tejtel SK, Muscal E. Immune thrombocytopenia following multisystem inflammatory syndrome in children (MIS-C) – a case series. Pediatr Hematol Oncol. 2021;38(7):663–668. doi:10.1080/08880018.2021.1917737
24. Rosenzweig JD, McThenia SS, Kaicker S. SARS‐CoV‐2 infection in two pediatric patients with immune cytopenias: a single institution experience during the pandemic. Pediatr Blood Cancer. 2020;67(9). doi:10.1002/pbc.28503
25. Schoettler ML, Graham D, Tao W, et al. Increasing observation rates in low-risk pediatric immune thrombocytopenia using a standardized clinical assessment and management plan (SCAMP ®): schoettler et al. Pediatr Blood Cancer. 2017;64(5):e26303. doi:10.1002/pbc.26303
26. Zhang Y, Zeng X, Jiao Y, et al. Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thromb Res. 2020;193:110–115. doi:10.1016/j.thromres.2020.06.008
27. Behlivani E, Tragiannidis A, Hatzipantelis E, Panagopoulou P. Immune thrombocytopenia secondary to COVID‐19 infection: report of two cases. Pediatr Blood Cancer. 2021;68(10). doi:10.1002/pbc.29175
28. Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Compr Clin Med. 2020;2(11):2048–2058. doi:10.1007/s42399-020-00521-8
29. Quinn R, Murakhovskaya I. SARS-CoV-2 and autoimmune cytopenia. Hemato. 2021;2(3):463–476. doi:10.3390/hemato2030029
30. Gloude NJ, Dandoy CE, Davies SM, et al. Thinking beyond HLH: clinical features of patients with concurrent presentation of hemophagocytic lymphohistiocytosis and thrombotic microangiopathy. J Clin Immunol. 2020;40(5):699–707. doi:10.1007/s10875-020-00789-4
31. Staffa SJ, Joerger JD, Henry E, Christensen RD, Brugnara C, Zurakowski D. Pediatric hematology normal ranges derived from pediatric primary care patients. Am J Hematol. 2020;95. doi:10.1002/ajh.25904
32. Diorio C, McNerney KO, Lambert M, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4(23):6051–6063. doi:10.1182/bloodadvances.2020003471
33. Yazdanpanah N, Rezaei N. Autoimmune complications of COVID‐19. J Med Virol. 2022;94(1):54–62. doi:10.1002/jmv.27292
34. Berchtold P, McMillan R, Tani P, Sommerville-Nielsen S, Blanchette VS. Autoantibodies against platelet membrane glycoproteins in children with acute and chronic immune thrombocytopenic purpura. Blood. 1989;74(5):1600–1602. doi:10.1182/blood.V74.5.1600.1600
35. Bobircă A, Bobircă F, Ancuța I, et al. COVID-19—a trigger factor for severe immune-mediated thrombocytopenia in active rheumatoid arthritis. Life. 2022;12(1):77. doi:10.3390/life12010077
36. Bao C, Tao X, Cui W, et al. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp Hematol Oncol. 2020;9(1):16. doi:10.1186/s40164-020-00172-4
37. Liebman HA. Viral-associated immune thrombocytopenic purpura. Hematology. 2008;2008(1):212–218. doi:10.1182/asheducation-2008.1.212
38. Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. doi:10.1016/j.eclinm.2020.100433
39. Soares AC, Loggetto SR, Manga FCM, Faustino LR, Braga JAP. Outcome of SARS-CoV-2 and immune thrombocytopenia in a pediatric patient. Hematol Transfus Cell Ther. 2021;43(1):101–103. doi:10.1016/j.htct.2020.09.145
40. Chen W, Li Z, Yang B, et al. Delayed‐phase thrombocytopenia in patients with coronavirus disease 2019 (COVID‐19). Br J Haematol. 2020;190(2):179–184. doi:10.1111/bjh.16885
41. Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi:10.1007/s00277-020-04019-0
42. Alonso-Beato R, Morales-Ortega A, Fernández FJD, et al. Immune thrombocytopenia and COVID-19: case report and review of literature. Lupus. 2021;30(9):1515–1521. doi:10.1177/09612033211021161
43. Dalkıran T, Kandur Y, Kara EM, Dağoğlu B, Taner S, Öncü D. Thrombotic Microangiopathy in a Severe Pediatric Case of COVID-19. Clin Med Insights Pediatr. 2021;15:117955652110498. doi:10.1177/11795565211049897
44. Oi SSP, Muniz MPR, Faria IM, et al. Multisystemic inflammatory syndrome and thrombotic microangiopathy as complications of COVID-19 in a child: a case report. Front Pediatr. 2021;9:659069. doi:10.3389/fped.2021.659069
45. Chen YT, Shao SC, Hsu CK, Wu IW, Hung MJ, Chen YC. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):346. doi:10.1186/s13054-020-03009-y
46. Kari JA, Shalaby MA, Albanna AS, Alahmadi TS, Alherbish A, Alhasan KA. Acute kidney injury in children with COVID-19: a retrospective study. BMC Nephrol. 2021;22(1):202. doi:10.1186/s12882-021-02389-9
47. Icenogle T. COVID-19: infection or autoimmunity. Front Immunol. 2020;11:2055. doi:10.3389/fimmu.2020.02055
48. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259. doi:10.1001/jama.2020.10369
49. Rabinowicz S, Leshem E, Pessach IM. COVID-19 in the pediatric population—review and current evidence. Curr Infect Dis Rep. 2020;22(11):29. doi:10.1007/s11908-020-00739-6
50. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;m2094. doi:10.1136/bmj.m2094
51. Algarni AS, Alamri NM, Khayat NZ, Alabdali RA, Alsubhi RS, Alghamdi SH. Clinical practice guidelines in multisystem inflammatory syndrome (MIS-C) related to COVID-19: a critical review and recommendations. World J Pediatr. 2022;18(2):83–90. doi:10.1007/s12519-021-00499-w
52. Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi:10.1182/bloodadvances.2019000812
53. Song F, Al-Samkari H. Management of adult patients with Immune Thrombocytopenia (ITP): a review on current guidance and experience from clinical practice. JBM. 2021;12:653–664. doi:10.2147/JBM.S259101
54. Slichter SJ, Dumont LJ, Cancelas JA, et al. Safety and efficacy of cryopreserved platelets in bleeding patients with thrombocytopenia. Transfusion. 2018;58(9):2129–2138. doi:10.1111/trf.14780
55. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. doi:10.1182/blood-2008-07-162503
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
