Back to Journals » International Medical Case Reports Journal » Volume 11
Severe neutropenia in a breastfed infant: a case report and discussion of the differential diagnosis
Authors van den Broek L , van der Werff ten-Bosch J, Cortoos P , van Steijn S , van den Akker M
Received 11 May 2018
Accepted for publication 11 August 2018
Published 15 November 2018 Volume 2018:11 Pages 333—337
DOI https://doi.org/10.2147/IMCRJ.S173826
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Leonie van den Broek,1 Jutte van der Werff-ten Bosch,2 Pieter-Jan Cortoos,3 Susanne van Steijn,1 Machiel van den Akker1,2
1Department of Pediatrics, Queen Paola Children’s Hospital, Antwerp, Belgium; 2Department of Pediatric Hematology Oncology, UZ Brussel, Brussels, Belgium; 3Pharmacy, UZ Brussel, Brussels, Belgium
Abstract: Neonatal neutropenia is regularly seen with variable etiology. We describe a breastfed infant with maternal medication use as a probable cause of neonatal neutropenia. An 8 days old exclusively breastfed female infant of Arab-Berber descent was referred to our hospital because of an infection of the umbilicus. Complete blood count showed a picture of severe isolated neutropenia. After initiating intravenous antibiotic treatment, the infection quickly resolved, but the isolated neutropenia persisted. Bone marrow aspiration indicated severe congenital neutropenia. The mother was known to have Crohn’s disease, treated with methylprednisolone and adalimumab up to 3 months before delivery, and latent tuberculosis, for which she used isoniazid postnatally. Breast-feeding was terminated and filgrastim was started, with an increase of the neutrophilic count. After several weeks, filgrastim could be terminated. Bone marrow and complete blood count were repeated and were completely normal. This case report describes a very young breastfed female infant with severe neutropenia, causing an infection, in which maternal adalimumab use could not be excluded as a possible cause. Maternal isoniazid use is highly unlikely.
Keywords: congenital neutropenia, neonate, breast-feeding, adalimumab, isoniazid
Introduction
Neutrophils are the most abundant white blood cells in blood and play a critical role in recruiting and activating cells of the immune system, besides being a key player in the frontline defense against invading pathogens as part of the innate immune system. Reduction in neutrophils below an absolute count of 0.5×109/L is termed severe neutropenia or agranulocytosis.1
Neutropenia can be the result of a decreased production of neutrophils, an increased neutrophil destruction, or a combination of these mechanisms (Box 1).2 One of the causes is drug induced. In drug-induced immune neutropenia, drug-dependent antibodies are formed against neutrophil membrane glycoprotein, causing neutrophil destruction. Severe neutropenia or agranulocytosis associated with exposure to nonchemotherapy drugs is seen in up to 15.4 cases per million population per year.2
  | Box 1 Causes of neonatal neutropenia Notes: Adapted with permission from Nittala S, Subbarao GC, Maheswari A. Evaluation of Neutropenia in preterm infants. J Matern Featal Neonatal Med. 2012;25(Suppl 5):100–103. Copyright © 2012 Taylor & Francis Ltd; www.tandfonline.com.23 |
We report a very young breastfed female infant with severe neutropenia, in which adalimumab and isoniazid (INH) were used by the mother.
Case presentation
An 8 days old exclusively breastfed female infant was referred to our hospital because of an infection of the umbilicus without fever. She was the second child of nonconsanguineous parents, both of Arab-Berber descent, born after 39 weeks of pregnancy, complicated by intrauterine growth restriction. Birth weight 2.570 kg (SD –1.6), length 48 cm (SD –0.8), and head circumference 32 cm (SD –1.7). The mother was known to have Crohn’s disease, treated with oral methylprednisolone, in a gradually reducing dose with a maximum of 32 mg daily, and adalimumab, 40 mg subcutaneously every 2 weeks for up to 3 months before delivery. Additional investigations revealed a latent tuberculosis (positive interferon gamma release assay), for which she used INH 300 mg once a day, in combination with pyridoxine 125 mg, which both were started immediately after delivery. Family history is negative for hematologic diseases, syndromes, or early unexplained death.
Physical examination of the neonate was, besides a local infection of the umbilicus, normal for her age. No (skeletal) malformations, cutaneous, or nail abnormalities; hepatosplenomegaly; or hypotonia were noted. However, complete blood count showed a picture of severe isolated neutropenia (hemoglobin 18.2 g/dL, mean corpuscular volume 97, platelets 254×109/L, leukocytes 7.56×109/L, and absolute neutrophil count [ANC] 0.04×109/L; C-reactive protein 73.1 mg/L). The child was admitted and broad-spectrum intravenous antibiotic treatment (ampicillin and cefotaxime) was started. Cultures of the umbilicus revealed the growth of Staphylococcus aureus. Urine and blood cultures remained negative.
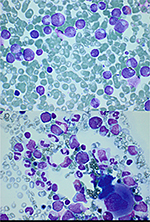
The infection improved; however, the isolated neutropenia persisted. No viral etiology (TORCH, which includes Toxoplasmosis, Other [syphilis, varicella-zoster, parvovirus B19], Rubella, cytomegalovirus, and herpes infections, [para]influenza, respiratory syncytial virus, adenovirus) could be demonstrated. Testing for antineutrophil antibodies was not done, as the tests often show false-positive and false-negative results.3 Bone marrow aspiration revealed a severe dysgranulopoiesis, characterized by a maturation stop after meta-/myelocyte stage (Figure 1), indicating severe congenital neutropenia. Maternal usage of adalimumab during pregnancy can cause neutropenia because it can cross the placenta from the maternal circulation into the fetal circulation. Also, agranulocytosis due to INH, used by the mother while breast-feeding, could not be excluded. Tuberculosis was excluded in the child, and the complete blood count of the mother did not show neutropenia. As a probability scale, we use the Naranjo algorithm,4 and this case was scored for both medications separately. INH was scored “3,” while adalimumab was scored “4,” both as a “possible” likelihood to be responsible for the neutropenia (Naranjo scores: 9 or 10 indicate “definitely”; 5–8 rate the likelihood as “probable”; 1–4 “possible”; <1 “doubtful”).
Breast-feeding was terminated, and filgrastim (Neupogen®, Amgen Inc., Thousand Oaks, CA, USA) 5 µg/kg subcutaneously was started. A very slow increase of ANC was seen, and so filgrastim dose was increased to 10 µg/kg subcutaneously, with good improvement. ANC increased to a maximum of 35.85×109/L. Two months after birth, filgrastim was terminated which, initially, led to a decrease of ANC, before it stabilized in the normal range (Figure 2). Two weeks after terminating, the filgrastim a new bone marrow aspiration (Figure 1) and a complete blood count was repeated. Both were normal, which excluded severe congenital neutropenia.
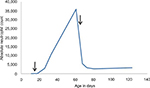  | Figure 2 ANC (cells/µL) of the patient (the arrows symbolize the start and the terminating of filgrastim) over several months. Abbreviation: ANC, absolute neutrophil count. |
Discussion
Adalimumab is a chimeric, monoclonal immunoglobulin G1 antibody directed against human TNF-α.5 Neutropenia is a common adverse reaction and may occur in up to 16% of patients receiving anti-TNF therapies, possibly due to a direct toxic effect on bone marrow.6 Adalimumab can cross the placenta from the maternal circulation into the fetal circulation,5 and peak levels (fetus) are reached during the third trimester of pregnancy.7–9 Consequently, these infants may be at increased risk for infection.10 Elimination of adalimumab is relatively long, half-life is about 2 weeks.7,9
In our case, the mother did not use adalimumab during the third trimester. Despite this, a case series demonstrated that significant levels of anti-TNF agents can be measured in cord blood, even when adalimumab was discontinued 8 weeks before delivery, and can be 2- to 3-fold higher than in the peripheral blood of the mother.8,9
In 2014, Guiddir et al5 reported four cases of severe neutropenia, all complicated by a skin infection, after maternal use of infliximab, which has similar anti-TNF-α activity as adalimumab, but a shorter half-life of 7–12 days.11 Similarly to our case, anti-TNF therapy was not given during the third trimester. We did not measure adalimumab levels in the mother or in the child. However, as far as we know, there is no other literature which describes a neonatal neutropenia due to the use of adalimumab during pregnancy.
INH, a pyridine derivative of nicotinamide, is the most widely used first-line anti-tuberculosis drug and the mainstay in the treatment of latent tuberculosis.12–14 It is excreted in breast milk at relatively small concentrations15 and is considered to be compatible with breast-feeding by the American Academy of Pediatrics and Centers for Disease Control and Prevention.14–16 Well-known adverse reactions of INH are an asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity.12 Neutropenia is a known adverse reaction, but less often described. INH is excreted in breast milk at relatively small concentrations, but limited data are available about the subsequent exposure to the nursing infant.14,15 Normally, peak plasma concentrations of INH are achieved around one hour after ingestion and a similar trend is seen in breast milk, indicating that INH is quickly absorbed and transferred to breast milk with a mean milk: plasma ratio of 0.9. The mean relative dose of INH (1.2%) transmitted to the infant via breast milk is below the 10% notional level of concern. Under normal circumstances, these amounts of drug transfer to breast milk are not directly toxic since they are much lower than the INH dose prescribed to the infant as a prophylactic dose of 10 µg/kg. These data suggest that INH therapy is safe during breast-feeding.14 However, pyridoxine supplements (1–2 mg/kg) are still recommended for breastfed infants if the mother uses INH.17,18
Although concentrations are low and toxic concentrations are rarely reached, pharmacological activity is possible.14 Toddywalla et al19 reported that INH is capable of suppressing hepatic drug metabolizing activity in infants of mothers on chronic therapy, and Pariente-Khayat et al20 reported that the maturation of N-acetyltransferase 2, which is the main route of metabolizing INH,14 occurs in the first 4 years of life.14,17,20
Finally, the rate of acetylation INH is also genetically determined with a bimodal distribution of slow and fast acetylators.20 Given the fact that the slow acetylator phenotype is the most common (72%) one in Moroccan/North-African populations, it is very well possible that for the mother peak concentrations in plasma and breast milk have been high and half-life time prolonged.21 Still, we cannot explain the severe neutropenia due to the most likely insignificant INH exposure to the infant via breast milk, which has been described by Garessus et al.22 When we consequently rule out INH as a possible cause of the congenital neutropenia, the Naranjo score of adalimumab will change to “7,” a “probable” likelihood to be responsible for the neutropenia.
Although we realize a neonatal alloimmune neutropenia provoked by the maternal production of neutrophil-specific allo-antibodies is a more common cause, and, as said before, unfortunately we cannot prove that the persistence of adalimumab is the cause of the severe neutropenia in our child, we think this may be a good explanation. We would like to call for awareness of the side effects of the use of adalimumab in pregnancy, even in the first and second trimester. For each case, an individual risk/benefit analysis is advised. If INH-induced neutropenia is suspected, it is wise to examine the INH concentration in plasma and breastmilk before intervening in the INH treatment of the mother.22
Conclusion
Adalimumab can cross the placenta from the maternal circulation into the fetal circulation, and peak levels are reached during the third trimester of pregnancy. Despite the advice of using adalimumab only in the first and second trimester, significant levels of anti-TNF agents are possible.
INH is excreted in breast milk at relatively small concentrations, and so it is considered to be compatible with breast-feeding. Toxic concentrations are rarely reached, but pharmacologically active plasma concentrations in breastfed infants due to suppressing hepatic drug metabolizing activity and N-acetyltransferase 2 immaturity in infants are theoretically possible. No evidence is found in the literature to support this assumption yet.
We reported an exclusively breastfed infant with severe neutropenia and infection possibly due to maternal adalimumab, which illustrates that thoughtfulness with the prescription of adalimumab in pregnancy, even in the first and second trimester, is important. For each case, an individual risk/benefit analysis is advised.
Author contributions
Leonie van den Broek drafted the initial manuscript. Pieter-Jan Cortoos participated in the writing of the manuscript. Machiel van den Akker carried out the initial analyses and coordinated and supervised the writing of the manuscript. Jutte van der Werfften Bosch, Pieter-Jan Cortoos, Susanne van Steijn, and Machiel van den Akker critically reviewed the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Curtis BR. Drug-induced immune neutropenia/agranulocytosis. Immunohematology. 2014;30(2):95–101. | ||
Maheshwari A. Neutropenia in the newborn. Curr Opin Hematol. 2014;21(1):43–49. | ||
Clay ME, Schuller RM, Bachowski GJ. Granulocyte serology: current concepts and clinical signifcance. Immunohematology. 2010;26(1):11–21. | ||
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. | ||
Guiddir T, Frémond ML, Triki TB, et al. Anti-TNF-α therapy may cause neonatal neutropenia. Pediatrics. 2014;134(4):e1189–e1193. | ||
Bessissow T, Renard M, Hoffman I, Vermeire S, Rutgeerts P, van Assche G. Review article: non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther. 2012;36(4):312–323. | ||
Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol. 2009;104(1):228–233. | ||
Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11(3):286–292quiz e24. | ||
Zelinkova Z, de Haar C, de Ridder L, et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2011;33(9):1053–1058. | ||
Baddley JW, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [I]: anti-tumor necrosis factor-α agents). Clin Microbiol Infect. 2018;24(Suppl 2):S10–S20. | ||
Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46(8):645–660. | ||
World Health Organization. Guidelines on the Management of Latent Tuberculosis. Geneva: World Health Organization; 2015. | ||
Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent mycobacterium tuberculosis infection. N Engl J Med. 2015;372(22):2127–2135. | ||
Singh N, Golani A, Patel Z, Maitra A. Transfer of isoniazid from circulation to breast milk in lactating women on chronic therapy for tuberculosis. Br J Clin Pharmacol. 2008;65(3):418–422. | ||
Tran JH, Montakantikul P. The safety of antituberculosis medications during breastfeeding. J Hum Lact. 1998;14(4):337–340. | ||
Centers for Disease Control and Prevention. Latent Tuberculosis Infection: A Guide for Primary Health Care Providers. Atlanta: Centers for Disease Control and Prevention; 2014. | ||
Baquero-Artigao F, Mellado Pena MJ, del Rosal Rabes T, et al. Spanish Society for Pediatric Infectious Diseases guidelines on tuberculosis in pregnant women and neonates (ii): prophylaxis and treatment. Anales Pediatria. 2015;83(4):286.e1–286.e7. | ||
Loto OM, Awowole I. Tuberculosis in pregnancy: a review. J Pregnancy. 2012;2012:379271. | ||
Toddywalla VS, Patel SB, Betrabet SS, Kulkarni RD, Kombo I, Saxena BN. Can chronic maternal drug therapy alter the nursing infant’s hepatic drug metabolizing enzyme pattern? J Clin Pharmacol. 1995;35(10):1025–1029. | ||
Pariente-Khayat A, Rey E, Gendrel D, et al. Isoniazid acetylation metabolic ratio during maturation in children. Clin Pharmacol Ther. 1997;62(4):377–383. | ||
Guaoua S, Ratbi I, Laarabi FZ, et al. Distribution of allelic and genotypic frequencies of NAT2 and CYP2E1 variants in Moroccan population. BMC Genet. 2014;15:156. | ||
Garessus Edg MH, Gundert-Remy U. Influence of fast and slow metabolizer status on the pharmacokinetics of isoniazid in lactating women and breast-fed infants. Naunyn-Schmiedeberg’s Arch Pharmacol. 2018;391:S75. | ||
Nittala S, Subbarao GC, Maheswari A. Evaluation of Neutropenia in preterm infants. J Matern Featal Neonatal Med. 2012;25(Suppl 5):100–103. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.