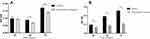Back to Journals » Nature and Science of Sleep » Volume 14
Severe Intermittent Hypoxia Modulates the Macrophage Phenotype and Impairs Wound Healing Through Downregulation of HIF-2α
Authors Chen L, Gao Y , Li Y, Wang C, Chen D, Gao Y, Ran X
Received 13 July 2022
Accepted for publication 23 August 2022
Published 31 August 2022 Volume 2022:14 Pages 1511—1520
DOI https://doi.org/10.2147/NSS.S382275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Lihong Chen,1 Yunyi Gao,2 Yan Li,1 Chun Wang,1 Dawei Chen,1 Yun Gao,1 Xingwu Ran1
1Innovation Center for Wound Repair, Diabetic Foot Care Center, Department of Endocrinology and Metabolism, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 2Department of Medical Affairs, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China
Correspondence: Xingwu Ran, Innovation Center for Wound Repair, Diabetic Foot Care Center, Department of Endocrinology and Metabolism, West China Hospital, Sichuan University, 37 Guo Xue Lane, Chengdu, 610041, People’s Republic of China, Tel +86 028-85422046, Email [email protected]
Background: Obstructive sleep apnea is prevalent in patients with diabetic foot ulcers, while the effect of intermittent hypoxia on wound healing is unclear. The objective of this study was to investigate the effect of severe intermittent hypoxia on wound healing.
Methods: C57BL/6 mice were exposed to 5 weeks of severe intermittent hypoxia or normoxia. The wound healing rate were assessed. The gene expression of CD206 and HIF-2α was tested in vivo and in vitro. Inflammatory factors in RAW264.7 macrophages were measured to investigate the effect of intermittent hypoxia on macrophage polarization. The proliferation of HUVECs and HaCaT cells was also assessed after exposure to intermittent hypoxia.
Results: Severe intermittent hypoxia decreased wound healing at day 3. The expression of CD206 and HIF-2α was significantly decreased after exposure to severe intermittent hypoxia. In vitro, severe intermittent hypoxia significantly promoted M1 phenotype polarization of RAW264.7 macrophages and increased the expression of proinflammatory factors (IL-1β and TNF-α). Severe intermittent hypoxia also decreased the proliferation of HUVECs cultured in endothelial cell medium and HaCaT cells cultured in high glucose DMEM.
Conclusion: Severe intermittent hypoxia could lead to M1 but not M2 macrophage polarization through downregulation of HIF-2α, and then lead to impaired wound healing.
Keywords: wound and injuries, intermittent hypoxia, wound healing, inflammation, hypoxia inducible factor
Introduction
Diabetic foot ulcers (DFUs) are very common in patients with diabetes mellitus, with a lifetime incidence estimated to be between 19% and 34%.1 It is one of the most serious complications of patients with diabetes, with foot amputation required in up to 20% of these patients.2 DFUs are associated with a 2.5-fold higher risk of death than diabetic patients without foot ulcers.1 DFUs impose a considerable burden on patients and health care service systems.
Obstructive sleep apnea (OSA) is a common form of sleep-disordered breathing. It is characterized by episodic sleep state-dependent collapse of the upper airway, resulting in periodic reductions or cessation in ventilation, with consequent intermittent hypoxia, hypercapnia, or arousals from sleep.3 The prevalence of OSA is estimated to be 4% in men and 3% in women in the general population.4 However, it is more prevalent in patients with DFU, with more than 90% of patients having OSA (AHI≥5 events/h). Of people with OSA, the severity of moderate to severe accounted for more than half of the population.5
Given the high prevalence of OSA in patients with DFU, it is imperative to investigate the impact of OSA on wound healing. A previous study in patients with DFUs showed that a STOP-BANG score ≥4 significantly increased the relative risk of poor wound healing.6 Another study by Andres et al found that healing of amputation site wounds was not significantly impaired by prior diagnosis of OSA;7 the use of continuous positive airway pressure treatment may explain the higher wound healing rate observed in patients with SDB, although only a small proportion of patients (28%) received treatment.7 In our previous study, we also did not find an association between AHI and wound healing, while that study revealed that sleep fragmentation and hypoxemia of OSA in patients with DFUs were strong predictors of poor wound healing, high ulcer recurrence, and increased risk of death.8 Thus, it is imperative to investigate whether OSA would further impair wound healing in DFUs.
Chronic intermittent hypoxia is one of the selected features of OSA and is commonly used as a model of human disease.9 It was found that mild intermittent hypoxia was protective, while severe intermittent hypoxia exacerbated tissue injury after artery occlusion.10,11 Similar findings were revealed in vitro experiments. Campillo et al revealed that the frequency and magnitude of intermittent hypoxia could modulate endothelial wound healing in vitro.12 Therefore, it is important to distinguish models between mild and severe intermittent hypoxia when investigating the effect of intermittent hypoxia.
Wound healing is a critical physiological process to maintain skin integrity and involves perfect interactions of numerous cell types and molecules.13 Among the cell types involved in wound healing, macrophages play a central role. Inflammatory macrophages are engaged in the inflammation response after wounding, while alternatively activated macrophages are essential for transition from inflammation stage to proliferation stage.14 Vascular endothelial cells and fibroblasts are involved in the angiogenesis and formation of granulation tissue.15 Re-epithelialization by epithelial cells occurs to close the skin gap and restores the barrier function of the skin.15 The impact of OSA on wound healing might be introduced through the influence on the cell types.
In this study, we employed severe but not mild intermittent hypoxia to simulate the impact of OSA on wound healing. The aim of this study was to investigate the impact of severe intermittent hypoxia on wound healing.
Methods
Animals
Experiments were performed in 10-week-old male C57BL/6 mice (Chengdu Dossy Experimental Animal Co. Ltd). The average weight was 23.3±0.8 gram, with mean blood glucose 8.3±1.1mmol/L. Severe intermittent hypoxia was applied for 5 weeks. Then, two excisional full-thickness 6-mm wounds were created on the dorsum of the mice. The wound area was recorded at day 0, 3, 7, and 10. The skin samples were flash frozen and stored at −80°C until analysis. The animal studies were approved by the Ethics Review Committee of Experimental Animal, West China Hospital, Sichuan University (Reference No. 2021410A). We followed the Guide for the Care and Use of Laboratory Animals on the use of laboratory animals.
Protocols for Severe Intermittent Hypoxia
All mice were housed at room temperature and subjected to a 12 h light/dark cycle. Intermittent hypoxia was induced using an animal hypoxic control machine (ProOx-100, Tow, China).16 Briefly, mice were randomly assigned to intermittent hypoxia or sham groups. In the severe intermittent group, oxygen levels within the chamber rapidly transitioned from ambient oxygen concentration (20.9%±0.5%) to low oxygen (5%±0.5%) over 30 sec, maintained for 30 sec, rapidly returned to ambient oxygen and maintained for 30 sec. The low oxygen was generated with the rapid mixture of pure oxygen and nitrogen from two separate tanks, and the oxygen concentration was assessed with a real-time oxygen monitoring device of the hypoxic control machine (ProOx-100). On average, 30 episodes/h of intermittent hypoxia were induced. Cyclic hypoxia was induced from 9:00 to 17:00 every day for 5 weeks. (Supplementary Figure 1A) Mice had free access to water and food.17
Cell Culture and in vitro Severe Intermittent Hypoxia Protocol
HaCaT cells, purchased from BeNa Culture Collection, were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, China) supplemented with 10% fetal bovine serum (Gibco, South America) and 1% penicillin/streptomycin (Gibco, China) and maintained at 37°C in humidified air with 5% CO2. HUVECs, purchased from Sciencell research laboratories, were maintained in endothelial cell medium (ScienCell, US). RAW264.7 cells (macrophages), purchased from Guangzhou Jennio Biotech Co., Ltd, were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum.
Advanced Tyrode’s solution (Solarbio, China) was flushed with N2 for 15 minutes to become hypoxic medium (3.1–4.2% oxygen concentration). Hypoxia experiments were performed using a costumed airtight chamber. The chamber was flushed with 99% N2 pressurized gas until the partial pressure of O2 in the chamber was 3%. For severe intermittent hypoxia, the plates were changed with N2-saturated hypoxic medium and moved promptly to the hypoxia chamber for 5 minutes. The plates were then removed from the chamber and placed in a room air (20.9% O2) environment for 10 minutes. The 5-minute hypoxia and 10-minute normoxia cycles were repeated 12 times.18 (Supplementary Figure 1B) Cell proliferation was determined using Cell Counting Kit-8 assays. Lysates were extracted for quantitative reverse transcriptase-polymerase chain reaction (RT-PCR).
Quantitative Reverse Transcriptase-Polymerase Chain Reaction
Skin samples were flash frozen and stored at −80°C until analysis. Total RNA was extracted using TRIzol reagent (Invitrogen, CA). cDNA was synthesized using the PrimeScriptTM RT reagent kit with gDNA Eraser (Takara Bio Inc., JA), and qPCR was performed with a LightCycler 96 (Roche, Switzerland) using TB Green Premix Ex TaqTM II (Takara Bio Inc., JA). The following primers were used in reactions: HIF-2α F: 5’-GTGACATGATCTTTCTGTCGGAA-3’ and R: 5’-CGCAAGGATGAGTGAAGTCAAA-3’, IL-1β F: 5’-GAAATGCCACCTTTTGACAGTG-3’ and R: 5’-TGGATGCTCTCATCAGGACAG-3’, IL-6 F: 5’-CTGCAAGAGACTTCCATCCAG-3’ and R: 5’-AGTGGTATAGACAGGTCTGTTGG-3’, TNF-α F: 5’-CTGAACTTCGGGGTGATCGG-3’ and R: 5’-GGCTTGTCACTCGAATTTTGAGA-3’, IL-10 F: 5’-GCTCTTACTGACTGGCATGAG-3’ and R: 5’-CGCAGCTCTAGGAGCATGTG-3’, CD206 F: 5’-CTCTGTTCAGCTATTGGACGC-3’ and R: 5’-CGGAATTTCTGGGATTCAGCTTC-3’; VEGFA F: 5’-GCACATAGAGAGAATGAGCTTCC-3’ and R: 5’-CTCCGCTCTGAACAAGGCT-3’. Expression levels were normalized to the expression of β-actin (a housekeeping gene).
Statistics
Data are expressed as the mean ± standard deviation. The P values ≤ 0.05 were considered significant. Statistical analysis was performed with Stata version 13 (Stata Corp, College Station, TX). Multilevel mixed-effects models were used to compare wound healing among groups. Other intergroup comparisons were made using either t-test or ANOVA as appropriate.
Results
Severe Intermittent Hypoxia Impairs Wound Healing
After 5 weeks of severe intermittent hypoxia, wounds were created in all mice at the dorsum. We observed the area of the wound for 10 days. Exposure to severe intermittent hypoxia significantly decreased the wound area reduction at day 3 (P =0.006) (Figure 1). The gene expression of HIF-2α was significantly decreased after exposure to severe intermittent hypoxia (P= 0.025) (Figure 2A). We evaluated the macrophage phenotype using CD206 as a marker of M2 macrophages. The expression of CD206 was significantly decreased in mice that were exposed to severe intermittent hypoxia (P= 0.016) (Figure 2B). This indicated that severe intermittent hypoxia significantly inhibited the expression of M2 phenotype macrophages. In addition, the relative expression of VEGFA was lower at day 3 after intermittent hypoxia than that of control (P< 0.001, Supplementary Figure 2).
Severe Intermittent Hypoxia Promotes M1 Polarization of RAW264.7 Macrophages and the Expression of Proinflammatory Factors
We evaluated the M1 and M2 polarization of RAW264.7 macrophages after severe intermittent hypoxia exposure. The gene expression of CD86 was significantly increased after severe intermittent hypoxia (P <0.001) (Figure 3A), whereas the expression of CD206 did not change after severe intermittent hypoxia (P =0.682) (Figure 3B). This indicated that exposure to severe intermittent hypoxia could promote M1 polarization of macrophages.
We also evaluated the gene expression of IL-1β, IL-6, and TNF-α, which are proinflammatory factors, and IL-10, which is an anti-inflammatory factor. The gene expression of IL-1β and TNF-α was significantly increased after severe intermittent hypoxia (P =0.002, P <0.001) (Figure 3C and E), whereas the expression of IL-6 and IL-10 did not change after severe intermittent hypoxia (P =0.092, P =0.682) (Figure 3D and F). Thus, severe intermittent hypoxia could promote inflammation.
Severe Intermittent Hypoxia Decreased the Proliferation of HUVECs and HaCaT Cells
To investigate the impact of severe intermittent hypoxia on wound healing-related cells, we assessed the proliferation of HUVECs and HaCaT cells using the CCK-8 test. The results showed that the proliferation of HUVECs was significantly decreased after severe intermittent hypoxia (P <0.001) (Figure 4A). The proliferation of HaCaT cells cultured in high glucose DMEM medium was also decreased (P <0.001) (Figure 4B).
Discussion
The results of the current study demonstrated that exposure to severe intermittent hypoxia, a pathognomonic feature of OSA, could lead to downregulation of HIF-2α and M1 macrophage polarization but not M2, eventually leading to impaired wound healing in mice. In vitro, this study also revealed that exposure to severe intermittent hypoxia might induce decreased proliferation of HUVECs and HaCaT cells.
To the best of our knowledge, this is the first study suggesting that exposure to severe intermittent hypoxia could induce impaired wound healing in animal models. This is similar with previous work by Maltese et al6 demonstrating that probable OSA, as identified from the STOP-Bang questionnaire, predicted poor wound healing in patients with DFUs. It is well established that OSA is associated with daytime sleepiness and cardiovascular disease.19 Furthermore, a large body of observational evidence indicates that OSA is associated with glucose intolerance, insulin resistance, and type 2 diabetes mellitus.20,21 OSA may contribute to ulcer development through the increased risk of diabetic polyneuropathy, impaired glucose metabolism, and obesity.22,23 The current study extends previous observations by demonstrating that exposure to severe intermittent hypoxia could lead to impaired wound healing. This might further delay the wound healing of DFUs.
In this study, we found that exposure to severe intermittent hypoxia could induce downregulation of HIF-2α, which is consistent with previous studies.24–27 In fact, previous work has shown that, unlike continuous hypoxia, intermittent hypoxia results in differential regulation of HIF-1α and HIF-2α.24–27 Intermittent hypoxia leads to downregulation of HIF-2α via a calpain-dependent signaling pathway and results in oxidative stress.24,28 HIF-2α can induce macrophages to polarize to the M2 phenotype by adjusting the levels of inducible nitric oxide synthase and arginase 1.29 HIF-2α can also upregulate mitochondrial superoxide dismutase and inhibit the generation of reactive oxygen species (ROS),24,30 thereby inhibiting the activation of macrophages and exerting anti-inflammatory effects.30 Reduction of HIF-2α caused by severe intermittent hypoxia may cause an increase in ROS levels. Increased ROS production can cause activation of the NF-κB pathway, upregulate adhesion molecules, inflammatory factors, and adipokines, promote the activation of platelets and white blood cells, and then promote inflammation.31,32 ROS can also cause mitochondrial damage and apoptosis.33 Of note, in addition to HIF-2α, other HIF subunits also have important role in different stages of wound healing.34 HIF-1 and HIF-2 have same downstream targets such as VEGF that facilitate angiogenesis, while they also have different role in inflammation.28,35
It is well established that macrophages are critical to normal wound healing and tissue regeneration.36 Macrophages are categorized as proinflammatory (M1) or anti-inflammatory (M2). M1 macrophages are observed in initial tissue damage responses, facilitating innate immunity and wound debridement.37 M2 macrophages predominate later in repair, expressing vascular endothelial growth factor and transforming growth factor beta, assisting in the resolution of inflammation, and promoting vascularization and tissue formation and remodeling.38–40 Continuous activation of M1 macrophages can cause an increase in inflammatory factors, leading to chronic inflammation of the wound. Downregulated HIF-2α levels after exposure to severe intermittent hypoxia could polarize macrophages to the M1 phenotype rather than the M2 phenotype and thus lead to chronic inflammation.
The fact that severe intermittent hypoxia induced downregulation of M2 macrophages in the wound is consistent with previous studies in other tissues. Severe intermittent hypoxia could induce a proinflammatory phenotype of visceral adipose tissue in mice with M1 macrophage polarization.41 In vitro, our study demonstrated that severe intermittent hypoxia could act as a potent proinflammatory stimulus, inducing macrophage polarization to an M1 but not M2 phenotype and resulting in Il-1β and TNF-α expression. The proinflammatory potential of severe intermittent hypoxia was also revealed in TH1 cell lines and bone marrow-derived macrophages.22,42,43 Thus, exposure to severe intermittent hypoxia could decrease anti-inflammatory M2 macrophage polarization through the downregulation of HIF-2α. The expression of proinflammatory factors, such as ROS, could further lead to continuous chronic inflammation and tissue injury, which in turn leads to delayed wound healing. In addition, the study also revealed that the level of VEGF was also decreased after intermittent hypoxia. This might also be a mechanism that intermittent hypoxia interferes the wound healing.
Our study also revealed that exposure to severe intermittent hypoxia could significantly decrease the proliferation of HUVECs and HaCaT cells in high glucose medium. The study by Campillo et al12 demonstrated that the frequency and magnitude of intermittent hypoxia markedly altered endothelial wound closure in vitro, with reduced wound closure after exposure to severe intermittent hypoxia. Another study also revealed that moderate and severe intermittent hypoxia diminished the mobilization, chemotactic and angiogenetic ability of endothelial progenitor cells.44 Chronic severe intermittent hypoxia-induced mitochondrial dysfunction could mediate endothelial oxidative stress and apoptosis.45,46 Re-epithelization involving keratinocytes and angiogenesis involving vascular endothelial cells are critical to wound healing.13,47 The regulation of severe intermittent hypoxia on these two cells might also contribute to impaired wound healing.
The results we report here have implications for clinical practice. It is well known that infection and lower extremity artery disease are the most complicating factors of DFUs. However, the prevalence of OSA in patients with DFUs is relatively high.5 Furthermore, as shown by our study, exposure to severe intermittent hypoxia might impair wound healing, which, if neglected, might become an obstacle to timely wound closure in patients with OSA. Therefore, it is imperative to pay attention to OSA in the management of DFUs, particularly those with a severe degree of OSA.
The current study has several limitations that merit discussion. First, we only investigated the impact of severe intermittent hypoxia on wound healing, which is one aspect of OSA. In fact, sleep fragmentation, another important feature of OSA, can promote inflammation by increasing proinflammatory cytokines, such as interleukin-1 and tumor necrosis factor-α,48,49 and decreasing chemokines, such as interleukin-8.50 In turn, chronic inflammation can lead to impaired angiogenesis and disorganized granulation, hence compromising wound healing in these patients.51 Thus, OSA might have a more profound impact on wound healing. We summarized the findings of this study and potential mechanism of OSA on wound healing in Figure 5. Second, the cycles of severe intermittent hypoxia in in vitro experiments were relatively short. Third, in this study, we employed acute wound models to investigate the effect of severe intermittent hypoxia on wound healing. It would be better to employ a chronic wound model to simulate clinical chronic wounds, such as DFUs.
Conclusion
Severe intermittent hypoxia could lead to M1 but not M2 macrophage polarization through downregulation of HIF-2α and eventually lead to impaired wound healing. The regulation of severe intermittent hypoxia on the proliferation of HUVECs and HaCaT cells might also contribute to impaired wound healing. Further research is needed to ascertain whether the identification and treatment of OSA could improve wound healing in patients with chronic wounds, especially in patients with DFUs.
Acknowledgments
The work was partial supported by 1.3.5 Project for disciplines of excellence, West China Hospital, Sichuan University [Grant No. ZYGD18025], Science and Technology Bureau of Sichuan Province [Grant No. 2021JDKP004], Health Medical Big Data Application and Innovation Project in Sichuan [Grant No.2018gfgw001], Science and Technology Bureau of Chengdu city [Grant No. 2017-CY02 to 00028-GX].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas: took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. doi:10.1056/NEJMra1615439
2. Xu Z, Ran X. Diabetic foot care in China: challenges and strategy. Lancet Diabetes Endocrinol. 2016;4:297–298. doi:10.1016/S2213-8587(16)00051-6
3. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi:10.1152/physrev.00043.2008
4. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi:10.1056/NEJM199304293281704
5. Chen L, Ma W, Tang W, et al. Prevalence of obstructive sleep apnea in patients with diabetic foot ulcers. Front Endocrinol (Lausanne). 2020;11:416. doi:10.3389/fendo.2020.00416
6. Maltese G, Fountoulakis N, Drakatos P, et al. Elevated obstructive sleep apnoea risk score is associated with poor healing of diabetic foot ulcers: a prospective cohort study. Diabetic Med. 2018;35:1494–1498. doi:10.1111/dme.13780
7. Andrews KL, Dib M, Shives TC, Hoskin TL, Liedl DA, Boon AJ. The effect of obstructive sleep apnea on amputation site healing. J Vasc Nurs. 2012;30:61–63. doi:10.1016/j.jvn.2011.12.003
8. Chen L, Ma W, Covassin N, et al. Association of sleep-disordered breathing and wound healing in patients with diabetic foot ulcers. J Clin Sleep Med. 2021;17:909–916. doi:10.5664/jcsm.9088
9. Veasey S. Insight from animal models into the cognitive consequences of adult sleep-disordered breathing. ILAR J. 2009;50:307–311. doi:10.1093/ilar.50.3.307
10. Jackman KA, Zhou P, Faraco G, et al. Dichotomous effects of chronic intermittent hypoxia on focal cerebral ischemic injury. Stroke. 2014;45:1460–1467. doi:10.1161/STROKEAHA.114.004816
11. Beguin PC, Joyeux-Faure M, Godin-Ribuot D, Levy P, Ribuot C. Acute intermittent hypoxia improves rat myocardium tolerance to ischemia. J Appl Physiol. 2005;99:1064–1069. doi:10.1152/japplphysiol.00056.2005
12. Campillo N, Falcones B, Montserrat JM, et al. Frequency and magnitude of intermittent hypoxia modulate endothelial wound healing in a cell culture model of sleep apnea. J Appl Physiol. 2017;123:1047–1054. doi:10.1152/japplphysiol.00077.2017
13. Singer AJ, Clark RA, Epstein FH. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi:10.1056/NEJM199909023411006
14. Eming SA, Murray PJ, Pearce EJ. Metabolic orchestration of the wound healing response. Cell Metab. 2021;33:1726–1743. doi:10.1016/j.cmet.2021.07.017
15. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. doi:10.1152/physrev.00067.2017
16. Xu C, Xu J, Zou C, et al. Chronic intermittent hypoxia regulates camkii-dependent MAPK signaling to promote the initiation of abdominal aortic aneurysm. Oxid Med Cell Longev. 2021;2021:2502324. doi:10.1155/2021/2502324
17. Polak J, Shimoda LA, Drager LF, et al. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: partial improvement with cessation of the exposure. Sleep. 2013;36:
18. Koyama T, Temma K, Akera T. Reperfusion-induced contracture develops with a decreasing [Ca2+]i in single heart cells. Am J Physiol. 1991;261:H1115–H22. doi:10.1152/ajpheart.1991.261.4.H1115
19. Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med. 2019;380:1442–1449. doi:10.1056/NEJMcp1816152
20. Huang T, Lin BM, Stampfer MJ, Tworoger SS, Hu FB, Redline S. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. cohorts. Diabetes Care. 2018;41:2111–2119. doi:10.2337/dc18-0675
21. Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013;1:329–338. doi:10.1016/S2213-2600(13)70039-0
22. Schaefer E, Wu W, Mark C, et al. Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol Commun. 2017;1:326–337. doi:10.1002/hep4.1045
23. Altaf QA, Ali A, Piya MK, Raymond NT, Tahrani AA. The relationship between obstructive sleep apnea and intra-epidermal nerve fiber density, PARP activation and foot ulceration in patients with type 2 diabetes. J Diabetes Complications. 2016;30:1315–1320. doi:10.1016/j.jdiacomp.2016.05.025
24. Nanduri J, Wang N, Yuan G, et al. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106:1199–1204. doi:10.1073/pnas.0811018106
25. Lam SY, Tipoe GL, Liong EC, Fung ML. Hypoxia-inducible factor (HIF)-1alpha and endothelin-1 expression in the rat carotid body during intermittent hypoxia. Adv Exp Med Biol. 2006;580:
26. Lam SY, Tipoe GL, Liong EC, Fung ML. Differential expressions and roles of hypoxia-inducible factor-1alpha, −2alpha and −3alpha in the rat carotid body during chronic and intermittent hypoxia. Histol Histopathol. 2008;23:271–280. doi:10.14670/HH-23.271
27. Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–4328. doi:10.1074/jbc.M407706200
28. Prabhakar NR, Peng YJ, Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J Clin Invest. 2020;130:5042–5051. doi:10.1172/JCI137560
29. Takeda N, O’Dea EL, Doedens A, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi:10.1101/gad.1881410
30. Dehn S, DeBerge M, Yeap XY, et al. HIF-2alpha in resting macrophages tempers mitochondrial reactive oxygen species to selectively repress MARCO-dependent phagocytosis. J Immunol. 2016;197:3639–3649. doi:10.4049/jimmunol.1600402
31. Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–1484. doi:10.1183/09031936.00086608
32. Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia – revisited – the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45. doi:10.1016/j.smrv.2014.07.003
33. Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM. Biological and physiological role of reactive oxygen species–the good, the bad and the ugly. Acta Physiol. 2015;214:329–348. doi:10.1111/apha.12515
34. Mima A. Hypoxia-inducible factor-prolyl hydroxylase inhibitors for renal anemia in chronic kidney disease: advantages and disadvantages. Eur J Pharmacol. 2021;912:174583. doi:10.1016/j.ejphar.2021.174583
35. Mima A, Horii Y. Treatment of renal anemia in patients with hemodialysis using hypoxia-inducible factor (HIF) stabilizer, roxadustat: a short-term clinical study. Vivo. 2022;36:1785–1789. doi:10.21873/invivo.12892
36. He L, Marneros AG. Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am J Pathol. 2013;182:2407–2417. doi:10.1016/j.ajpath.2013.02.032
37. Slauch JM. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol Microbiol. 2011;80:580–583. doi:10.1111/j.1365-2958.2011.07612.x
38. Zhu Z, Ding J, Ma Z, Iwashina T, Tredget EE. Alternatively activated macrophages derived from THP-1 cells promote the fibrogenic activities of human dermal fibroblasts. Wound Repair Regen. 2017;25:377–388. doi:10.1111/wrr.12532
39. Willenborg S, Lucas T, Van Loo G, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. J Blood. 2012;120:613–625. doi:10.1182/blood-2012-01-403386
40. Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care. 2012;1:10–16. doi:10.1089/wound.2011.0307
41. Murphy AM, Thomas A, Crinion SJ, et al. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J. 2017;49:1601731. doi:10.1183/13993003.01731-2016
42. Zhou J, Bai W, Liu Q, Cui J, Zhang W. Intermittent hypoxia enhances THP-1 monocyte adhesion and chemotaxis and promotes M1 macrophage polarization via RAGE. Biomed Res Int. 2018;2018:1650456. doi:10.1155/2018/1650456
43. Fitzpatrick SF, King AD, O’Donnell C, Roche HM, Ryan S. Mechanisms of intermittent hypoxia-mediated macrophage activation – potential therapeutic targets for obstructive sleep apnoea. J Sleep Res. 2021;30:e13202. doi:10.1111/jsr.13202
44. Song T, Chen M, Wang X, et al. Intermittent hypoxia: friend or foe on endothelial repair in mouse model. Exp Lung Res. 2021;47:211–225. doi:10.1080/01902148.2021.1891355
45. Yan YR, Zhang L, Lin YN, et al. Chronic intermittent hypoxia-induced mitochondrial dysfunction mediates endothelial injury via the TXNIP/NLRP3/IL-1β signaling pathway. Free Radic Biol Med. 2021;165:401–410. doi:10.1016/j.freeradbiomed.2021.01.053
46. Chen Q, Lin G, Huang J, et al. Inhibition of miR-193a-3p protects human umbilical vein endothelial cells against intermittent hypoxia-induced endothelial injury by targeting FAIM2. Aging. 2020;12:1899–1909. doi:10.18632/aging.102729
47. Velnar T, Gradisnik L. Tissue augmentation in wound healing: the role of endothelial and epithelial cells. Med Arch. 2018;72:444–448. doi:10.5455/medarh.2018.72.444-448
48. McLain JM, Alami WH, Glovak ZT, et al. Sleep fragmentation delays wound healing in a mouse model of type 2 diabetes. Sleep. 2018;41:zsy156.
49. Dumaine JE, Ashley NT. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am J Physiol Regul Integr Comp Physiol. 2015;308:R1062–9. doi:10.1152/ajpregu.00049.2015
50. Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol. 2018;124:190–200. doi:10.1152/japplphysiol.00547.2017
51. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi:10.1038/sj.jid.5700701
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.