Back to Journals » Clinical Interventions in Aging » Volume 15
Severe Aortic Stenosis and ATTRwt Amyloidosis – Beware in the Aging: A Case Report and Review of the Literature
Authors Adam R, Munteanu A, Mititelu R , Onciul S , Deleanu D, Iliescu VA, Popescu BA, Jurcut R
Received 12 June 2020
Accepted for publication 5 August 2020
Published 2 October 2020 Volume 2020:15 Pages 1863—1872
DOI https://doi.org/10.2147/CIA.S265103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Robert Adam,1,2 Alice Munteanu,3 Raluca Mititelu,4 Sebastian Onciul,2,5 Dan Deleanu,1 Vlad Anton Iliescu,2,6 Bogdan A Popescu,1,2 Ruxandra Jurcut1,2
1Department of Cardiology, “Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania; 2“Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; 3Department of Cardiology, Emergency University Central Military Hospital, Bucharest, Romania; 4Department of Nuclear Medicine, Emergency University Central Military Hospital, Bucharest, Romania; 5Department of Cardiology, Clinical Emergency Hospital, Bucharest, Romania; 6Department of Cardiovascular Surgery, “Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
Correspondence: Ruxandra Jurcut Email [email protected]
Abstract: Degenerative aortic valve (AV) disease is the most frequent valvular heart disease slowly progressing to severe aortic stenosis (AS) which usually requires aortic valve replacement. Another frequent condition, especially among elderly people, is cardiac amyloidosis (CA), particularly the wild-type transthyretin cardiac amyloidosis (ATTRwt). Since both of these diseases are considered a marker of ageing, there is a significant proportion of elderly patients who associate both severe AS and CA. Recent studies reported a high prevalence of both severe AS and CA (AS-CA) in elderly patients referred for TAVR of 13– 16%, carrying a worse prognosis. The present case illustrates the diagnostic algorithm and the management of ATTRwt CA in an elderly patient with severe paradoxical low-flow low-gradient AS, accompanied by a review of the current literature about the red flags which help identifying CA in patients with severe AS, as well as the prognosis and management of these disease association.
Keywords: aortic stenosis, cardiac amyloidosis, wild-type ATTR, transcatheter aortic valve replacement, conduction disorders
Introduction
Degenerative aortic valve (AV) disease is the most frequent valvular heart disease with a severity ranging from aortic sclerosis (without hemodynamic impact) slowly progressing to severe aortic stenosis (AS) which usually requires aortic valve replacement.1 In patients older than 75 years, AS is present in 12.4% of the population, with severe forms in 3.4% of the elderly.2 According to the European and American guidelines, symptomatic severe AS requires AV replacement either by surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR), with a mean survival of 2–3 years in the absence of these procedures.3,4 TAVR is increasingly used in high5 and more recently in intermediate-risk population,6 studies evaluating now the indication even in low-risk population.7,8
Recent years saw the evolution of data regarding the wild-type form of transthyretin amyloidosis (ATTRwt) as a frequent form of cardiac amyloidosis (CA) among elderly people. ATTRwt is mainly affecting the heart, but it can also involve the soft tissues leading to spinal stenosis or bilateral carpal tunnel syndrome,9 being considered a marker of ageing and commonly affecting elderly men, with a prevalence of 25% in 256 patients aged over 85 on autopsy.10 Moreover, the presence of ATTRwt was described in 13% of heart failure with preserved ejection fraction (HFPEF) patients above 60 years old and with LV hypertrophy of more than 12 mm.11
A non-invasive algorithm12 represents a valuable alternative to cardiac biopsy for CA, by detecting features strongly suggestive of amyloidosis and was approved by the experts in this field;13 according to this diagnostic algorithm every patient with a high suspicion of CA should undergo 2 procedures: a bone-avid, phosphate-based isotope (99mTc-DPD, 99mTc-HMDP, 99mTc-PYP) whole-body scintigraphy (the same procedure as a bone scan) and a search for signs of plasma cell dyscrasia (serum and/or urinary light-chain assessment and determination of immunoglobulin light chains levels). A positive bone scintigraphy combined with the absence of plasma cell dyscrasia signs can diagnose ATTR with specificity and positive predictive value of 100%.12
Recent studies reported a high prevalence (13–16%) of concomitant severe AS and CA (AS-CA) in elderly patients referred for TAVR14–16 raising the issue of active screening and prognostic value of these findings. These studies reported a larger proportion of men and a preponderance of low-flow low-gradient AS phenotype with lower tissue Doppler (TDI) velocities in the AS-CA group.
Case Presentation
We present the case of a 78-year-old male known with AS and multiple hospitalizations in the last year due to decompensations of his heart failure, who was referred to our cardiology center for further investigations and treatment of the AV disease. On admission, the patient’s general status was severely altered with symptoms of angina, NYHA III functional class and extreme fatigue. The clinical examination revealed a pale and underweight patient with peripheral edemas, irregular heart rhythm and a significant systolic ejection murmur (grade III/VI) on the entire heart projection area with a maximum of intensity in the AV projection area and radiation to both carotid arteries.
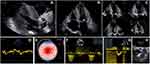
Biological investigations revealed a mild normocytic, normochromic anemia (hemoglobin 11.4 g/dL), normal lipid profile under statins, normal thyroid function (TSH 1.28 mcUI/mL), normal renal function for his age (creatinine 0.94 mg/dL, estimated glomerular filtration rate 77 mL/min/1.73m2) with no proteinuria and elevated cardiac biomarkers (NT-proBNP: 3125 pg/mL and high sensitivity troponin I: 64 pg/mL; N< 27 pg/mL). The chest X-ray showed an enlarged heart with small pleural effusion on the right side and signs of pulmonary congestion. The initial ECG showed atrial fibrillation with adequate ventricular response rate (70 beats per minute), pseudoinfarction pattern in the inferior (QS complex in DII, DIII, aVF) and anterior (QS complex in V1-V2) leads, a normal (but inconsistent to the hypertrophy revealed by imaging techniques) voltage of the QRS complex and nonspecific ST-T changes (Figure 1).
A transthoracic echocardiography (TTE) (Figure 2) has been performed for grading the severity of the AV disease which revealed a poor opening of the calcified tricuspid AV and a mild-to-moderate aortic regurgitation (AR), low transaortic velocities and pressure gradients (peak velocity 2.7 m/s, peak and mean pressure gradients of 30 mmHg and 17 mmHg, respectively) but with a calculated area of 0.8 cm2 (0.46 cm2/m2) confirmed by planimetry at transesophageal echocardiography as 0.7 cm2. The left ventricle (LV) was severely hypertrophied especially in the basal and mid segments (maximum wall thickness of the basal interventricular septum 24 mm) with a normal ejection fraction of 58%, but a small cavity with small end diastolic (66 mL, 38 mL/m2) and end systolic (38mL, 22 mL/m2) volumes resulting in a low stroke volume (SV) (29 mL/m2), leading to a diagnosis of paradoxical low-flow low-gradient severe AS.3 The patient associated severe longitudinal systolic dysfunction with a GLS of −9.1% and an apical sparing pattern of the bull’s eye view rising the suspicion of CA. Other echocardiographic findings on transthoracic echocardiography were the diastolic dysfunction with signs of elevated LV filling pressures (average mitral e’ 3 cm/s, E/e’ 30.5, LA volume index 59 mL/m2, peak velocity of the tricuspid regurgitation jet 3.2 m/s). The right ventricle (RV) free wall was hypertrophied (8 mm) with longitudinal and global systolic dysfunction (tricuspid annular plane systolic excursion 9 mm and peak tissue velocity of 7 cm/s, RV fractional area change 32%) and both atria were severely enlarged. A small pericardial effusion was another red flag for CA.
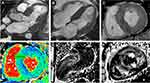
Given the CA suspicion, a whole-body scintigraphy using 99mTc-Hydroxymethylene Diphosphonate (99mTc-HMDP) was performed, which demonstrated important myocardial uptake of the bone tracer highly suggestive of ATTR CA, Perugini grade 3 (Figure 3). The patient also underwent cardiac magnetic resonance (CMR) imaging (Figure 4) which was consistent with CA: severe biventricular hypertrophy especially in the basal and mid segments of the LV, normal LV global systolic function but with a small cavity, elevated native T1 values (1148 ms) and extracellular volume (58%) measured at 1.5T scanner, abnormal Gadolinium kinetics with simultaneous nulling of both the blood and the myocardium on Look-Locker sequences and global subendocardial with transmural areas of late Gadolinium enhancement, especially in the LV basal and mid segments. Coronary angiography revealed normal coronary arteries without significant atherosclerotic lesions.
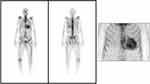 |
Figure 3 99mTc-HMDP whole-body scintigraphy planar image showing important myocardial uptake of the bone tracer (Perugini grade 3) which is highly suggestive of ATTR cardiac amyloidosis. |
The serum and urine light-chain assessment was negative, excluding light-chain amyloidosis and the TTR gene sequencing did not identify any mutation. Therefore, the final diagnosis was ATTRwt CA associated with paradoxical low-flow low-gradient severe degenerative AS.
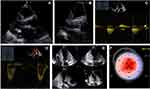
For this severely symptomatic AS patient, an AV replacement was scheduled choosing the transcatheter approach given the multiple comorbidities and frailty of the patient, according to the current guidelines.3 The patient successfully underwent a transfemoral TAVR with a 23 mm Edwards Sapien valve, with a well-positioned valve under fluoroscopic and transesophageal echocardiography guidance and a trivial post-procedural paraprosthethic regurgitation. He had no periprocedural complications and he was discharged from the cardiac surgery department without any symptoms at rest. The TTE at 1 month revealed a biological aortic prosthesis with normal aspect and function (Figure 5).
At the 3 months follow-up the patient was experiencing symptoms at minimal efforts, severe fatigability and mild edema. No syncope was reported. We noted low heart rates in the absence of beta-blockers or other bradycardia inducing drugs. The 24h ECG Holter monitoring reported an atrial tachycardia with average heart rate of 52 bpm, lowest heart rate of 25 bpm, longest pause of 3.9 sec and intermittent periods of complete heart block (Figure 6). Given the symptoms and the association of ATTRwt CA, the patient was implanted a single chamber pacemaker (VVI-R). He also received parenteral iron administration due to a severe hyposideremia and a higher dose of diuretics, reporting an improvement in symptomatology in the following months. He was also prescribed long-term treatment with Tafamidis 61 mg per day for the ATTRwt CA.
Discussion
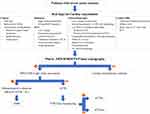
CA is a restrictive cardiomyopathy caused by the deposition of insoluble amyloid fibrils in the myocardium leading to severe and progressive heart failure.9 Given that both conditions, degenerative AV disease and ATTRwt CA are now considered markers of ageing, these two diseases can coexist in the same patient. However, since they share a common phenotype (LV hypertrophy, normal global systolic function, longitudinal systolic dysfunction and diastolic dysfunction) the presence of concomitant severe AS and CA may go unrecognized18 and clinical suspicion red flags is essential for timely diagnosis (Figure 7).
The first small preliminary study looking at the coexistence of degenerative AS-CA has been published in 2015 by Rapezzi’s group from Bologna who identified positive bone scan in 5 of the 43 patients (11.6%) with AS and one or more of the following echocardiographic red flags: increased thickness of the atrioventricular valves, interatrial septum or RV free wall, pericardial effusion and myocardial granular appearance, concluding that particular attention should be paid to patients with echocardiographic signs of myocardial infiltration, mismatch between LV wall thickness and ECG QRS voltage, severe longitudinal dysfunction and the low-flow low-gradient phenotype of AS.19
Later, Treibel et al performed intraoperative heart biopsies and CMR on 146 patients with severe AS requiring SAVR, identifying 6 patients (5.6%) with ATTRwt CA.20 At a median 2.3 years follow-up, 3 of the patients (50%) with CA had died versus 7.5% of the patients without CA, concluding that occult ATTRwt CA was associated with a poor outcome, the presence of TTR amyloid having the highest hazard ratio for death in the univariate analyses. Furthermore, Cavalcante et al14 reported that over a median follow-up of 18 months, 40 deaths (total 113 patients) occurred and the mortality in patients with AS-CA is higher than in patients with AS alone (56% vs 20%, p< 0.0001).
Four important studies followed these data in 2017,14,15 201816 and 202021 focusing especially on patients with severe AS referred for TAVR, which reported a prevalence of AS-CA of 16% (24 from 151 patients), 8% (9 from 113 patients), 13.9% (14 from 101 patients) and 8.4% (16 from 191 patients, 1 AL and 15 ATTRwt), respectively. These studies also gave us a better understanding of the red-flags which can be suggestive of CA, since there are important similarities in echocardiographic findings in both severe AS and CA. In their study, Nitsche et al tested the voltage to mass ratio, SV index and GLS as screening parameters for CA in AS patients concluding that while echocardiographic GLS did not reliably differentiate AS alone from AS-CA (AUC 0.643), the voltage to mass ratio and SV index showed modest discriminative power (AUC 0.770 and 0.773), which was comparable to the extracellular volume fraction by CMR (AUC 0.756).21 Furthermore, when looking at the AS phenotype, the authors reported a low-flow low-gradient (with normal or reduced LV ejection fraction) in 56.3% of AS-CA patients (12.5% EF< 50%, 43.8% EF> 50%) compared to 24.7% in the isolated AS patients.21 Castano et al also reported some important features of AS-CA: higher thickness of the interventricular septum (13 vs 11 mm, P= 0.007), LV mass index (130 vs 98 g/m2, P= 0.002), lower SV index (30 vs 36 mL/m2, P= 0.009), more advanced diastolic dysfunction (E/A 2.3 vs 0.9, p= 0.001 and lower deceleration time: 176 vs. 257 ms, p< 0.0001). In addition, the authors concluded that the average mitral annulus S’ measured by TDI best predicted AS-CA with a value <6 cm/s conferring 100% sensitivity for predicting a positive bone scan.15
Castano et al15 revealed that AS-CA patients present with an advanced ATTR cardiomyopathy so, in this case, initiating a disease-modifying treatment may be less efficient. In a 2018 multicentric, international, double-blind, placebo-controlled Phase 3 trial, the ATTR-ACT study, tafamidis showed lower all-cause mortality (29.5% vs. 42.9%) than placebo and lower rate of cardiovascular-related hospitalizations with a risk reduction of 32%.22 When looking at NYHA class subgroups (I and II vs III), the difference in all-cause mortality and frequency of hospitalizations favored tafamidis over placebo, except in patients with NYHA class III at baseline; the authors speculated that the higher hospitalization rate observed in this subgroup was attributable to longer survival with a more severe disease.22 Furthermore, in a prospective study enrolling 1240 patients with ATTR CA evaluated in the National Amyloidosis Centre, London, Chacko et al identified only 22 patients (1.77%) presenting severe AS among a total of 85 patients with AS (20 of the severe AS patients being in the ATTRwt group); the severe AS-CA coexistence was independently associated with poor prognosis, conferring a significantly shorter survival (22 vs 53 months, p= 0.001).23 The authors also reported a statistically significant difference in survival between the 5 patients who underwent TAVR and the rest of the 17 patients who did not receive any treatment for the severe AS (P=0.012).
Recently, Scully et al performed bone scintigraphy in 200 patients aged >75 with severe degenerative AS referred for TAVR and discovered AS-CA in 26 of these patients (13%).17 The authors also reported several significant differences between the 2 groups, the AS-CA patients being older (88 vs 85 years, P=0.001), with reduced quality of life, thicker LV walls (14 vs 13 mm, P=0.02), lower voltage (Sokolow–Lyon index 1.9 vs 2.5 mV, P=0.03) and voltage to mass ratio (0.017 vs 0.025 mV/g/m2, p= 0.03) and higher cardiac biomarkers (high sensitive Troponin T 41 vs 21 ng/L, p< 0.001; NTproBNP 3702 vs 1254 ng/L, P=0.001).17 During the evaluation, 149 patients (75%) underwent TAVR (16 AS-CA, 133 AS alone) and postintervention complications occurred at the same rate in both groups. At a follow-up of 19 months after the bone scintigraphy, 42 patients (21%) died with no significant differences between the AS-CA and AS alone groups (23% vs 21%, respectively, P=0.71). Moreover, the study population was divided into 4 groups by CA presence (AS-CA vs AS alone) and by management strategy (TAVR vs medical management) and, most importantly, TAVR significantly improved the outcome in both groups: TAVR vs medical management in AS-AC (P= 0.03), TAVR vs medical management in the AS alone group (P< 0.001), without significant differences between the groups by the CA presence status: TAVR in AS-CA vs AS alone (P= 0.48), medical management in AS-CA vs AS alone (P= 0.39).17 Specific evaluation of disease-modifying amyloid therapy in AS-CA is needed.17
TAVR may result in new-onset conduction abnormalities at both short and long time distances after the procedure,24,25 while CA is also known to be associated with conduction disorders.26 In an AS-CA study, patients with ATTR also had a higher incidence of right bundle branch block, but did not have an increased need for placement of a permanent pacemaker after TAVR compared with AS patients without ATTR (17.4% vs 14.4%, P=0.751).15 However, given the high percentage of patients with conduction defects in TAVR and ATTR patients, we suggest a close monitoring of these patients, based on clinical findings of bradycardia, with repeated ECGs, 24h ECG Holter monitoring or, if indicated, an electrophysiological study.
Conclusions
Given that both degenerative AS and ATTRwt are more frequent in the elderly and are considered a marker of ageing, their coexistence has been frequently reported. Patients with AS-CA have worse outcome, but TAVR was proven to improve it. The present case emphasizes on searching for CA in patients with severe AS with associated “red flags”, especially as ATTR diagnosis leads nowadays to disease-modifying therapies that can ameliorate the clinical course of these patients.
Abbreviations
AR, aortic regurgitation; AS-CA, aortic stenosis coexisting with cardiac amyloidosis; AS, aortic stenosis; ATTR, transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; AV, aortic valve; CA, cardiac amyloidosis; GLS, global longitudinal strain; LA, left atrium; LV, left ventricle; RV, right ventricle; SAVR, surgical aortic valve replacement; SV, stroke volume; TAVR, transcatheter aortic valve replacement; TDI, tissue Doppler imaging; TTE, transthoracic echocardiography.
Research Ethics
Written informed consent to publish has been obtained from the patient. Based on local requirements, an IRB is not required for a unique case report.
Acknowledgments
This work was supported by an unrestricted educational work from the Pfizer competitive grant program under grant contract no 2480/14.05.2020.
Disclosure
Dr Alice Munteanu reports personal fees, non-financial support from Servier, AstraZeneca, ViforPharma, AlfaSigma, and Boehringer Ingelheim, outside the submitted work. Dr Raluca Mititelu reports speaker fees from Pfizer, non-competing with the present work. Dr Ruxandra Jurcut reports speaker fees and unrestricted grants from Pfizer, non-competing with the present work. The authors report no other conflicts of interest in this work.
References
1. Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart. 2016;102(1):75–85. doi:10.1136/heartjnl-2014-307020
2. Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–1012. doi:10.1016/j.jacc.2013.05.015
3. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–2791.
4. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57–e185. doi:10.1016/j.jacc.2014.02.536
5. Gleason TG, Reardon MJ, Popma JJ, et al. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. 2018;72(22):2687–2696. doi:10.1016/j.jacc.2018.08.2146
6. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi:10.1056/NEJMoa1514616
7. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi:10.1056/NEJMoa1814052
8. Kolte D, Vlahakes GJ, Palacios IF, et al. Transcatheter versus surgical aortic valve replacement in low-risk patients. J Am Coll Cardiol. 2019;74(12):1532–1540. doi:10.1016/j.jacc.2019.06.076
9. Siddiqi OK, Ruberg FL. Cardiac amyloidosis: an update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28(1):10–21. doi:10.1016/j.tcm.2017.07.004
10. Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40(3):232–239. doi:10.1080/07853890701842988
11. Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–2594. doi:10.1093/eurheartj/ehv338
12. Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. doi:10.1161/CIRCULATIONAHA.116.021612
13. Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol. 2019;26(6):2065–2123.
14. Cavalcante JL, Rijal S, Abdelkarim I, et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J Cardiovasc Magn Reson. 2017;19(1):98. doi:10.1186/s12968-017-0415-x
15. Castano A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879–2887. doi:10.1093/eurheartj/ehx350
16. Scully PR, Treibel TA, Fontana M, et al. Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71(4):463–464. doi:10.1016/j.jacc.2017.11.037
17. Scully PR, Patel KP, Treibel TA, et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur Heart J. 2020;41(29):2759–2767. doi:10.1093/eurheartj/ehaa170
18. Griffin JM, Maurer MS. Cardiac amyloidosis in severe aortic stenosis: we can find it but what should we do? Eur J Heart Fail. 2020. doi:10.1002/ejhf.1798
19. Longhi S, Lorenzini M, Gagliardi C, et al. Coexistence of degenerative aortic stenosis and wild-type transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2016;9(3):325–327. doi:10.1016/j.jcmg.2015.04.012
20. Treibel TA, Fontana M, Gilbertson JA, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016;9(8). doi:10.1161/CIRCIMAGING.116.005066
21. Nitsche C, Aschauer S, Kammerlander AA, et al. Light-chain and transthyretin cardiac amyloidosis in severe aortic stenosis: prevalence, screening possibilities, and outcome. Eur J Heart Fail. 2020. doi:10.1002/ejhf.1756
22. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. doi:10.1056/NEJMoa1805689
23. Chacko L, Martone R, Bandera F, et al. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J. 2020;41(14):1439–1447. doi:10.1093/eurheartj/ehz905
24. Auffret V, Puri R, Urena M, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;136(11):1049–1069. doi:10.1161/CIRCULATIONAHA.117.028352
25. Mangieri A, Montalto C, Pagnesi M, et al. TAVI and post procedural cardiac conduction abnormalities. Front Cardiovasc Med. 2018;5:85. doi:10.3389/fcvm.2018.00085
26. Algalarrondo V, Dinanian S, Juin C, et al. Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm. 2012;9(7):1069–1075. doi:10.1016/j.hrthm.2012.02.033
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.