Back to Journals » Hepatic Medicine: Evidence and Research » Volume 11
Severe alcoholic hepatitis: current perspectives
Authors Philips CA , Augustine P, Yerol PK, Rajesh S , Mahadevan P
Received 31 May 2019
Accepted for publication 18 July 2019
Published 8 August 2019 Volume 2019:11 Pages 97—108
DOI https://doi.org/10.2147/HMER.S197933
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Cyriac Abby Philips,1 Philip Augustine,2 Praveen Kumar Yerol,3 Sasidharan Rajesh,4 Pushpa Mahadevan5
1The Liver Unit, Cochin Gastroenterology Group, Ernakulam Medical Centre, Cochin, Kerala, India; 2Gastroenterology, Cochin Gastroenterology Group, Ernakulam Medical Centre, Cochin, Kerala, India; 3Department of Gastroenterology, Government Medical College and Hospital, Thrissur, Kerala, India; 4Interventional Radiology, Hepatobiliary Division, Cochin Gastroenterology Group, Ernakulam Medical Centre, Cochin, Kerala, India; 5Clinical Pathology, VPS Lakeshore Hospital, Nettoor, Kerala, India
Abstract: Severe acute alcoholic hepatitis (AH) is a catastrophic disease in the natural history of alcoholic liver disease with a very high 180-day mortality. It can present as acute on chronic liver failure with worse prognosis in the presence of infections and higher grades of liver disease severity. The clinical scenario involves a patient with a recent history of heavy alcohol consumption within three months of presentation with jaundice and characteristic liver enzyme elevation pattern with coagulopathy, hepatic encephalopathy, variceal bleeding and sepsis that results in extrahepatic organ failures. Several liver disease severities and therapy response indicators are in clinical use. Even though not approved, the only recommended treatment option for patients with severe AH is corticosteroids, which is without long term survival benefit. Novel efficacious treatment options awaiting high-quality multi-center studies include liver transplantation (involves strict selection criteria), growth factor therapy and fecal microbiota transplantation. In this exhaustive review, we discuss the definitions, disease severity, histopathology, and treatment options – past, present, and future, in patients with severe alcoholic hepatitis.
Keywords: SAH, hepatitis, alcohol, gut microbiome, stool transplant, ACLF
Introduction
Alcoholic beverages are food products with empty calories. The alcohol content varies between different types of beverages, for example, 40–50 g/L in beer, to approximately 120 g/L in wine and prepacked cocktails, to 400–500 g/L in hard liquor. In general, a standard drink is defined as 5 ounces (oz) of wine, 3 oz of fortified wine, 12 oz of beer, or 1.5 oz of distilled beverage or hard liquor, that is said to contain 12–14 grams of alcohol. Pure alcohol provides approximately 7.1 kilocalories per gram (kcal/g). In the United States of America, a standard drink is 14 g, or 18 mL, or 0.6 fl oz of pure alcohol (Figure 1).1 In the olden times, cirrhosis in chronic alcohol users was thought to be due to malnutrition and not directly related to toxic effects of alcohol. Cirrhosis in such a situation was termed “nutritional cirrhosis”. Chronic alcohol use leads to alcoholic liver disease (ALD) that encompass three major disease conditions –alcoholic fatty liver, alcoholic hepatitis and cirrhosis. In ALD patients with cirrhosis, a continuation of alcohol consumption may lead to the syndrome of acute on chronic liver failure secondary to alcoholic hepatitis (AH).2 In up to 90% of heavy alcohol drinkers, the first and most common liver-related change noted is macrovesicular steatosis, that progress through microvesicular steatosis and in the presence of hepatocytic balloon degeneration, lobular neutrophilic inflammation and Mallory bodies and pericellular fibrosis develops into AH. The importance of the development of AH in a patient with ALD is that it is associated with a 40% 180-days mortality and a nine-times higher risk of progression to cirrhosis when compared to ALD patients with the only steatosis.3 Up to 90–100%of chronic heavy drinkers develop alcoholic fatty liver (AFL) in whom, continued intake leads to alcoholic steatohepatitis (ASH). The actual incidence of AH among those who develop AFL and ASH remains unknown. However, 8–20%of patients with ASH develop cirrhosis, in whom, continued heavy drinking can lead to severe AH in 40%, and can present with acute on chronic liver failure. Conversely, 70% of patients with AH can go on to develop cirrhosis with continued alcohol use. Two percent of alcoholic cirrhosis patients develop liver cancer every year. In patients who survive the acute episode of severe AH, relapse of alcohol use could be as high as 25% at one year and up to 35% at five years.4
 |
Figure 1 Descriptions and definition of standard drinks and their respective alcohol content. |
Development of AH is dependent on multiple mechanisms, In the presence of modifying factors such as gender, genetic predisposition, ethnicity, obesity, underlying or associated liver diseases, nutritional status, type and duration of drinking, and smoking status. Excessive alcohol consumption leads to hepatocyte swelling and death which releases damage-associated molecular patterns such as mitochondrial DNA and high-mobility group-1 protein. In the presence of alcohol, bacterial overgrowth in the intestines, leakiness of gut mucosal barrier and diversification of pathogenic microbial communities (dysbiosis) leads to elevated pathogen-associated molecular patterns such as lipopolysaccharide (LPS) and bacterial DNA. These activate innate immunity, induces production of inflammatory cytokines, activates Kupffer cells, macrophages and neutrophils leading to an expansion of inflammatory milieu within the liver microenvironment. Simultaneous activation of the adaptive immunity by reactive oxygen species (ROS) leads to the generation of protein adducts and increase in translocation of bacterial antigens. Hepatocyte chemokine production is directly induced in the presence of alcohol leading to increase in micro-RNA expression through nuclear factor-kB mediated transcription stimulating LPS triggered tumor necrosis factor production from Kupffer cells, increasing inflammation, leading to the clinicopathological syndrome AH.
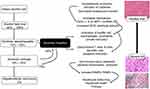
Despite understanding the complexities in progression and aetiopathogenesis of ALD (Figure 2), no approved treatments exist currently and management of a spectrum of ALD depends on regional choices, availability of expertise and treatment modalities. In this review article, we discuss exhaustively on definitions, diagnosis, histopathology, severity assessment and outcomes related to AH. We further discuss novel therapies that are currently under trial for the treatment of AH and present future directions in diagnosis and treatment.
Definition and diagnosis
Alcoholic hepatitis was once considered to occur in persons with excessive drinking over the decades. However, shorter periods of heavy alcohol use can also result in AH with most patients with AH usually consuming >100 g ethanol per day. Heavy drinking is ≥15 drinks per week for men and ≥ eight drinks per week for women. Binge drinking and drinking without adequate food intake portend risk for early development of AH. Binge drinking, as defined by the National Institute on Alcohol Abuse and Alcoholism is consumption of ≥5 drinks in men and ≥ four drinks in women in about two hours per week, resulting in a blood alcohol concentration of ≥0.08%. Risk of severe AH was found to be lower among wine drinkers compared to those drinking beer and distilled spirits.5 Alcoholic hepatitis is defined as a syndrome of acute liver injury in persons with known alcoholic liver disease or heavy alcohol use for >6 months presenting with recent onset or worsening jaundice (serum bilirubin >3 mg/dl) and hepatitis [aspartate aminotransferase (AST) >50 IU/ml but <500 IU/ml, and AST:alanine aminotransferase (ALT) ratio of>2] with <2 months of abstinence before the onset of jaundice in the absence of other known causes of liver disease. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) states that an average consumption of more than 3 drinks (approximately 40 g) per day for women and 4 drinks (approximately 60 g) per day for men are to be considered minimal thresholds for the diagnosis of AH and that heavy alcohol use should have occurred for more than six months, with fewer than two months of abstinence before the onset of jaundice. Serum bilirubin is usually >3 mg/dL with elevated AST and ALT not exceeding 400 IU/mL; and an AST to ALT ratio of >1.5. However, AST/ALT ratios of <1.5 are also seen in <2% of patients with histologically proven AH.6
Serum AST levels are higher than ALT levels in alcoholic hepatitis due to the reduction of ALT activity in pyridoxine depleted hepatocytes. The ethanol-related mitochondrial injury also leads to release of mitochondrial AST that increases total levels. Arbitrarily ≥ three drinks (60 grams) and ≥4 (40 grams) per day for women and men respectively, is considered the minimal threshold for AH development. Based on expert recommendation, AH can be further characterized as – a) definite AH: in which both clinical criteria and confirmation of diagnosis on liver biopsy are met; b) probable AH: defined in a patient who has both alcohol use disorder and hepatitis, and investigations have ruled out other causes of liver disease but a biopsy confirmation is lacking; and c) possible AH: defined in patients in whom the clinical diagnosis of AH is suspicious, in the presence of other clinical events such as recent variceal bleeding, ischemic hepatitis, drug-induced liver injury; associated viral infections; or in the presence of atypical AST and ALT pattern. In the latter, a liver biopsy becomes mandatory for diagnosis of AH, rule out additional insults and for inclusion into AH related clinical trials (Figure 3).7 A systematic review of randomized trials showed that 84.5% of patients had confirmed histologic AH and an improved diagnostic accuracy of 96% when total bilirubin was>4.7 mg/dL. A recent retrospective study identified the combination of an elevated white cell count and a nodular liver surface in the absence of active infection to have a high likelihood of histologic AH for whom liver biopsy may not be necessary.8
Histopathology of alcoholic hepatitis
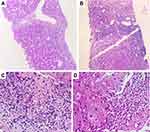
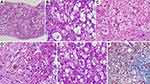
In AH, the classical histopathological findings include hepatocellular ballooning, perivenular steatosis (both small and large droplet), neutrophilic lobular inflammation along with neutrophilic satellitosis (clusters of polymorphonuclear cells around ballooned hepatocytes), presence of extensive Mallory-Denk bodies (MDB, previously known as “alcoholic hyaline”) and megamitochondria (Figure 4). Hepatocyte ballooning occurs due to increased hepatocellular fluid and fat droplet accumulation, depletion of cytoplasmic keratin 18 and deposition of MDBs within hepatocytes. Ballooned hepatocytes are not specific for AH, but are also notable in non-alcoholic fatty liver disease, Wilson’s disease and primary biliary cholangitis. Balloon degeneration is the predominant form of hepatocyte injury in AH. The hepatocytes are markedly swollen, contain rarefied cytoplasm with clumping of intermediate filaments that lose staining for cytokeratin 8 and 18. This leads to lytic necrosis leading to hepatocyte apoptosis. The apoptotic hepatocytes (councilman bodies or acidophil bodies) are shrunken with chromatic condensation with cellular and nuclear fragmentation. These are more commonly associated with NASH than AH.9 Micro (1–2 mm in diameter) as well as macrovesicular steatosis (happens over days, due to coalescing of fat droplets, exceeding 20 mm in diameter) are most prominent in the perivenular or zone 3 region on liver histological evaluation in AH. Their extension towards the portal tracts is associated with increasing severity of AH. Mallory- Denk bodies consist of clumps of eosinophilic ropy material in the hepatocyte cytoplasm usually perinuclear in location. They consist of misfolded and aggregated keratin filaments and undergoes degradation through the ubiquitin-proteosome pathway to produce ubiquitinated keratin and protein p62 and heat shock proteins 70 and 25. The amount of MDBs correlates with severity of AH and mortality. Lobular inflammation associated with AH is predominantly neutrophilic, even though lymphocytic mononuclear cells may also be recognized on biopsies. Portal-based Inflammation in AH is usually milder when compared to other forms of acute and chronic hepatitis. However, portal inflammation is more common and of higher grade in AH than in NASH. Alcoholic foamy degeneration is a rare and severe pattern of microvesicular steatosis seen in patients with severe AH and classically centrilobular in a location with minimal inflammation and only a few MDBs. In the clinical scenario, the patient has markedly elevated serum gamma-glutamyl transaminase levels, with or without elevation of other liver enzymes. Megamitochondria are eosinophilic round intracytoplasmic forms of mitochondria usually seen on hematoxylin and eosin staining. They are not specific for AH, but when present in the central of the lobule, provide diagnostic clues to alcohol as the inciting event for liver injury. Megamitochondria are also related to the amount of daily ethanol use and the length of abstinence before the time of biopsy, occurring in larger numbers in the former and decreasing in numbers in the latter. Fibrosis associated with severe AH starts in zone 3 or the perivenular region extending to form a pericellular/perisinusoidal pattern (or “chicken-wire” fibrosis). In advanced stages, the fibrosis progresses and extend to the portal tracts ultimately leading to a central-portal or portal-portal bridging pattern. Perivenular fibrosis must include an extension of fibrosis to at least two-thirds the perimeter of the terminal hepatic venule with the fibrous rim measuring over 4 mm in thickness. In advanced stages and with ongoing alcohol use, thick fibrous bands develop encasing regenerating nodules of hepatocytes. In later stages, orcein stain helps differentiate broad bands of fibrosis (orcein positive) from areas of hepatocellular reticulin collapse due to acute alcoholic hepatitis. Sclerosing hyaline necrosis (SHN) is pathognomonic for AH and is characterized by an extreme form of perivenular hepatocyte necrosis associated with concomitant fibrosis, resulting in terminal hepatic venules occlusion and portal hypertension in the absence of cirrhosis. Cholestasis is seen in alcoholic foamy degeneration. Vascular lesions associated with AH include, lymphocytic phlebitis, SHN or phlebosclerosis and venous-occlusive lesions. With abstinence from alcohol, steatosis rapidly disappears within weeks, followed by amelioration in inflammatory changes while Mallory-Denk bodies persist longer, for up to 6 months in some patients. Pericellular fibrosis can disappear altogether with the cessation of alcohol.10,11 The Chinese Association for the Study of Liver classification defines and categorizes histopathology of alcoholic liver disease. Accordingly, alcoholic fatty liver is divided into four grades based on the proportion of hepatocytes with fatty degeneration and similarly inflammation with or without necrosis and fibrosis are graded into four types. Necrosis is not a well-defined feature of AH, except in SHN, but forms part of the classification as per Chinese guidelines.12 The Chinese classification system do not have prognostic value and is not powered to assess severity of AH. No other histopathological classification systems exist for diagnosis and grading of AH.
Severity and clinical outcomes
The severity of malnutrition is strongly correlated with the prognosis of AH patients. Moderate protein-energy malnutrition (score of 60–79% of normal) was associated with 29% and severe malnutrition(score <60%) is associated with 45% mortality at 180-days respectively. It was shown that patients with moderate malnutrition and caloric intake >2,500 kcal/day given oxandrolone had a reduc.tion in 6-month mortality by 4% versus 28% who received a placebo. Nutritional deficiency and immunosuppressed state bring forth infections, which is one of the main complications as well as mortality in AH patients. Up to a quarter of patients with severe AH have the infection during admission to the hospital. A meta-analysis found that the cumulative incidence of infection was 20% in patients with severe AH, during steroid treatment. However, incidences as high as 50% to 67% during a 3-month follow-up are also reported in the literature. The one-year mortality was 14% and 76% in AH patients with mild or severe malnutrition respectively. Patients with severe AH and infections have an increased mortality of 30% at two months and those who develop infections while on corticosteroid and are responders, then, their survival becomes similar to that of non-responders. Patients with AH often deteriorate after hospital admission within the first week, despite abstinence - which may be due to reperfusion liver injury and acute severe malnutrition accompanying the loss of calorie intake with alcohol abstinence.13 Serste and colleagues found that patients with severe AH and infections can develop the syndrome of ACLF. In their study, the 28-day cumulative incidence of death in patients with higher grades of ACLF was >70% and >90% at six months. Hence, in patients with AH, underlying malnutrition and evidence of sepsis are strong factors for disease severity and mortality.14
The discriminant function (DF) identifies patients with AH with significant risk for early mortality. Patients with DF>32 are considered to have severe AH. Patients with AH with hepatic encephalopathy are also considered to have severe disease irrespective of the DF score. DF has low specificity (<40–62%) and sensitivity (67s–100%) for 30-day mortality. Patients with DF<32 also have relatively high 28-day mortality of 17%.
An admission model for end-stage liver disease (MELD) score ≥21 in the first week and the first-week change of MELD score by ≥2 points are effective for predicting in-hospital mortality. In patients with MELD score >30.5 the sensitivity for predicting 30-day mortality was 100%.
The Glasgow Alcoholic Hepatitis Severity Score [GAHS, ranging from 5 to 12, utilizes age, blood urea, white cell count, total bilirubin and international normalized ratio (INR)] ≥9 points characterize severe AH. The one-month survival in patients with GAHS <9 was 87% compared to 46% in those with score ≥9.
A predictive score based on multivariate analysis of age, serum bilirubin, INR, and serum creatinine (ABIC) during admission with a cut-off value of 6.71 to 9, identified patients with low (0%), intermediate (30%), and high risk (75%) of death at three months. The ABIC score was also useful in determining 1-year mortality.
The Beclere model initially formulated to determine survival in alcoholic cirrhosis was utilized to assess long-term survival in AH patients receiving corticosteroids. The study found that no difference existed in the 1- and 2-year survival in the observed placebo-randomized group using the Beclere model.
The model for alcoholic hepatitis to grade severity in an Asian patient cohort (MAGIC) was the first prognostic model derived from an Asian population with AH. It highlighted the prognostic role of hyperkalemia in AH and demonstrated early improvement in liver function and its association with better survival.15
It was reported that CTP score compared to the MELD score had similar predictive power for 3- and 6-month mortality in AH patients. Change in CTP score was not found to predict outcomes well. A simple score, known as an early change in bilirubin levels (ECBL), defined as the change in bilirubin level at seven days compared to baseline during treatment with steroids was shown to predict mortality. 95% of patients with ECBL continue to have a reduction in liver disease severity during treatment. The six-month survival in patients with ECBL was 82% versus 23% in those without bilirubin reduction. Severe AH patients with 25% reduction in serum bilirubin from baseline at seven days of corticosteroid treatment have better survival than non-responders.
In a recent study, Hanouneh et al identified novel breath biomarkers in patients with AH. Trimethylamine (TMA), acetone and pentane were significantly higher in AH subjects. Combination of pentane and TMA levels had excellent accuracy in diagnosing AH. A logistic model, named the TAP score, with cut-off ≥36 identified patients with AH (90% sensitivity and 80% specificity). However, the correlation of ‘breath-print score ‘and severity of liver disease was only moderate when compared to the MELD score.
The MELD + Lille combination model was found to predict survival better than other combination models. Patients with AH and MELD score of 21 and Lille score at 0.45 had 15.3% and 23.7% mortality rate at 2 and six months, respectively. The presence of acute kidney injury (AKI) also strongly correlates with three-month mortality in patients with AH. In a retrospective series, the most accurate predictors of AKI in patients with severe AH were the presence of systemic inflammatory response syndrome, serum bilirubin >16 mg/dL and INR >1.7.16
Das et al described a potential new marker of AH severity. In a prospective cohort study determining the molecular ellipticity (ME) of circulating albumin-bilirubin complex using circular dichroism spectroscopy, it was found that ME was significantly higher in patients with SAH versus those with alcoholic cirrhosis. Importantly, ME was higher in patients who died (2.92 mdeg) compared with those who survived (1.47 mdeg), performing better than MELD and DF scores. An optimal cut off for ME for predicting poor outcomes was 1.84 mdeg (sensitivity 86%, specificity of 87%).17
The Lille score identifies patients with severe AH who respond to corticosteroid therapy. Patients with Lille score>0.45 are non-responders with a predicted poor six-month survival of <25%. Lille score on day 4 is equivalent to day 7 in predicting 3-month mortality in patients with severe AH. Lille response can also be divided into complete responders (score ≤0.16), partial responders (0.16–0.56), and null responders (≥0.56) with one-month survival rates of 91%, 79% and 53%, respectively.18
Only one scoring system was based on liver histopathology and its association with disease severity and outcomes. The Alcoholic Hepatitis Histologic Score system, which has no diagnostic value, consist of patterns of bilirubinostasis, neutrophil infiltration, degree of fibrosis and presence of megamitochondria which independently predicts three-month mortality. Patients with low (0–3 points), moderate (4–5 points), or high (6–9 points) scores had mortality rates of 3%, 19% and 51%, respectively. Bilirubinostasis (canalicular/ductular pattern correlates with development of bacterial infections) and severe fibrosis were associated with a poorer prognosis in AH, while intense neutrophilic infiltration and presence of proliferating hepatocytes were associated with better prognosis.19
Other factors independently associated with higher mortality from AH include older age, alcohol consumption >120 g/d, upper gastrointestinal bleed, bilirubin to gamma-glutamyl transferase ratio >1, interleukin (IL) −6 levels ≥38.66 pg/mL and in-vitro measure of steroid sensitivity.20,21
Treatment
Randomized controlled trials have shown that improvement in nutritional status through enteral nutrition was associated with amelioration in liver disease severity and improved survival in the short term in patients with AH. Studies have shown that medical treatment, combined with nutritional therapy resulted in lower 180-day mortality in AH patients with moderate malnutrition continued on nutritional supplementation; addition of 80 g of amino acids combined with 3000 Kcal/day and 100 g protein/day improved survival and four-week regimen of peripheral parenteral nutrition revealed rapid reduction in bilirubin levels independent of drug treatments in patients with severe AH. Moreno et al showed that in patients with severe AH, <21.5 Kcal/kg/day intake resulted in a lower survival rate and increased risk of infections and acute kidney injury at six months. AH patients in whom dietary intake included ≥65 g/day of lipids and ≥77.6 g/day of protein had lower mortality at the end of six months.22,23
Both the American Association for the Study of Liver Disease (AASLD) and European Association for the Study of the Liver (EASL) practice guidelines recommend the use of corticosteroids (ie, prednisolone 40 mg daily for four weeks) for patients with severe AH. The Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial, the largest randomized clinical trial, studied short- and long-term mortality of patients with severe AH. It demonstrated no reduction in all-cause mortality at one month for patients treated with steroid or pentoxifylline, except for non-significant mortality benefit at 28 days in patients treated with steroid, unsustained at the end of 3 months and 1-year.
In a large, randomized, double-blinded, placebo-controlled trial in patients with AH, significant improvement in short-term survival was noted with pentoxifylline use due to significant decrease in occurrence of hepatorenal syndrome. In another randomized trial on pentoxifylline in comparison to corticosteroids, an important observation was significant reduction in mortality of patients receiving the former which was also attributed to significant reduction in the development of hepatorenal syndrome. A Cochrane meta‐analysis showed that pentoxifylline reduced liver related mortality due to hepatorenal syndrome, but subsequent trial sequential analysis was not supportive.24–26
A meta-analysis of 22 randomized clinical trials demonstrated improved survival in patients with severe AH treated with steroids and the combination therapy with steroid and N-acetylcysteine was found to have the most benefit in improving short term survival.27,28 Vergis et al demonstrated that at a cut-off of 18.5 pg/mL, the level of circulating bacterial deoxyribonucleic acid before corticosteroid treatment in patients with severe AH could identify those with a high risk of infection.29
Spahr et al demonstrated the role of granulocyte colony stimulating factor(GCSF) therapy to induce proliferation of hepatic progenitors in patients with AH and background alcohol-related cirrhosis. Over four weeks of treatment, significant increases in CD 34+ and HGF expression were notable. Serial liver biopsies showed increased proliferative progenitor cells. Even though well tolerated, no clinical improvement was observed at 30 days follow up. A randomized controlled trial performed by the same group utilized bone marrow stem cell transfusion in patients with AH and decompensated alcohol-related cirrhosis. The authors found that differences in inflammation on 4-week follow-up biopsies and improvement in MELD scores after 3 months of follow-up were not significant between both groups after stem cell transfusion.30–32 Singh et al, in two pilot studies, randomized patients to either receive G-CSF at 5 µg/kg for 5 days along with standard medical therapy (pentoxifylline with nutrition) or standard medical therapy alone. Improvement of CTP, MELD, and DF score for up to 3 months in the GCSF group was noted with a survival benefit in GCSF treated patients. Addition of N-acetyl cysteine did not increase survival benefit.33,34
Randomized controlled studies in patients with severe AH on anti-tumor necrosis factor (TNF) inhibitors, was found to have a higher probability for severe infection and mortality among patients in the former group.35 Metadoxine, an antioxidant, that improves glutathione availability and inhibits hepatic steatosis was found to improve one- and three-month survival rates when added to steroids in patients with AH. Notably, hepatorenal syndrome and hepatic encephalopathy events were significantly fewer in patients treated with metadoxine.36 Many therapies studied for AH are without proven efficacy in clinical trials. These include vitamin E, silymarin, colchicine, amlodipine, propylthiouracil, anabolic steroids, and insulin and glucagon combinations (Box 1).
The French-Belgian liver transplant (LT) inclusion criteria for severe AH include those with Lille score ≥0.45 or worsening liver function one week, without prior episodes of AH, absence of severe coexisting medical or psychiatric conditions, presence of supportive family members, social integration and a lifelong commitment to alcohol abstinence and an absolute consensus among four team circles of medical and paramedical staff prior to listing. Those undergoing LT had a six-month and 2-year survival of 78% vs 24% and 72% vs 24% in the non-transplanted group respectively. This meant that early liver transplantation could improve survival in a highly selected group of patients with the first episode of severe AH. Two studies from America, had reproduced these findings with a more pronounced survival benefit. The American Consortium of Early Liver Transplantation for Alcoholic Hepatitis published the combined experience of 12 centers, totaling 147 patients over close to a decade. The reported 1- and 3-year survival rates following transplantation was 94% and 84%, respectively. Alcohol relapse was 25% for any alcohol use, and 10% for sustained use 12-months post-LT. Although promising, the LT for severe AH remains a difficult option due to the intensive screening and selection process required and as of now.37–39
Novel perspectives
Extracorporeal cellular therapy (ELAD) utilizing hepatoblastoma‐derived C3A cells that express anti‐inflammatory proteins and growth factors has been under study as a liver support device in patients with severe AH. In a prior phase 2 study, no survival benefit in end‐stage liver disease while a non-significant positive trend toward improved survival in a subset of AH patients was noted. Another study was conducted to evaluate the safety and efficacy of ELAD on overall survival (OS) in patients with severe AH. ELAD treatment was conducted continuously for 120 hrs or stopped in the presence of early response at 72 hrs. The primary objective was safety and efficacy of ELAD concerning OS at 3-months and secondary objectives included evaluation of the proportion of survivors at three months. The study failed its primary and secondary endpoint in patients with severe AH and MELD ranging from 18 to 35. In a sub-group analysis of patients with MELD <28, ELAD was associated non-significant trend toward higher OS at three months (68.6% versus 53.6%; p=0.08). This has prompted a new trial to study the potential benefit of ELAD in younger subjects with mild to moderate renal function abnormality and less severe coagulopathy.40
Currently, evaluation of novel and combined therapies for severe AH are ongoing, featuring trials ranging from phase II to IV. Novel therapies under consideration include anti-inflammatory agents – Anakinra, Canakinumab (anti interleukin 1-beta antibody), Selonsertib (oral inhibitor of apoptosis signal-regulating kinase −1), Cenicriviroc (antagonist to chemokine CCR2/5) and bovine colostrum coupled to anti-lipopolysaccharide IgG antibody; regenerative agents such as interleukin – 22 and GCSF and others such as Lactobacillus rhamnosus, obetocholic acid and zinc supplementation. Other therapies feature a combination of amoxicillin and clavulanic acid, probiotics and rifaximin and recently micro-RNA inhibitors. Study on safety and efficacy of Emricasan in severe AH was terminated due to concern for high systemic drug levels that could have exceeded levels in toxicology studies. Mathurin et al, in a randomized Phase 2 trial, demonstrated that one-month therapy with Selonsertib in combination with prednisolone was safe in patients with severe AH but did not improve mortality or liver function when compared to prednisolone alone.41–44
The role of gut-liver axis and modulation of intestinal microbiota in alcoholic hepatitis
Alcoholic hepatitis is associated with dysbiosis of intestinal microbiota wherein the normal eubiotic state is modified to involve predominantly, pathobionts in the presence of continued ethanol use. Studies have shown that AH related dysbiosis was associated with an increase in Bifidobacteria, Streptococci and Enterobacteria and a decrease in anti-inflammatory Clostridium leptum or Faecalibacterium prausnitzii. A reduction in the relative abundance of the protective strains was associated with an increase in disease severity. Eubiosis in such a situation can be achieved through modulation of the pathobiont rich intestinal microbiota in either indirectly, in the form of modulation by diet, antibiotics, prebiotics, probiotics; or directly through fecal microbiota transplantation (FMT) which includes the utilization of bacterial and host metabolites as therapeutic targets.45 Kirpich et al, in an open-label randomized controlled trial, showed that probiotics rich in Bifidobacterium bifidum and Lactobacillus plantarum strains in comparison to placebo in hospitalized patients with AH led to a reduction in hepatic enzymes that was associated with an increase in probiotic strains. In another open-label randomized trial, the addition of Lactobacillus casei Shirota 3 strain three times daily for one month improved neutrophilic phagocytic capacity in alcoholic liver disease patients compared to baseline. Han et al in a placebo-controlled trial demonstrated that supplementation with 1.5 g of Bacillus subtilis and Enterococcus feacium per day for one-week improved liver function, decreased systemic inflammation and endotoxemia in patients with AH.
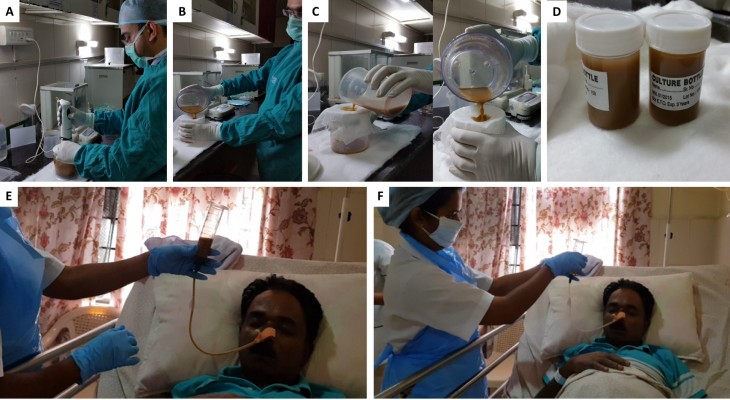
In the first pilot study of FMT in steroid ineligible severe AH, Philips et al demonstrated an improvementin1- year survival rate in FMT-treated patients compared to historical controls (87.5% vs 33.3%). The FMT was given daily for seven consecutive days through a fluoroscopically placed nasoduodenal tube. In 8 patients (Figure 5). In this study, the relative abundance of Proteobacteria was high that of Actinobacteria was low in patients with severe AH at baseline. A year after FMT, there was a significant reversal between the two, along with coexistence of donor and recipient species at 6–12 months post-FMT. This meant that new species from the donor, which were less pathogenic and beneficial, coexisted with pre-existing bacterial communities in the recipient. Alteration in the relative abundance of pathogenic species Klebsiella pneumonia (10% to <1% at one year), and non-pathogenic species, such as Enterococcus villorum (9–23% at six months) and Megasphaera elsdenii (10–60% at one year) were also notable. Post FMT, the deranged metabolic functionality of bacterial communities in the gut were seen to change, with notable downregulation of methane metabolism, bacterial invasion of the epithelial cells, inflammatory arachidonic acid metabolism, cellular toxic and oxidative stress-related geraniol degradation and reduction in volatile organic compounds and aromatic amino acid generation.46 In a retrospective observational study comparing FMT to other modalities of treatment in AH, the proportions of patients surviving at the end of 3 months in the steroids, nutrition, pentoxifylline, and FMT group were 38%, 29%, 30%, and 75% (p=0.036). Patients receiving FMT had a lower relative risk and hazard ratios for death compared to other groups which were associated with distinct and beneficial modulation of bacterial communities and associated metabolic functions.47 Development of ACLF in severe AH was associated with a poor outcome with progressive worsening of clinical events and lower rates of response to corticosteroid use. In patients with severe AH and non-responders to steroids with ACLF grades 0, 1 and (2+3) the 90-day survival rates were 68.1%, 45.8% and 36.7%. In a recently published study in patients with AH related ACLF, Philips and colleagues showed that, at the end of 548 days follow up, the proportion of patients surviving, after FMT, in the lower (ACLF 0+1) and higher grades (ACLF 2+3), were 72.7% and 58.3%, respectively, higher than any medical therapy currently in use.48
Future studies on FMT in AH could improve on the current protocol, identify better methods for fecal transfer, utilize specific groups of bacterial species for targeted therapy or identify specific metabolites to modify through gut bacterial modulation in the form of precision metagenomic medicine. Currently, FMT remains part of clinical research under strict trial monitoring. Even though survival benefit with FMT in severe AH is remarkable, the intermediate and long-term effect of FMT on patients is still unknown.
Summary and conclusions
Severe alcoholic hepatitis is a catastrophic disease without approved therapy. Even though international societies recommend corticosteroid treatment as the first line in the management of this critically ill cohort of patients, the benefits of such therapy are short lived. None of the second-line therapies have shown to reduce mortality beyond one month of the disease presentation. The use of GCSF for alcoholic hepatitis cannot be recommended until larger studies both from the Western and Eastern regions showcase reproducible outcomes. A large number of novel treatments and combination therapies are in clinical trials, pending result review. Healthy donor FMT has been shown to have long term survival benefit in a specific group of severe AH patients, but is still in infancy, pending further trials from both basic science and translational research.
 |
Box 1 Summary of treatments for alcoholic hepatitis |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug Alcohol Rev. 2011;31(2):200–205. doi:10.1111/j.1465-3362.2011.00374.x
2. Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27(3):220–231.
3. Frazier TH, Stocker AM, Kershner NA, Marsano LS, McClain CJ. Treatment of alcoholic liver disease. Therap Adv Gastroenterol. 2011;4:63–81. doi:10.1177/1756283X10378925
4. Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. doi:10.1038/s41572-018-0014-7
5. Dugum MF, McCullough AJ. Acute alcoholic hepatitis, the clinical aspects. Clin Liver Dis. 2016;20:499–508. doi:10.1016/j.cld.2016.02.008
6. Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology. 2016;150(4):785–790. doi:10.1053/j.gastro.2016.02.042
7. Singal AK, Louvet A, Shah VH, Kamath PS. Grand rounds: alcoholic hepatitis. J Hepatol. 2018;69:534–543. doi:10.1016/j.jhep.2018.05.001
8. Roth NC, Saberi B, Macklin J, et al. Prediction of histologic alcoholic hepatitis based on clinical presentation limits the need for liver biopsy. Hepatol Commun. 2017;1(10):1070–1084. doi:10.1002/hep4.1103
9. Caldwell S, Ikura Y, Dias D, et al. Hepatocellular ballooning in NASH. J Hepatol. 2010;53(4):719–723. doi:10.1016/j.jhep.2010.01.032
10. Sakhuja P. Pathology of alcoholic liver disease, can it be differentiated from nonalcoholic steatohepatitis? World J Gastroenterol. 2014;20(44):16474–16479. doi:10.3748/wjg.v20.i44.16474
11. Theise ND. Histopathology of alcoholic liver disease. Clin Liver Dis. 2013;2:64–67. doi:10.1002/cld.172
12. Li YM, Fan JG, Wang BY, et al. Guidelines for the diagnosis and management of alcoholic liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18: 167–170). J Dig Dis. 2011;12(1):45–50. doi:10.1111/j.1751-2980.2010.00477.x
13. Gustot T, Fernandez J, Szabo G. Sepsis in alcohol-related liver disease. J Hepatol. 2017;67(5):1031–1050. doi:10.1016/j.jhep.2017.06.013
14. Sersté T, Cornillie A, Njimi H, et al. The prognostic value of acute-on-chronic liver failure during the course of severe alcoholic hepatitis. J Hepatol. 2018;69(2):318–324. doi:10.1016/j.jhep.2018.02.022
15. Lee M, Kim W, Choi Y, et al. Spontaneous evolution in bilirubin levels predicts liver-related mortality in patients with alcoholic hepatitis [published correction appears in PLoS One. 2016;11(11):e0167184]. PLoS One. 2014;9(7):e100870. doi:10.1371/journal.pone.0100870
16. Rahimi E, Pan JJ. Prognostic models for alcoholic hepatitis. Biomark Res. 2015;3:20. doi:10.1186/s40364-015-0046-z
17. Das S, Maras JS, Maiwall R, et al. Molecular ellipticity of circulating albumin-bilirubin complex associates with mortality in patients with severe alcoholic hepatitis. Clin Gastroenterol Hepatol. 2018;16:1322–1332. doi:10.1016/j.cgh.2017.11.022
18. Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60(2):255–260. doi:10.1136/gut.2011.237727
19. Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:
20. Farooq MO, Bataller R. Pathogenesis and management of alcoholic liver disease. Dig Dis. 2016;34:347–355. doi:10.1159/000444545
21. Dhanda AD, Di Mambro AJ, Hunt VL, et al. Long-term outcome in patients with severe alcoholic hepatitis can be reliably determined using an in vitro measure of steroid sensitivity. Hepatology. 2015;61(3):1099. doi:10.1002/hep.27211
22. Rossi RE, Conte D, Massironi S. Diagnosis and treatment of nutritional deficiencies in alcoholic liver disease: overview of available evidence and open issues. Dig Liver Dis. 2015;47:819–825. doi:10.1016/j.dld.2015.05.021
23. Moreno C, Deltenre P, Senterre C, et al. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology. 2016;150:903–910.e8. doi:10.1053/j.gastro.2015.12.038
24. Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(6):1637–1648. doi:10.1053/gast.2000.20189
25. De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15(13):1613–1619. doi:10.3748/wjg.15.919
26. Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev. 2009;CD007339.
27. Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–1628. doi:10.1056/NEJMoa1412278
28. Singh S, Murad MH, Chandar AK, et al. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta-analysis. Gastroenterology. 2015;149:958–70. e12. doi:10.1053/j.gastro.2015.06.006
29. Vergis N, Atkinson SR, Knapp S, et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology. 2017;152(5):1068–1077.e4. doi:10.1053/j.gastro.2016.12.019
30. Spahr L, Lambert JF, Rubbia-Brandt L, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221–229. doi:10.1002/hep.22317
31. Spahr L, Chalandon Y, Terraz S, et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One. 2013;8:e53719. doi:10.1371/journal.pone.0053719
32. Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109:1417–1423. doi:10.1038/ajg.2014.154
33. Singh V, Keisham A, Bhalla A, et al. Efficacy of Granulocyte Colony-Stimulating Factor and N-acetylcysteine therapies in patients with severe alcoholic hepatitis. Clin Gastroenterol Hepatol. 2018;16:1650–1656.e2. doi:10.1016/j.cgh.2018.01.040
34. Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–1397. PMID: 15122768. doi:10.1002/hep.20206
35. Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960. doi:10.1053/j.gastro.2008.08.057
36. Higuera-de la Tijera F, Servín-Caamaño AI, Serralde-Zúñiga AE, et al. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol. 2015;21:4975–4985. doi:10.3748/wjg.v21.i16.4975
37. Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi:10.1056/NEJMoa1105703
38. Im GY, Kim-Schluger L, Shenoy A, et al. Early liver transplantation for severe alcoholic hepatitis in the United States–A single-center experience. Am J Transplant. 2016;16:841–849. doi:10.1111/ajt.13586
39. Lee BP, Chen PH, Haugen C, et al. Three-year results of a pilot program in early liver transplantation for severe alcoholic hepatitis. Ann Surg. 2017;265:20–29. doi:10.1097/SLA.0000000000001831
40. Thompson J, Jones N, Al-Khafaji A, et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: a multinational, prospective, controlled, randomized trial. Liver Transpl. 2018;24(3):380–393. doi:10.1002/lt.24986
41. Singal AK, Shah VH. Current trials and novel therapeutic targets for alcoholic hepatitis. J Hepatol. 2019;70(2):305–313. doi:10.1016/j.jhep.2018.10.026
42. Vergis N, Atkinson SR, Thursz MR. The future of therapy for alcoholic hepatitis - Beyond corticosteroids. J Hepatol. 2019;70(4):785–787. doi:10.1016/j.jhep.2019.01.016
43. Peeraphatdit TB, Simonetto DA, Shah VH. Exploring new treatment paradigms for alcoholic hepatitis by extrapolating from NASH and cholestasis. J Hepatol. 2018;69(2):275–277. doi:10.1016/j.jhep.2018.05.012
44. Mathurin P, Dufour JF, Bzowej NH, et al. Selonsertib in combination with prednisolone for the treatment of severe alcoholic hepatitis: a phase 2 randomized controlled trial. Oral abstracts (Abstracts 1–299). Hepatology. 2018;68:1–183. doi:10.1002/hep.30256
45. Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(4):235–246. doi:10.1038/s41575-018-0099-1
46. Philips CA, Pande A, Shasthry SM, et al. Healthy donor faecal microbiota transplantation in steroid ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol. 2017;15:600–602. doi:10.1016/j.cgh.2016.10.029
47. Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol. 2018;37(3):215–225. doi:10.1007/s12664-018-0859-4
48. Philips CA, Augustine P, Padsalgi G, Ahamed R, Jose A, Rajesh S. Only in the darkness can you see the stars: severe alcoholic hepatitis and higher grades of acute-on-chronic liver failure. J Hepatol. 2019;70(3):550–551. doi:10.1016/j.jhep.2018.10.004
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.