Back to Journals » Journal of Inflammation Research » Volume 16
Serum YKL-40 Levels and White Matter Hyperintensities in Patients with Acute Ischemic Stroke
Authors Shi G, Ke D, Gong P, Yu P, Zhou J, Wang M, Zhang X , Wang X, Guo M, Xu M, Zhou R
Received 22 November 2022
Accepted for publication 18 January 2023
Published 25 January 2023 Volume 2023:16 Pages 311—319
DOI https://doi.org/10.2147/JIR.S398701
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Guomei Shi,1,2,* Dongdong Ke,2,3,* Pengyu Gong,4,5 Peng Yu,2,6 Junshan Zhou,5 Meng Wang,5 Xiaohao Zhang,5 Xiaorong Wang,1,2 Minwang Guo,1,2 Mingyang Xu,1,2 Rujuan Zhou1,2
1Department of Neurology, the Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China; 2Stroke Center, the Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China; 3Department of Rehabilitation, the Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China; 4Department of Neurology, Affiliated Hospital of Nantong University, Nantong, Jiangsu, People’s Republic of China; 5Department of Neurology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 6Department of Radiology, the Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Rujuan Zhou, Tel +86-13951158499, Email [email protected]
Background: White matter hyperintensity (WMH) is associated with risk of acute ischemic stroke (AIS) and poor outcomes after AIS. The purpose of this prospective study was to evaluate the association between serum YKL-40 levels and WMH burden in patients with AIS.
Methods: From February 2020 to March 2021, a total of 672 consecutive AIS patients with magnetic resonance imaging data were prospectively recruited form two centers. Serum YKL-40 levels were quantified using enzyme-linked immunosorbent assay. The burden of WMH was semiquantitatively measured by the Fazekas visual grading scale. According to severity of overall WMH, patients were dichotomized into none–mild WMH group (Fazekas score 0– 2) or moderate–severe WMH group (Fazekas score 3– 6). Besides, based on severity of periventricular WMH (PV-WMH) and deep WMH (D-WMH), patients were categorized as none–mild (Fazekas score 0– 1) or moderate–severe (Fazekas score 2– 3).
Results: Among the 672 patients, 335 (49.9%) participants were identified with moderate–severe overall WMH, 326 (48.5%) with moderate–severe PV-WMH and 262 (39.0%) with moderate–severe D-WMH. Compared with the first quartile of serum YKL-40, the adjusted odds ratio (OR) of the fourth quartile for moderate–severe PV-WMH was 2.473 (95% confidence interval [CI] 1.316– 4.646; P=0.005). No significant association was observed between YKL-40 and overall WMH (OR 0.762; 95% CI 0.434– 1.336; P=0.343) or D-WMH (OR 0.695; 95% CI 0.413– 1.171; P=0.172).
Conclusion: Our results suggested that higher YKL-40 levels appeared to be associated with PV-WMH, but not with overall WMH or D-WMH in patients with AIS.
Keywords: acute ischemic stroke, YKL-40, white matter hyperintensities, biomarkers
Background
Stroke is reported to be one of the leading causes of disability and death around the world, especially in China.1 White matter hyperintensity (WMH), a standout neuroimaging feature of chronic cerebral small vessel disease (CSVD),2 is associated with an increased risk of ischemic stroke, poor clinical prognosis after stroke, as well as recurrent stroke.3,4 To date, the underlying pathophysiology of WMH is poorly understood. Although age and traditional vascular risk factors are widely regarded as the main risk factors for WMH,2,5 they are not responsible for all the pathogenesis and progression of WMH. Therefore, identifying novel related risk factors is crucial and would improve current understanding of the etiology and pathogenesis of WMH in patients with acute ischemic stroke (AIS).
Neuroinflammatory process is getting more and more attention for its pivotal role in the progression of both acute and chronic cerebrovascular disease.6,7 Several inflammatory biomarkers have been identified as candidate risk factors for WMH, such as vascular cell adhesion molecule-1, lipoprotein-associated phospholipase A2, and so on.8,9 YKL-40, also called chitinase-3-like-1 protein (CHI3L1) or breast regression protein 39 (BRP-39), is a novel biomarker of inflammation and plays a crucial role in angiogenesis, tissue fibrosis, inflammation, oxidative tissue injury, and extracellular remodeling responses.10,11 Elevated YKL-40 levels have been reported to be associated with atherosclerosis,12 cardiovascular disease13 and cerebrovascular disease.14 Recently, the cerebrospinal fluid (CSF) YKL-40 levels had been recognized as a pathophysiological biomarker for neurological diseases, such as Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, multiple sclerosis, and amyotrophic lateral sclerosis.15,16 Nevertheless, the relationship between serum YKL-40 and WMH burden in AIS patients remained unknown and was waiting for neurologists to explore.
We hypothesized that increased serum YKL-40 levels may be linked to severity of WMH. Based on this hypothesis, a prospective cross-sectional study was conducted to assess the correlation between serum YKL-40 levels at admission and severity of WMH in patients with AIS. Additionally, we explored the associations of YKL-40 with WMH burden in different regions.
Materials and Methods
Study Population
Between February 2020 and March 2021, a total of 672 AIS patients admitted to Stroke Center of the Taixing People’s Hospital and Nanjing First Hospital were consecutively recruited. The Ethics Committees of the two participating centers reviewed and approved the study. Informed consent was acquired from each participant/representative. This study was also complied with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
Patients were enrolled if they (1) aged 18 years or older, (2) were admitted within 48 hours of symptom onset, (3) underwent brain magnetic resonance imaging (MRI) including T2 fluid-attenuated inversion recovery (FLAIR) sequence. We excluded patients with (1) bilateral cerebral hemisphere infarction, (2) history of brain operation or trauma, (3) abnormalities on brain MRI (eg, neoplasm, hydrocephalus, or autoimmune encephalitis).
Data Collection
For each participant, we collected demographic characteristics and past medical history including age, gender, hypertension, diabetes mellitus, lipid metabolism disorders, atrial fibrillation, coronary heart disease, previous stroke or transient ischemic attack (TIA), smoking, and drinking. Besides, we recorded each participant’s laboratory data including levels of leucocyte, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), and hyper-sensitive C-reactive protein (hs-CRP). Clinical assessment (blood pressure, previous antiplatelet, previous statin, and stroke etiology) of each AIS patient was also collected. Stroke etiology was classified in light of Trial of ORG 10,172 in Acute Stroke Treatment (TOAST) criteria.17
Measurement of YKL-40
Blood samples were collected within the first 24 hours of admission after overnight fasting. Serum samples were centrifuged (1500 rpm, 4°C, 15 min) and frozen at −80°C until later analysis. Serum concentrations of YKL-40 were determined with a four-parameter curve using a commercial enzyme-linked immunosorbent assay kit (Cat No. ab255719, Abcam). Intra-panel calibration was performed in line with the manufacturer’s instructions, where the calculated minimal detectable dose is 3.9 pg/mL. The mean intra-assay and inter-assay coefficients of variation for YKL-40 were shown to be 2.4% and 1.7%, respectively.
Imaging Assessment
Brain MRI was performed with two different 3.0 Tesla scanners (Verio Dot, Siemens, Erlangen, Germany and Ingenia, Philips, Best, Netherlands). Detailed MRI acquisition included T1-weighted sequence, T2-weighted sequence and T2 FLAIR sequence. WMH can be localised to two anatomically distinct regions: the area adjacent to the ventricles (periventricular WMH, PV-WMH) and the area under the cortex (deep WMH, D-WMH).18 Both PV-WMH and D-WMH were semiquantitatively measured at T2-weighted as well as FLAIR sequences using the Fazekas visual grading scale,19 which ranges from 0 to 3, by two experienced neuroradiologists who were blinded to all clinical data. We categorized the severity of PV-WMH and D-WMH as none–mild (Fazekas score 0–1) or moderate–severe (Fazekas score 2–3). The sum of PV-WMH and D-WMH scores was used to characterize the severity of overall WMH. All participants were dichotomized into none–mild (Fazekas score 0–2) and moderate–severe (Fazekas score 3–6) groups according to severity of overall WMH.
Statistical Analysis
Statistical analyses were conducted by SPSS (version 26; SPSS Inc, Chicago, IL, USA). Continuous variables of an abnormal distribution were presented as the medians (interquartile range [IQR]), and categorical variables were presented as numbers (percentages [%]). Continuous variables were compared using analysis of variance, Kruskal–Wallis H-test, Student’s t-test or Mann–Whitney U-test, as appropriate. Categorical variables were compared using Pearson’s chi-square test or Fisher exact test, as appropriate. Spearman rank correlation was used to identify the association between serum YKL-40 levels and Fazekas scores. Univariate and multivariate logistic regression analyses were performed to explore the relationship between serum YKL-40 levels and the severity of overall WMH, PV-WMH as well as D-WMH. Adjustments were made for age, gender, and the variables with P < 0.1 in univariate analyses. All tests were 2-tailed, and statistical significance threshold was set at P < 0.05.
Results
Baseline Clinical Characteristics
A total of 672 AIS patients were consecutively recruited from February 2020 to March 2021 in our study. Among the included patients, the median age was 70 years, and 419 (62.4%) patients were male. The median level of serum YKL-40 at admission was 134.26 ng/mL. There were 337 (50.1%) patients with none–mild overall WMH (Fazekas score 0–2) and 335 (49.9%) patients with moderate–severe overall WMH (Fazekas score 3–6). Baseline clinical characteristics of participants by the degree of overall WMH are shown in Table 1. When comparing patients with none–mild overall WMH, those with moderate–severe overall WMH tended to be older (P=0.001) and had higher levels of FBG (P=0.014), higher proportions of hypertension (P=0.001), previous stroke or TIA (P=0.006) and subtype of large-artery atherosclerosis (P=0.002); while the levels of serum YKL-40 (P=0.754) were not significantly different.
 |
Table 1 Baseline Characteristics of Participants by the Degree of Overall WMH |
Associations of YKL-40 with Overall WMH, PV-WMH, and D-WMH
Table 2 demonstrates Fazekas scores among patients according to quartiles of YKL-40. Compared with the lowest quartile of serum YKL-40, patients in the fourth quartile had higher Fazekas scores of overall WMH (P=0.029) and PV-WMH (P=0.001). However, there was no significant difference in D-WMH among the four groups (P=0.973).
 |
Table 2 Fazekas Scores Among Patients According to Quartiles of YKL-40 |
We further assessed the associations of PV-WMH and D-WMH with serum YKL-40 levels separately. There were 346 (51.5%) patients with none–mild PV-WMH and 326 (48.5%) patients with moderate–severe PV-WMH. Compared with patients in none–mild PV-WMH group, those in moderate–severe PV-WMH group were older and had a higher prevalence of hypertension, previous stroke or TIA and subtype of large-artery atherosclerosis; lower levels of TG; and higher levels of FBG, hs-CRP and YKL-40 (Table 3). When grouped according to severity of D-WMH, 410 (61.0%) and 262 (39.0%) patients were in the none–mild and moderate–severe D-WMH groups, respectively. Patients with moderate–severe D-WMH were older and had higher proportion of hypertension, previous stroke or TIA, smoking and subtype of large-artery atherosclerosis; and higher levels of FBG (Table 3).
 |
Table 3 Characteristics of Participants the Degree of PV-WMH and D-WMH |
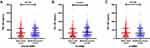
Levels of YKL-40 in the overall WMH, PV-WMH, and D-WMH groups are depicted in Figure 1. Serum YKL-40 levels of AIS patients were higher in moderate–severe PV-WMH group than those in none–mild PV-WMH group (150.21 [81.13, 261.60] versus 130.94 [63.53, 232.33] ng/mL, P=0.007). Furthermore, when comparing with first quartile of serum YKL-40, the adjusted odds ratio (OR) of the fourth quartile for moderate–severe PV-WMH was 2.473 (95% confidence interval [CI] 1.316–4.646; P=0.005) after adjusting for age, gender, and variables with P < 0.1 in univariate analysis. However, there was no significant association between YKL-40 with overall WMH or D-WMH (Table 4).
 |
Table 4 Logistic Regression Analyses of YKL-40 with White Matter Hyperintensity |
Discussion
In this prospective cross-sectional study, we demonstrated that baseline serum YKL-40 levels were statistically associated with severity of PV-WMH in patients with AIS. This relationship was independent of age, gender, and other potential confounding variables. However, no significant associations were found between YKL-40 with overall WMH or D-WMH.
Our study showed a prevalence of 49.9% for moderate–severe overall WMH, 48.5% for moderate–severe PV-WMH and 39.0% for moderate–severe D-WMH, which were in parallel with previous researches,20,21 while higher than findings (36.2% for moderate–severe PV-WMH and 15.0% for moderate–severe D-WMH) by Zong and colleagues.22 Differences in age distributions of the participants may be the main reason for this discrepancy. The mean age was 62.3 ± 11.5 years in the study by Zong and colleagues,22 which was lower than that in our study (69.0 ± 12.0 years). Previous studies have demonstrated that increasing age was the most prominent risk factor for WMH,23,24 and our results also confirmed this. In addition, our present study revealed that both PV-WMH and D-WMH were significantly associated with hypertension, previous stroke or TIA, as well as higher blood glucose levels, which were consistent with previous results.25
The precise pathophysiological mechanisms of WMH have not been fully elucidated, but may include hypoxia-ischemia, blood–brain barrier (BBB) breakdown, endothelial dysfunction, oxidative stress, venous collagen deposition.26,27 In recent years, increasing evidence indicates an extraordinarily critical role of inflammation in the progression of WMH.9,24,28,29 As a glycoprotein mediating inflammation, YKL-40 has been reported to correlated with pulmonary diseases, diabetes mellitus, coronary heart disease, Alzheimer’s disease and stroke.10,11,14 However, evidence for the correlation between YKL-40 and CSVD is limited. In a mouse model of WMH induced by chronic cerebral hypoperfusion, the expression of YKL-40 was shown to be elevated.30 A recently published community-based study with 960 Chinese stroke-free participants observed a significant association between elevated levels of endothelial-related inflammatory biomarkers including YKL-40 and increased WMH volume.9 In another study with 42 sporadic CSVD patients, CSF levels of YKL-40 were positively correlated with the WMH load.31 The results of our study suggested that serum YKL-40 levels at baseline, in AIS patients remained significantly associated with PV-WMH after adjusting for potential confounding risk factors, which has never been reported to our knowledge.
Although the exact mechanisms linking elevated YKL-40 and PV-WMH are still unclear, some possible explanations have been proposed. First of all, evidence from both Danish general population and Chinese stroke patients have shown that YKL-40 levels are positively correlated with increasing age,32,33 and age is recognized as a prominent risk factor for PV-WMH,23,24 which could partly explain the association between YKL-40 and PV-WMH. Secondly, overexpression of YKL-40 has been shown to up-regulate matrix metalloproteinase-9,34 which is best known as a major contributor to degrade extracellular matrix and disrupt BBB permeability,35,36 and further participate in the progression of WMH. Thirdly, as an endothelial-related inflammatory mediator, YKL-40 could activate endothelial cells to induce intercellular adhesion molecule-1 expression,37 which would further recruit leukocytes and release pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin-1β and interferon-γ, leading to endothelial dysfunction and BBB disruption.38,39 Additionally, according to the latest research,30 YKL-40 expression in astrocytes was elevated in white matter lesions caused by chronic hypoxia, and downregulating the expression of YKL-40 significantly alleviate white matter injury, suggesting that YKL-40 is implicated in the process of white matter injury. Taken together, the mechanisms mentioned above might be significant contributors to YKL-40 with PV-WMH.
Whereas our study suggested that elevated serum YKL-40 levels were associated with PV-WMH, no significant association was found between YKL-40 and D-WMH. To date, only a few studies investigated the differences between PV-WMH and D-WMH. In a prospective cross-sectional study involving 595 AIS patients, Yu et al showed that plasma phenylacetylglutamine levels in the fourth quartile was significantly associated with moderate–severe overall WMH (95% CI 1.134–4.018; P=0.019) and PV-WMH (95% CI 1.174–4.226; P=0.014), but not D-WMH (95% CI 0.981–3.372; P=0.057).21 Similarly, another recent study which detected the relationships of trimethylamine N-oxide with PV-WMH and D-WMH in a large cohort of TIA/AIS patients found that elevated trimethylamine N-oxide levels were associated with a higher risk of WMH burden, and more closely associated with PV-WMH than D-WMH.40 We speculate that the discrepancy may be attributed to anatomical, histological as well as pathophysiological differences between PV-WMH and D-WMH. Previous histopathological studies have demonstrated that PV-WMH contained more immunoreactive microglia and astrocytes than D-WMH.41,42 Pathology studies have also shown that PV-WMH is more likely related to inflammation and chronic hypoperfusion,43 while D-WMH is more linked to ischemic damage.44 Our data provided clinical evidence for the different pathological processes underlying PV-WMH and D-WMH, and further investigations about the detailed mechanisms are required.
Several potential limitations of our study should be mentioned. First, the samples were collected from AIS patients, thereby our results may not be able to reflect the general population. Second, due to the cross-sectional observational nature of this study, we cannot establish a causal relationship between YKL-40 and WMH. Third, as reported by a previous study, YKL-40 levels of AIS patients increased on the first day and peaked on the second day after admission.14 However, our study only monitored YKL-40 levels at baseline and did not examine the dynamic changes of YKL-40, which may have provided more valuable information about the mechanism underlying the association between YKL-40 and WMH burden in AIS patients. Additionally, although the Fazekas visual rating scale is widely used to assess WMH burden, it is not as precise as quantitative evaluation. Therefore, our results should be interpreted with caution. Whereas YKL-40 was associated with PV-WMH severity in our study, it is not a risk factor for PV-WMH.
Conclusions
Despite its inherent shortcomings, our data suggest that in AIS patients, elevated serum YKL-40 levels appear to be associated with PV-WMH severity, but not with D-WMH severity. Future studies are needed to validate the relationship and to further explore the exact mechanism linking YKL-40 and WMH.
Data Sharing Statement
The relevant data supporting the conclusions of this study are available on reasonable request to Rujuan Zhou.
Ethics Approval and Consent to Participate
The protocol was reviewed and approved by the Ethical Committee of the Taixing People’s Hospital and Nanjing First Hospital. All participants or their legal representatives provided informed consent. The protocol was also conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made significant contributions to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Doctoral Science Foundation of Taixing People’s Hospital Foundation Project (trybs2022001), the Young Scientific and Technological Talents Promotion Project of Jiangsu Association for Science and Technology (TJ2021019), and the Key Program of Kangda College of Nanjing Medical University Science and Technique Development Foundation Project (KD2020KYJJZD021).
Disclosure
All the authors declare that there is no conflict of interest for this work.
References
1. Ma Q, Li R, Wang L., et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2021;6(12):e897–e906. doi:10.1016/s2468-2667(21)00228-0
2. Han F, Zhai FF, Wang Q, et al. Prevalence and risk factors of cerebral small vessel disease in a Chinese population-based sample. J Stroke. 2018;20(2):239–246. doi:10.5853/jos.2017.02110
3. Derraz I, Abdelrady M, Ahmed R, et al. Impact of white matter hyperintensity burden on outcome in large-vessel occlusion stroke. Radiology. 2022;304(1):145–152. doi:10.1148/radiol.210419
4. Ryu WS, Schellingerhout D, Hong KS, et al. White matter hyperintensity load on stroke recurrence and mortality at 1 year after ischemic stroke. Neurology. 2019;93(6):e578–e589. doi:10.1212/wnl.0000000000007896
5. Rist PM, Buring JE, Rexrode KM, Cook NR, Rost NS. Prospectively collected lifestyle and health information as risk factors for white matter hyperintensity volume in stroke patients. Eur J Epidemiol. 2019;34(10):957–965. doi:10.1007/s10654-019-00546-x
6. Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2018;137(5):693–714. doi:10.1007/s00401-018-1930-z
7. Evans LE, Taylor JL, Smith CJ, Pritchard HAT, Greenstein AS, Allan SM. Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc Res. 2021;117(13):2575–2588. doi:10.1093/cvr/cvab284
8. Shoamanesh A, Preis SR, Beiser AS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84(8):825–832. doi:10.1212/wnl.0000000000001279
9. Zhang DD, Cao Y, Mu JY, et al. Inflammatory biomarkers and cerebral small vessel disease: a community-based cohort study. Stroke Vasc Neurol. 2022;7(4):302–309. doi:10.1136/svn-2021-001102
10. Yeo I, Lee C, Han S, Yun J, Hong J. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol Ther. 2019;203:107394. doi:10.1016/j.pharmthera.2019.107394
11. Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther. 2020;5(1):201. doi:10.1038/s41392-020-00303-7
12. Wang Y, Li B, Jiang Y, et al. YKL-40 Is associated with ultrasound-determined carotid atherosclerotic plaque instability. Front Neurol. 2021;12:622869. doi:10.3389/fneur.2021.622869
13. Pala S, Sari M, Kahveci G, Alizade E, Arslantas U, Uslu A. Plasma YKL-40 elevation on admission and follow-up is associated with diastolic dysfunction and mortality in patients with acute myocardial infarction. Cardiol Res Pract. 2018;2018:8701851. doi:10.1155/2018/8701851
14. Park H, Jun C, Jeon S, et al. Serum YKL-40 levels correlate with infarct volume, stroke severity, and functional outcome in acute ischemic stroke patients. PLoS One. 2012;7(12):e51722. doi:10.1371/journal.pone.0051722
15. Baldacci F, Lista S, Palermo G, Giorgi FS, Vergallo A, Hampel H. The neuroinflammatory biomarker YKL-40 for neurodegenerative diseases: advances in development. Expert Rev Proteomics. 2019;16(7):593–600. doi:10.1080/14789450.2019.1628643
16. Dichev V, Kazakova M, Sarafian V. YKL-40 and neuron-specific enolase in neurodegeneration and neuroinflammation. Rev Neurosci. 2020;31(5):539–553. doi:10.1515/revneuro-2019-0100
17. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi:10.1161/01.str.24.1.35
18. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. doi:10.2214/ajr.149.2.351
19. Fazekas F, Niederkorn K, Schmidt R, et al. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19(10):1285–1288. doi:10.1161/01.str.19.10.1285
20. Gong X, Shan W, Yuan K, et al. Dietary Inflammatory Index and Leukoaraiosis in Patients with Ischemic Stroke. J Nutr Health Aging. 2020;24(5):473–477. doi:10.1007/s12603-020-1351-2
21. Yu F, Feng X, Li X, et al. Gut-Derived Metabolite Phenylacetylglutamine and White Matter Hyperintensities in Patients With Acute Ischemic Stroke. Front Aging Neurosci. 2021;13:675158. doi:10.3389/fnagi.2021.675158
22. Zong L, Yao M, Ni J, et al. Kidney function is associated with severity of white matter hyperintensity in patients with acute ischemic stroke/TIA. BMC Neurol. 2016;16(1):193. doi:10.1186/s12883-016-0714-0
23. De Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70(1):9–14. doi:10.1136/jnnp.70.1.9
24. Li T, Huang Y, Cai W, et al. Age-related cerebral small vessel disease and inflammaging. Cell Death Dis. 2020;11(10):932. doi:10.1038/s41419-020-03137-x
25. Giese AK, Schirmer MD, Dalca AV, et al. White matter hyperintensity burden in acute stroke patients differs by ischemic stroke subtype. Neurology. 2020;95(1):e79–e88. doi:10.1212/wnl.0000000000009728
26. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483–497. doi:10.1016/s1474-4422(13)
27. Wu X, Ya J, Zhou D, Ding Y, Ji X, Meng R. Pathogeneses and Imaging Features of Cerebral White Matter Lesions of Vascular Origins. Aging Dis. 2021;12(8):2031–2051. doi:10.14336/ad.2021.0414
28. Low A, Mak E, Rowe JB, Markus HS, O’Brien JT. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. 2019;53:100916. doi:10.1016/j.arr.2019.100916
29. Gao Y, Li D, Lin J, et al. Cerebral small vessel disease: pathological mechanisms and potential therapeutic targets. Front Aging Neurosci. 2022;14:961661. doi:10.3389/fnagi.2022.961661
30. Yuan J, Chen L, Wang J, et al. Adenosine A2A Receptor Suppressed Astrocyte-Mediated Inflammation Through the Inhibition of STAT3/YKL-40 Axis in Mice With Chronic Cerebral Hypoperfusion-induced White Matter Lesions. Front Immunol. 2022;13:841290. doi:10.3389/fimmu.2022.841290
31. Huss A, Abdelhak A, Mayer B, et al. Association of Serum GFAP with Functional and Neurocognitive Outcome in Sporadic Small Vessel Disease. Biomedicines. 2022;10(8):1869. doi:10.3390/biomedicines10081869
32. Kjaergaard AD, Bojesen SE, Nordestgaard BG, Johansen JS. YKL-40 and alcoholic liver and pancreas damage and disease in 86,258 individuals from the general population: cohort and Mendelian randomization studies. Clin Chem. 2014;60(11):1429–1440. doi:10.1373/clinchem.2014.229096
33. Shi G, Chen W, Gong P, et al. The Relationship Between Serum YKL-40 Levels on Admission and Stroke-Associated Pneumonia in Patients with Acute Ischemic Stroke. J Inflamm Res. 2021;14:4361–4369. doi:10.2147/JIR.S329612
34. Libreros S, Garcia-Areas R, Shibata Y, Carrio R, Torroella-Kouri M, Iragavarapu-Charyulu V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int J Cancer. 2012;131(2):377–386. doi:10.1002/ijc.26379
35. Qin W, Li J, Zhu R, et al. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway. Aging. 2019;11(23):11391–11415. doi:10.18632/aging.102537
36. Zozulya A, Weidenfeller C, Galla HJ. Pericyte-endothelial cell interaction increases MMP-9 secretion at the blood-brain barrier in vitro. Brain Res. 2008;1189:1–11. doi:10.1016/j.brainres.2007.10.099
37. Yasuda T, Kaneto H, Katakami N, et al. YKL-40, a new biomarker of endothelial dysfunction, is independently associated with albuminuria in type 2 diabetic patients. Diabetes Res Clin Pract. 2011;91(2):e50–2. doi:10.1016/j.diabres.2010.11.015
38. Bui TM, Wiesolek HL, Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108(3):787–799. doi:10.1002/jlb.2mr0220-549r
39. Simka M. Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis. Curr Neurovasc Res. 2009;6(2):132–139. doi:10.2174/156720209788185605
40. Chen Y, Xu J, Pan Y, et al. Association of Trimethylamine N-Oxide and Its Precursor With Cerebral Small Vessel Imaging Markers. Front Neurol. 2021;12:648702. doi:10.3389/fneur.2021.648702
41. Simpson JE, Ince PG, Higham CE, et al. Microglial activation in white matter lesions and nonlesional white matter of ageing brains. Neuropathol Appl Neurobiol. 2007;33(6):670–683. doi:10.1111/j.1365-2990.2007.00890.x
42. Fadul MM, Heath PR, Cooper-Knock J, et al. Transcriptomic Analysis of Age-Associated Periventricular Lesions Reveals Dysregulation of the Immune Response. Int J Mol Sci. 2020;21(21):7924. doi:10.3390/ijms21217924
43. Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi:10.1212/wnl.43.9.1683
44. Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37(6):1391–1398. doi:10.1161/01.Str.0000221308.94473.14
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.