Back to Journals » Clinical Interventions in Aging » Volume 10
Serum total prostate-specific antigen values in men with symptomatic prostate enlargement in Nigeria: role in clinical decision-making
Authors Nnabugwu I , Ugwumba F, Enivwenae O, Udeh EI, Otene C, Nnabugwu C
Received 5 September 2014
Accepted for publication 8 November 2014
Published 30 December 2014 Volume 2015:10 Pages 89—93
DOI https://doi.org/10.2147/CIA.S73814
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Ikenna I Nnabugwu,1,2 Fred O Ugwumba,1 Oghenekaro A Enivwenae,2 Emeka I Udeh,1 Chris O Otene,2 Chinwe A Nnabugwu3
1Urology Unit, Department of Surgery, College of Medicine, University of Nigeria, Nsukka, 2Urology Unit, Department of Surgery, 3Department of Medical Laboratory Services, Federal Medical Centre, Asaba, Nigeria
Background: Prostatic enlargement is a common cause of bladder outlet obstruction in men in Nigeria. Malignant enlargements must be differentiated from benign enlargements for adequate treatment of each patient. High serum total prostate-specific antigen (tPSA) levels suggest malignancy, but some of the biopsies done due to a serum tPSA value >4 ng/mL would be negative for malignancy because of the low specificity of tPSA for prostate cancer. This study aims to compare the histologic findings of all prostate specimens obtained from core needle biopsy, open simple prostatectomy, and transurethral resection of the prostate with the respective serum tPSA values in an attempt to decipher the role of serum tPSA in the management of these patients.
Methods: The case notes of patients attended to from April 2009 to March 2012 were analyzed. Essentially, the age of the patient, findings on digital rectal examination, abdominopelvic ultrasonography report on the prostate, serum tPSA, and histology reports from biopsy or prostatectomy specimens as indicated were extracted for analysis.
Results: The relationship between age, findings on digital rectal examination, serum tPSA, abdominopelvic ultrasonography report, and histology are compared. A statistically significant relationship existed between a malignant histology and age 65 years and older, suspicious findings on digital rectal examination, suspicious ultrasonography findings, and serum tPSA >10 ng/mL, but not tPSA >4 ng/mL.
Conclusion: In Nigerian patients with symptomatic prostate enlargement, serum tPSA should be seen as a continuum with increasing risk of prostate malignancy.
Keywords: serum total prostate-specific antigen, symptomatic prostate enlargement, prostate histology
Introduction
Prostatic enlargement is a common cause of bladder outlet obstruction among men in Nigeria. The enlargement usually leads to lower urinary tract symptoms,1 irrespective of whether it is benign or malignant. With wide acceptance of serum prostate-specific antigen (PSA) estimation,2 the ability to differentiate malignant from benign prostatic enlargement with or without symptoms before instituting definitive treatment has increased.3 However, an entirely new range of challenges have been encountered as a result of the poor sensitivity of serum total PSA (tPSA).3–5 The need to carry out prostate biopsy to confirm malignant prostate enlargement in cases of elevated serum tPSA comes to the fore.6 Some of these prostate biopsies will be negative for malignant cells or identify low-volume, low-grade adenocarcinoma of uncertain clinical significance.7,8 The sensitivity of serum tPSA in detecting prostate cancer has been put at 25% in screening programs8 and as high as 99.6% in patients with symptoms.9
In Nigeria, a serum tPSA value >4.0 ng/mL has been widely accepted as an indication to exclude adenocarcinoma of the prostate through biopsy of the prostate,10 a cut-off value adopted from studies done mostly outside Nigeria.11–13
Serum tPSA varies with body mass index, intravascular volume, prostate volume, benign and malignant diseases of the prostate. As a result, some studies have applied serum tPSA values of 3.0 ng/mL instead of >4.0 ng/mL in the Asian continent as a cut-off point for subsequent prostate biopsy.5,14 In Nigeria, Anunobi et al found that among patients with serum tPSA 4.0–49.9 ng/mL, core needle biopsy specimens revealed many benign prostates.15
Clinical adenocarcinoma of the prostate varies between races16,17 and studies have shown that Africa has a high prevalence of the disease.1,18,19 It is important that malignancy is excluded in all cases of symptomatic prostatic enlargement, so this study aims to relate the histologic findings of all prostate specimens obtained from core needle biopsy, open simple prostatectomy, and transurethral resection of the prostate to the respective serum tPSA values in an attempt to decipher the role of serum tPSA in the clinician’s decision-making.
Materials and methods
This was a hospital-based retrospective study from April 2009 to March 2012, ie, a period of 36 months. The case notes of patients who presented with and were evaluated for symptomatic prostate enlargement were retrieved and the relevant information contained therein was extracted for analysis. When biopsy was indicated, digitally guided transrectal extended sextant core needle biopsy of the prostate was offered due to the nonavailability of transrectal ultrasonography-guided biopsy. A disposable semiautomatic size 16 G or 18 G Trucut® biopsy needle was used for each prostate biopsy. The indication for biopsy was a suspicious digital rectal examination (DRE) finding, suspicious prostate from abdominopelvic ultrasonography (USS), or elevated serum tPSA (>4 ng/mL). In patients with a working diagnosis of benign prostatic hyperplasia (BPH) requiring surgical management, simple retropubic prostatectomy, transvesical prostatectomy, or transurethral resection of the prostate was offered. All specimens were sent for histologic analysis by a pathologist.
The age and nationality of the patients were noted from the case notes. In addition, the DRE finding, serum tPSA result, USS assessment of the prostate, and the histologic report on the various specimens were extracted from each patient’s case notes for analysis. The data analysis was done using Statistical Package for the Social Sciences version 20 software (IBM Corporation, Armonk, NY, USA). The Pearson correlation was used to test for association between numeric data. The chi-square test was used for nonparametric tests (Yates correction for continuity was employed when necessary). The level of significance was set at a two-tailed P-value of <0.05.
Results
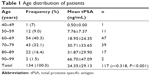
Case notes for 134 patients were identified, with only 117 having documented tPSA. The number of patients in each age group and the average tPSA for the respective age groups are shown in Table 1.
  | Table 1 Age distribution of patients |
DRE findings
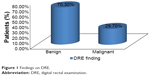
DRE was documented for 128 patients. Findings were benign in 90 (70.3%), and suspicious or suggestive of malignancy in 38 (29.7%), as shown in Figure 1.
  | Figure 1 Findings on DRE. |
Serum tPSA
The result for serum tPSA was documented for 117 patients. The average value was 24.35±29.13 ng/mL, with a range of 0.4–100 ng/mL. As shown in Table 2, 74.4% of tPSA values were >4 ng/mL while 25.6% were ≤4 ng/mL.
  | Table 2 Serum total prostate-specific antigen |
USS scanning
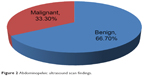
USS was done in 110 patients, with the results presented in Figure 2. The findings were inconclusive in two patients. In the remaining 108 patients, findings were benign in 72 (66.7%) and suspicious in 36 (33.3%).
  | Figure 2 Abdominopelvic ultrasound scan findings. |
Prostate biopsy
Forty-six patients had histology results (Table 3). Adenocarcinoma was confirmed in 26 (56.5%), while 19 (41.3%) showed benign features. One patient (2.2%) had high-grade prostatic intraepithelial neoplasia.
  | Table 3 Histology on transrectal core needle prostate biopsy |
Histology of prostatectomy specimens
A total of 23 patients underwent prostatectomy over the study period; 19 (82.6%) had open prostatectomy and four (17.4%) had transurethral resection of the prostate. Seven of the 23 patients who had prostatectomy underwent prostate biopsy due to serum tPSA >4 ng/mL. The histology reports were BPH. Subsequent histology reports of prostatectomy specimens from these seven patients confirmed BPH in six and adenocarcinoma (Gleason score 2+3) in one. Table 4 shows the histologic report of the 23 prostatectomy specimens: 18 (78.3%) were nodular hyperplasia (BPH), four (17.4%) were chronic inflammatory cell infiltration on a background of nodular hyperplasia (BPH + prostatitis), and one (4.3%) was adenocarcinoma (Gleason score 2+3).
  | Table 4 Histology of prostatectomy specimens |
Summary of histology reports
Thirty-nine patients underwent prostate biopsy due to suspicious prostates (suspicious DRE, suspicious ultrasound findings, or tPSA >4 ng/mL), while 16 patients had simple prostatectomy without prior biopsy since there was no indication for biopsy, giving a total of 55 patients. Of the 39 prostates that were biopsied, 26 were adenocarcinoma, one was high-grade prostatic intraepithelial neoplasia, and 12 were benign. Seven of the 12 cases that were benign underwent prostatectomy. Thus, seven patients had prostate biopsy initially and prostatectomy at a later date, resulting in 62 histologic reports that were analyzed (Figure 3). The histologic reports on all the prostate specimens were categorized for patient age, DRE findings, serum tPSA values, and USS reports (Table 5).
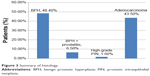  | Figure 3 Summary of histology. |
There were nine histologic reports for patients with serum tPSA >4 ng/mL and 10 ng/mL. The DRE findings, USS findings, and duration of follow-up for these patients were analyzed (Table 6). Four with benign DRE and USS findings had benign histology, while three adenocarcinoma cases had either suspicious DRE or USS findings.
Discussion
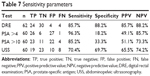
Case notes for 134 patients seen within the study period could be retrieved for analysis. All were for Nigerians of mean age 69.97±9.50 (45–94) years with a modal range of 60–69 years (Table 1). They all had symptomatic enlargement of the prostate. One hundred and twenty-eight DREs of the prostate were performed, with 90 (70.3%) and 38 (29.7%) feeling benign and malignant, respectively (Figure 1). Twenty-four (85.7%) of the 28 prostates that were histologically confirmed as adenocarcinoma of the prostate were suspicious or malignant on DRE (Table 5). A similar result was obtained by Badmus et al1 in Nigeria (92.1%), but not by Ma and Wan20 in the People’s Republic of China (31.25%) or Palmerola et al21 in the USA (44%). In contrast, of the 34 benign histology reports, four (11.8%) had suspicious DRE findings. The high sensitivity (85.7%), specificity (88.2%), positive predictive value (85.7%), and negative predictive value (88.2%) of DRE in this analysis (Table 7) may be due to late presentation, by which time locoregional features of the disease were well established.
USS assessment was documented conclusively for 108 prostates, with 72 (66.7%) showing benign features and 36 (33.3%) showing malignant features (Figure 2). Table 5 shows that 18 of the 28 malignant prostates (69.2%) had a suspicious ultrasonography report. In Southwestern Nigeria, Badmus et al1 obtained a value of 78.8%. In contrast, of the 34 histologically benign prostates, ten (30.3%) were reported as suspicious for malignancy by ultrasonography.
A total of 117 tPSA values were documented. There was a significant positive correlation between age and serum tPSA (r=0.318, P<0.001; Table 1). A similar correlation was documented by Mochtar et al for patients with a symptomatic benign prostate.22 tPSA values ranged from 0.4 to 100 ng/mL, with a mean of 24.35±29.13 ng/mL and a median of 12.7 ng/mL. Eighty-seven of these 117 tPSA values (74.4%) were >4 ng/mL and 30 (25.6%) were ≤4 ng/mL (Table 2). Applying a serum tPSA of >4 ng/mL, the sensitivity and specificity of tPSA for detecting prostate cancer were 96.3% and 18.2%, respectively, with a negative predictive value of 85.7%. These were similar to the findings of Lokuhetty et al.9 Therefore, in this study, using a serum tPSA >4 ng/mL alone, 81.8% of benign prostates would have been subjected to unnecessary transrectal biopsy. However, applying a serum tPSA of >10 ng/mL alone, with a sensitivity of 85.2% and a specificity of 33.3%, the negative predictive value decreased to 73.3%. Similarly, from the study by Abbiyesuku et al23 only 37% and 54.3% of histologically benign prostatic enlargements had tPSA <4 μg/L and <10 μg/L, respectively. Thompson et al13 concluded that there is no cutpoint of PSA with simultaneous high sensitivity and high specificity for monitoring healthy men for prostate cancer, but rather a continuum of prostate cancer risk at all PSA values. The inference from the review by Schröder and Roobol24 is similar to the conclusion of Thompson et al. In our study of men with symptomatic prostatic enlargement, serum tPSA values also form a continuum with increasing risk of prostate cancer. It may be argued that it is necessary to have followed-up the patients with tPSA >4 ng/mL, but benign histological report, for longer periods to ensure they do not turn out to be cases of false negative histological reports. However, de-novo prostate cancer can also occur in the residual prostates after simple prostatectomy for benign prostate enlargement.
Conclusion
Among Nigerian men with symptomatic prostate enlargement, serum tPSA >4 ng/mL and 10 ng/mL may not be interpreted in isolation as an indication for prostate biopsy, since from this analysis 81.8% of benign prostate specimens had values >4 ng/mL. However, with a negative predictive value of 85.7% for serum tPSA >4 ng/mL (Table 7), symptomatic patients with tPSA ≤4 ng/mL could be safely managed as benign prostate enlargement in the absence of suspicious DRE and USS findings. Applying serum tPSA >10 ng/mL in isolation as a cut-off may appear to have reduced the number of benign prostates that would have been biopsied by 15.1%, but also increased the missed malignant prostates by 3.6%. The sensitivity of DRE and USS in detecting suspicious features in symptomatic prostate enlargement should not be overlooked, especially in our community, where patients directly bear the cost of medical treatment. Serum tPSA should be considered as an invaluable component of the triad (DRE, ultrasonography, PSA) in the management of patients with symptomatic prostate enlargement. DRE and ultrasonography findings are important in deciding which prostate to biopsy when serum tPSA is between 4 ng/mL and 10 ng/mL (Table 6).
Disclosure
The authors report no conflicts of interest in this work.
References
Badmus TA, Adesunkanmi AK, Yusuf BM, et al. Burden of prostate cancer in Southwestern Nigeria. Urology. 2010;76(2):412–416. | ||
National Institute of Clinical Excellence. Prostate cancer diagnosis and treatment. Available from: http://www.nice.org.uk/nicemedia/pdf/CG58FullGuideline.pdf. Accessed November 24, 2014. | ||
Bensalah K, Lotan Y, Karam JA, Shariat SF. New circulating biomarkers for prostate cancer. Prostate Cancer Prostatic Dis. 2008;11(2):112–120. | ||
Wallner LP, Morgenstern H, McGree ME, et al. The effects of body mass index on changes in prostate-specific antigen levels and prostate volume over 15 years of follow-up: implications for prostate cancer detection. Cancer Epidemiol Biomarkers Prev. 2011;20(3):501–508. | ||
Lee SE, Hong SK, Park HZ, et al. Higher body mass index is associated with lower risk of prostate cancer detection via multi (≥12)-core prostate biopsy in Korean men. Urology. 2010;76(5):1063–1066. | ||
Moussa AS, Li J, Soriano M, Klein EA, Dong F, Jones JS. Prostate biopsy clinical and pathological variables that predict significant grading changes in patients with intermediate and high grade prostate cancer. BJU Int. 2009;103(1):43–48. | ||
Pepe P, Aragona F. Incidence of insignificant prostate cancer using free/total PSA: results of a case-finding protocol on 14,453 patients. Prostate Cancer Prostatic Dis. 2010;13(4):316–319. | ||
Tubaro A, De Nunzio C, Mariani S, et al. Reduction of prostate-specific antigen after tamsulosin treatment in patients with elevated prostate-specific antigen and lower urinary tract symptoms associated with low incidence of prostate cancer at biopsy. Urology. 2010;76(2):436–441. | ||
Lokuhetty MD, Wijesinghe HD, Abeysuriya DT, Samarasinghe UC, Perera ND. Transrectal ultrasound guided prostate biopsies: a single centre experience in Sri Lanka. Ceylon Med J. 2009;54(1):6–9. | ||
Ajape AA, Ibrahim KO, Fakeye JA, Abiola OO. An overview of cancer of the prostate diagnosis and management in Nigeria: the experience in a Nigerian tertiary hospital. Ann Afr Med. 2010;9(3):113–117. | ||
Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156–1161. | ||
Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6–4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277(18):1452–1455. | ||
Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/mL or lower. JAMA. 2005;294(1):66–70. | ||
Hong SK, Oh JJ, Byun S, et al. Impact of diabetes mellitus on the detection of prostate cancer via contemporary multi (≥12)-core prostate biopsy. Prostate. 2012;72(1):51–57. | ||
Anunobi CC, Akinde OR, Elesha SO, Daramola AO, Tijani KH, Ojewola RW. Prostate diseases in Lagos, Nigeria: a histologic study with tPSA correlation. Niger Postgrad Med J. 2011;18(2):98–104. | ||
Mettlin CJ, Murphy G. The national cancer base report on prostate cancer. Cancer. 1994;74(5):1640–1648. | ||
Bowa K. An overview of the diagnosis and management of prostate cancer in Nigeria: experience from a north-central state of Nigeria. Ann Afr Med. 2010;9(3):111–112. | ||
Osegbe DN. Prostate cancer in Nigerians: facts and nonfacts. J Urol. 1997;157(4):1340–1343. | ||
Bowa K, Kachimba JS, Labib M, Mudenda V, Chikwenya M. The changing pattern of urological cancers in Zambia. Med J Zambia. 2009;35(4):157–159. | ||
Ma H, Wan B. [Relationship of the positive rate of TRUS guided prostate biopsies with routine diagnostic findings in detecting prostate cancer]. Zhonghua Nan Ke Zue. 2013;19(1):40–43. Chinese. | ||
Palmerola R, Smith P, Elliot V, et al. The digital rectal examination (DRE) remains important – outcomes from a contemporary cohort of men undergoing an initial 12–18 core prostate needle biopsy. Can J Urol. 2012;19(6):6542–6547. | ||
Mochtar CA, Kiemeney LA, van Riemsdijk MM, et al. Prostate-specific antigen as an estimator of prostate volume in the management of patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2003;44(6):695–700. | ||
Abbiyesuku FM, Shittu OB, Oduwole OO, Osotimehin BO. Prostate specific antigen in the Nigerian African. Afr J Med Med Sci. 2000;29(2):97–100. | ||
Schröder FH, Roobol MJ. Defining the optimal prostate-specific antigen threshold for the diagnosis of prostate cancer. Curr Opin Urol. 2009;19(3):227–231. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.