Back to Journals » OncoTargets and Therapy » Volume 12
Serum starvation induces cell death in NSCLC via miR-224
Authors Wang G, Han J, Zhuang L, Li S, Gong Q, Chen Y
Received 6 September 2018
Accepted for publication 2 November 2018
Published 22 May 2019 Volume 2019:12 Pages 3955—3964
DOI https://doi.org/10.2147/OTT.S186613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Guoqin Wang,1 Jiangqiong Han,2 Li Zhuang,1 Shijuan Li,1 Quan Gong,1 Yunlan Chen3
1Department of Alleviative Treatment; 2Department of Integrative Medicine; 3Department of Cadres Convalescence, Yunnan Cancer Hospital, The Third Affiliated Hospital of Kunming Medical University, Kunming 650118, People’s Republic of China
Purpose: Increasing evidence suggests that microRNAs (miRNAs) may be involved in the occurrence and progression of non-small cell lung cancer (NSCLC). In the present study, we used serum-starved A549 cells emulating tumor under a nutrient depletion stress in the microenvironment.
Patients and methods: We first detected the expression level of miR-224 between tumor tissues and the adjacent normal tissues. We analyzed the expression levels of miR-224 and its predicted target phosphatase and tensin homolog (PTEN) using quantitative real-time PCR (qRT-PCR) in starved A549 cells. Following transfection with miR-224 mimic or inhibitor in starved A549 cells, MTT assay, Annexin V FITC/PI staining, and LC-3 immunofluorence staining were performed to investigate the roles of miR-224 on proliferation, apoptosis, and autophagy. Next, the expression of apoptosis-related protein Bax and Bcl-2, autophagy-related proteins LC3, PI3K signaling, and target PTEN were measured using qRT-PCR and Western blot assays. The direct interaction between miR-224 and PTEN was validated with a dual luciferase assay.
Results: We found that the expression level of miR-224 in tumor tissues was significantly higher when compared with the adjacent normal tissues. We discovered a reciprocal expression pattern between miR-224 and PTEN in starved A549 cells, and transfection with miR-224 mimic led to down-regulation of PTEN. A dual luciferase assay further confirmed the direct interaction between miR-224 and 3ʹUTR of PTEN. Transfection with miR-224 mimic in starved A549 cells resulted in enhanced cell proliferation, reduced apoptosis, and autophagy, accompanied by increased expression of anti-apoptotic protein Bcl-2, decreased expression of pro-apoptotic protein Bax, and autophagy-related protein LC3. Activation of PI3K was observed in miR-224 mimic transfected cells. The reverse effects by the miR-224 inhibitor in all experiments were observed.
Conclusion: Taken together, we proved that miR-224 might play essential roles in cellular functions of nutrient-depleted A549 cells possibly through regulating the target PTEN and downstream signal PI3K, suggesting the potential of miR-224 to be a therapeutic target for NSCLC therapy.
Keywords: miR-224, NSCLC, serum starvation, PTEN, PI3K
Introduction
Lung cancer is the most common type of cancer, with high incidence and mortality rates in the world.1 Approximately 85% of lung cancer cases are non-small cell lung cancer (NSCLC).2 Tumor microenvironment is characterized by hypoxia and nutrient depletion, which impacts metabolism and biological functions of tumor cells.3 Fetal bovine serum (FBS) is the most widely used supplement in cell culture medium, providing essential nutrients for normal cell growth in vitro. Serum starvation is considered as an effective means to nutrient depletion, which can simulate the tumor microenvironment of cancers, including A549 cells.4 Despite that the short-time starvation can reduce tumor growth and sensitize tumor cells to chemo-therapeutic agents,4 tumor cells can struggle to adapt to the loss of nutrient supply by adjusting a series of specific signal pathways or molecules and reprogramming their metabolism, which thereby supports their continuous proliferation.5 Therefore, more efforts are necessary to investigate the mechanisms of adaptation of tumor cells to insufficient nutrient supply and to explore the specific targets, which may provide a novel therapeutic strategy for cancers, including NSCLC.
MicroRNAs (miRNAs) are small (22 nucleotides in length) single-stranded noncoding RNAs that are involved in the regulation of gene expression at the post-transcriptional level by specific binding to 3´UTR of the target mRNA, leading to translational repression or degradation.6,7 miRNAs play essential roles in biological behaviors of tumor cells including proliferation, apoptosis, differentiation, autophagy, angiogenesis, and metastasis, thereby in strong association with the severity of cancers and outcome of clinical therapy in patients.8–10 Changes in miRNA expression patterns are important indicators of cancers.9,10 Increasing evidence suggests that miR-224 is significantly up-regulated in NSCLC tissues and positively associated with occurrence and metastasis of NSCLC.11–13 MiR-224 is also reportedly to be a key factor for poor prognosis and chemo-resistance.11,14 In vitro experiments demonstrate that miRNA-224 promotes proliferation and invasion of A549 cells.12,15 On the contrary, some researchers identified lower expression level of miR-224 in NSCLC tissues.16 Until now, the functions of miR-224 in A549 cells in serum-starved condition remain unclear.
The current study was designed to confirm the expression pattern of miR-224 in NSCLC tissues; analyze the effects of miR-224 on the cell viability, apoptosis, and autophagy of A549 cells under serum-starved condition; further explore the possible signaling pathway involved in these cellular functions; and finally identified phosphatase and tensin homolog (PTEN) as its direct target. This will enable us to fully understand the role of miR-224 in pathological functions and mechanisms in A549 cells under serum-starved condition.
Material and methods
Samples
Thirty pairs of NSCLC tissues and adjacent normal tissues (≥5 cm) were collected from patients hospitalized in Yunnan Cancer Hospital, the Third Affiliated Hospital of Kunming Medical University from May 2015 to January 2017. The information of the patients were as follows: gender, male (n=16), female (n=14); age 43–65 years. The samples obtained from surgery were immediately stored in liquid nitrogen at −80°C. The patients enrolled in this study all signed and handed over the confirmed consent. The study was approved by the Ethics Committee of Yunnan Cancer Hospital, the Third Affiliated Hospital of Kunming Medical University.
Cell viability
The impact of miR-224 on cell viability was analyzed using MTT assay (Bio-Rad, USA). Briefly, cells of each group in a 96-well cell plate (cells/well) were incubated for different periods of time (12, 24, and 48 hrs). Ten microliters of MTT reagent (5 mg/mL) were added to each well and incubated for 4 hrs at 37°C. The supernatants were aspirated, and the formazan crystals were dissolved in dimethylsulfoxide. The optical density of each well was measured using an ELISA reader (Bio-Rad, USA) at a wavelength of 490 nm.
Western blot
To obtain total protein extracts from each group, cells were washed with ice-cold PBS and lysed with RIPA lysis buffer (Beyotime, Shanghai, China) on ice. Cells were homogenized and centrifuged. Supernatants were collected and boiled with loading buffer. Cell lysates were subjected to 10% dodecyl sulfate polyacrylamide gels and proteins were transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat milk and incubated with anti-PTEN (ab32199, 1: 1/10,000, Abcam, USA), anti-Bax (ab32503, 1: 2,000, Abcam, USA), anti-Bcl-2 (ab32124, 1: 1,000, Abcam, USA), anti-AKT (ab106693, 1: 1,000, Abcam, USA), anti-PI3K (ab151549, 1: 1,000, Abcam, USA), anti-PI3K p85 (ab191606, 1: 1,000, Abcam, USA), anti-microtubule associated protein 1 light chain 3 (LC3)B-II/I (ab51520, 1: 3,000, Abcam, USA), and anti-GAPDH (ab9485, 1: 2,500, Abcam, USA) antibodies. The membranes were washed and then incubated with horseradish peroxidase–-conjugated secondary antibodies (ab6721, 1: 2,000, Abcam, USA). The resulting light emission was detected with Novex® ECL Chemiluminescent Substrate Reagent Kit (Thermo Fisher Scientific, Massachusetts, USA) and documented with a ChemiDoc™ XRS+ imaging system (Bio-Rad, California, USA).
Detection of cell apoptosis
The Annexin-V/Fluorescein isothiocyanate (FITC) and propidium iodine (PI) method (BD Biosciences, NJ, USA) was used to identify apoptotic cells. In short, cells seeded on a 24-well plate (cells/well) were detached from the plate surface using tripsin and washed with cold PBS. The cells were centrifuged and re-suspended in cold binding buffer. One hundred microliters of the sample solution were transferred to a 5 mL culture tube and incubated with 5 μL FITC-conjugated Annexin V and 5 μL of PI for 15 mins at room temperature in darkness. Four hundred microliters of binding buffer were added to each sample tube and subjected to FACS Calibur flow cytometer (Becton Dickinson, CA, USA). Results were analyzed by CellQuest software (BD Biosciences, NJ, USA). Each independent experiment was performed in triplicate.
Detection of autophagy using GFP-LC3
A549 cells were transfected with p-LC3 plasmid, a high specific fluorescent marker of autophagy, to measure autophagy levels. Transfected cells plated on microscope slides were washed with PBS and fixed with 3% paraformaldehyde. The fixed cells were strained with anti-LC and the primary antibody was then detected with DyLight 488-conjugated antiserum. Cell nuclei were counterstained with DAPI. The slides were mounted and the fluorescence was visualized under a Leica confocal laser scanning microscope.
Quantitative real-time PCR (qRT-PCR)
Total RNA from cultured cells was extracted using TRIZOL reagent (Thermo Fisher Scientific, Massachusetts, USA). cDNA was amplified in a reverse transcription reaction using PrimeScript™ RT reagent Kit (TaKaRa, Dalian, China). SYBR Green qRT-PCR assay was carried out R using SYBR® Premix Ex Taq™ kit (TaKaRa, Dalian, China) on an ABI 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with the specific primer sets for PTEN, Bax, Bcl-2, and PI3K. The expression of miR-224 was analyzed using the Hairpin-itTM miRNAs qPCR Quantitation Kit (GenePharma, Shanghai, China). GAPDH and U6 were used as internal controls for mRNAs and miR-224, respectively. Relative expression of the transcript levels was calculated using the 2−ΔΔCt method. Primers (synthesized by Genscript Nanjing Inc., China) are listed in Table 1. Each group was repeated three times.
 | Table 1 Oligonucleotide primers used for Q-PCR |
Cell culture and treatment
Human NSCLC cell line A549 was purchased from cell bank at Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, USA), supplemented with 10% FBS (Invitrogen, Carlsbad, USA), 100 IU/mL of penicillin and 100 mg/mL of streptomycin (Invitrogen, Carlsbad, USA), and incubated at 37°C in a humidified atmosphere of 5% CO2. Cells were starved in medium without FBS for 4 hrs prior to the experiments.
Cells were transfected with miR-224 mimic/NC or inhibitor/NC (RiboBio, Guangzhou, China) using Lipofectamine 2000 following the manufacturer’s instructions and subjected to the starvation condition for 4 hrs. According to the different treatments, the cells were divided into five groups as follows: control group (no treatment), miR-224 inhibitor group, miR-224 inhibitor negative control (NC) group, miR-224 mimic group, and miR-224 mimic NC group. The expression of miR-224 and the target gene PTEN in each group was detected by qRT-PCR assay.
Dual luciferase activity assay
3ʹUTR region of PETN containing predicted miR-224 specific binding site was PCR amplified and cloned into the firefly luciferase reporter vector (Thermo Fisher Scientific, Inc.) to obtain the wild-type luciferase reporter plasmid p-PETN-wt. For generation of the mutant reporter plasmid p-PETN-mut, nucleotides were mutated lacking miR-224-binding sites using PCR.
MiR-224 targeting assay was performed in HEK-293T cells, co-transfecting the firefly luciferase reporter plasmid (wild type or mutated) with ranilla luciferase vector and miR-224 mimic using Lipofectamine 2000. Cells were lysed and their luciferase activities were assayed after 24 hrs using Dual-Luciferase Reporter Assay System (Promega, USA).
Results
miR-224 was overexpressed in NSCLC tissues
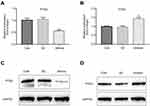
qRT-PCR was performed to examine the expression of miR-224. The result suggested that compared with adjacent normal tissues, the expression of miR-224 in NSCLC tissues was significantly increased (Figure 1, p<0.01).
miR-224 regulates proliferation, apoptosis, and autophagy of A549 cells under serum-starved condition
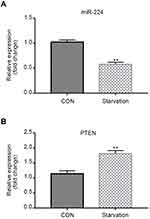
qRT-PCR data showed that miR-224 was down-regulated in A549 cells under serum-starved condition (Figure 2A). To understand the contribution of miR-224 to cellular functions of A549 under starvation condition, we transfected A549 cells with miR-224 mimic/NC or miR-224 inhibitor/NC. After confirming miR-224 expression by qRT-PCR (Figure 3A–B), we analyzed the cell proliferation capability associated with miR-224. Our data showed that there is a slight difference at 12 and 24 hrs at post-transfection, and a significant difference was observed at 48 hrs; miR-224 overexpression increased cell proliferation in a time-dependent manner (Figure 3C). As expected, the effect of miR-224 inhibitor on cell proliferation was reversed with that of miR-224 mimic (Figure 3D).
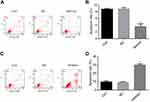
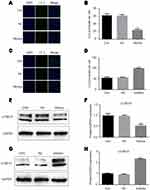
To identify the potential mechanisms responsible for the observed effects of miR-224 on cell proliferation, we performed autophagy analysis and evaluated apoptosis rates in A549 cells transfected with miR-224 mimic/NC or miR-224 inhibitor/NC. Our results showed that miR-224 overexpression reduced apoptosis in A549 cells (Figure 4A–D). In addition to the reduced apoptosis, we observed reduced autophagy in cells transfected with miR-224 mimic and increased autophagy following transfection with miR-224 inhibitor. Expression of LC-3B-II/I, an autophagy marker, was attenuated by miR-224 mimic and enhanced by the miR-224 inhibitor (Figure 5A–H). These data collectively suggested that the pro-proliferative effect of miR-224 might be possible due to repressed apoptosis and autophagy.
miR-224 regulates Bcl-2, Bax, and p-PI3K in A549 cells under serum-starved condition
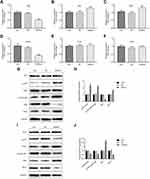
Next, we analyze the possible signal transduction involved in miR-224-mediated cellular responses. Bcl-2/Bax and PI3K signaling pathways have been reported to be related to cell proliferation, apoptosis, and autophagy. To investigate the effect of miR-224 on Bcl-2/Bax and PI3K signalings, we analyzed the expression of PI3K and p-PI3K in miR-224-transfected A549 cells. As shown in Figure 6A–F, miR-224 overexpression up-regulated expression of anti-apoptosis related Bcl-2 and down-regulated expression of pro-apoptosis-related Bax,17,18 whose effects were reversed by the miR-224 inhibitor. Moreover, similar effects were noted for protein expression levels of PI3K and AKT; however, a significant increase in protein expression of p-PI3K-p85 and p-AKT was induced by miR-224 mimic, and reduction in p-PI3K-p85 and p-AKT expression was observed in A549 cells transfecting with miR-224 inhibitor (Figure 6G–J, p<0.01). These results suggest that miR-224 could exert its cellular functions partially through activation of PI3K.
miR-224 directly targets PTEN
By using Targetscan tool, we predicted that miR-224 is highly likely to interact with 3´ UTR of PTEN. First, we observed that up-regulation of PTEN in serum-starved A549 cells and negatively correlated with the expression of miR-224 (Figure 2B). Then, we transfected miR-224 mimic or inhibitor into A549 cells under starvation. Our results showed that exogenous expression of miR-224 significantly suppressed PTEN expression at both mRNA and protein levels, whereas miR-224 inhibitor had the reverse effects (Figure 7A–D).
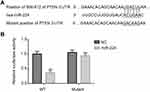
Next, to confirm whether PTEN is a direct target of miR-224, we subcloned PTEN 3´UTR containing the target sequences into a luciferase reporter vector, and the corresponding p-PTEN-mut was also constructed. The vectors were then co-transfected into the HEK293T cells with miR-224 mimic. It was found that miR-224 was bound to 3´UTR of PTEN and subsequently reduced the luciferase activity of reporter p-PTEN-wt; however, this effect was reversed following transfection of reporter p-PTEN-mut (Figure 8A and B).
Discussion
miRNAs are considered as pivotal regulators of gene expression at transcriptional and post-transcriptional levels. They participate in the pathology of cancers, functioning either as oncogenes or as tumor suppressors.9,10 Despite that one report suggests lower expression of miR-224 in NSCLC tissues is associated with poor prognosis,16 there are many studies identifying higher expression of miR-224 in NSCLC tissues and suggesting a prognostic value of miR-224 in NSCLC.11–14 In this study, we used serum deficiency emulating the nutrient depletion stress in the tumor microenvironment in vivo and identified lower expression of miR-224 in serum-starved condition.
Increased proliferation, reduced apoptosis, and autophagy are recognized as the key hallmarks in cancer cell development.19–21 In our study, miR-224 mimic has effectively increased the proliferation of A549 cells under serum-starved condition, which are similar to the results from most previous studies.11–14 Autophagy and apoptosis are closely related to cell death events. Notably, we have observed attenuated LC3-I/II accumulation and lower expression in A549 cells transfecting miR-224 mimic and the opposite effects in cells transfecting mR-224 inhibitor. Autophagy is also associated with apoptosis, which may be necessary for apoptosis initiation.22 The present study indicated that miR-224 overexpression suppressed apoptotic rate, accompanied with an up-regulated expression of anti-apoptotic protein Bcl-2 and down-regulated expression of pro-apoptotic protein Bax, whereas miR-224 down-regulation restored these effects. Therefore, cell viability or proliferation regulated by miR-224 is possibly dependent on miR-224-regulated autophagy and apoptosis.
Since miRNAs aberrant expression usually results in inhibition of their target genes, here we analyzed the expression level of PTEN, the target of miR-224. We observed a reciprocal expression pattern between the expression level of miR-224 and that of PTEN in A549 cells under serum-starved condition. Accordingly, we found that PTEN was higher expressed or lower expressed upon miR-224 silencing or overexpression, respectively. In addition, we confirmed the direct binding of miR-224 on PTEN 3´UTR through a dual luciferase assay. A growing evidence suggests that PTEN is strongly associated with improved apoptosis and suppressed proliferation in A549 cells.19,20 Moreover, overexpression, phosphorylation, and nuclear translocation of PTEN promote to induce autophagy of A549 cells.14,23 We thereby speculated that miR-224 exhibits its cellular functions in A549 cells possibly via negatively regulating the target PTEN.
The intracellular signaling pathway PI3K regulates diverse cellular functions such as proliferation, apoptosis, and autophagy in diverse types of cancer cells. Evidence has shown that PTEN/PI3K/AKT is involved proliferation and apoptosis of A549 cells, and PTEN functions via down-regulating PI3K/AKT pathway.24,25 Thus, an attempt has been made to investigate whether the effects of miR-224 on A549 cells under serum-starved condition is through PI3K pathway. Our data indicated that miR-224 mimic activated PI3K, which was suppressed by the miR-224 inhibitor. These results suggested that PI3K pathway might be involved in the mechanisms by which miR-224 functions in A549 cells.
Conclusion
The present study demonstrated that miR-224 overexpression inhibited cell proliferation and induced apoptosis and autophagy in A549 cells under serum-starved condition possibly by negatively regulating its target PTEN through PI3K pathway. Thus, these findings may provide a basis for a novel therapeutic strategy for clinical NSCLC therapy.
This study investigated the roles of miR-224 in NSCLC cells and tissues. However, the study was limited to cells. Further study should focus on clinical trial and animal experiment to verify the potential mechanisms of miR-224 regulating serum starvation-induced cell death.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi:10.1007/978-3-319-24223-1_1
2. Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81.
3. Kwon SJ, Lee YJ. Effect of low glutamine/glucose on hypoxia-induced elevation of hypoxia-inducible factor-1alpha in human pancreatic cancer MiaPaCa-2 and human prostatic cancer DU-145 cells. Clin Cancer Res. 2005;11:4694–4700. doi:10.1158/1078-0432.CCR-04-2530
4. Dong S, Khoo A, Wei J, et al. Serum starvation regulates E-cadherin upregulation via activation of c-Src in non-small-cell lung cancer A549 cells. Am J Physiol Cell Physiol. 2014;307:893–899. doi:10.1152/ajpcell.00132.2014
5. Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer. 2012;12:571. doi:10.1186/1471-2407-12-571
6. Hujie G, Zhou SH, Zhang H, et al. MicroRNA-10b regulates epithelial-mesenchymal transition by modulating KLF4/KLF11/Smads in hepatocellular carcinoma. Cancer Cell Int. 2018;18:10. doi:10.1186/s12935-018-0508-0
7. Li C, Jiang Y, Miao R, Qu K, Zhang J, Liu C. MicroRNA-1271 functions as a metastasis and epithelial-mesenchymal transition inhibitor in human HCC by targeting the PTP4A1/c-Src axis. Int J Oncol. 2018;52:536–546. doi:10.3892/ijo.2017.4224
8. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi:10.1038/nrg1379
9. Lages E, Ipas H, Guttin A, Nesr H, Berger F, Issartel JP. MicroRNAs: molecular features and role in cancer. Front Biosci (Landmark Ed). 2012;17:2508–2540.
10. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi:10.1038/nrc1997
11. Kulda V, Svaton M, Mukensnabl P, et al. Predictive relevance of miR-34a, miR-224 and miR-342 in patients with advanced squamous cell carcinoma of the lung undergoing palliative chemotherapy. Oncol Lett. 2018;15:592–599. doi:10.3892/ol.2017.7337
12. Cui R, Kim T, Fassan M, et al. MicroRNA-224 is implicated in lung cancer pathogenesis through targeting caspase-3 and caspase-7. Oncotarget. 2015;6:21802–21815. doi:10.18632/oncotarget.5224
13. Cui R, Meng W, Sun HL, et al. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc Natl Acad Sci USA. 2015;112:E4288–E4297. doi:10.1073/pnas.1502068112
14. Liu J, Xing Y, Rong L. miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway. Oncol Rep. 2018;39:1631–1639. doi:10.3892/or.2018.6268
15. Wang L, Liu W, Zhang YP, Huang XR. The miR-224 promotes non-small cell lung cancer cell proliferation by directly targeting RASSF8. Eur Rev Med Pharmacol Sci. 2017;21:3223–3231.
16. Zhu D, Chen H, Yang X, et al. Decreased microRNA-224 and its clinical significance in non-small cell lung cancer patients. Diagn Pathol. 2014;9:198. doi:10.1186/s13000-014-0198-4
17. Feliciano A, Castellvi J, Artero-Castro A, et al. miR-125b acts as a tumor suppressor in breast tumorigenesis via its novel direct targets ENPEP, CK2-alpha, CCNJ, and MEGF9. PLoS One. 2013;8:e76247. doi:10.1371/journal.pone.0076247
18. Sun KX, Xia HW. Pachymic acid inhibits growth and induces cell cycle arrest and apoptosis in gastric cancer SGC-7901 cells. Oncol Lett. 2018;16:2517–2524. doi:10.3892/ol.2018.8899
19. Ai X, Mao F, Shen S, Shentu Y, Wang J, Lu S. Bexarotene inhibits the viability of non-small cell lung cancer cells via slc10a2/PPARgamma/PTEN/mTOR signaling pathway. BMC Cancer. 2018;18:407. doi:10.1186/s12885-018-4242-8
20. Li J, Zhou Q, Liang Y, et al. miR-486 inhibits PM2.5-induced apoptosis and oxidative stress in human lung alveolar epithelial A549 cells. Ann Transl Med. 2018;6:209. doi:10.21037/atm.2018.06.09
21. Wang R, Zhang Q, Peng X, et al. Stellettin B induces G1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep. 2016;6:27071. doi:10.1038/srep27071
22. Hwang KE, Kim YS, Jung JW, et al. Inhibition of autophagy potentiates pemetrexed and simvastatin-induced apoptotic cell death in malignant mesothelioma and non-small cell lung cancer cells. Oncotarget. 2015;6:29482–29496. doi:10.18632/oncotarget.5022
23. Chen JH, Zhang P, Chen WD, et al. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy. 2015;11:239–252. doi:10.1080/15548627.2015.1009767
24. Lu XX, Cao LY, Chen X, Xiao J, Zou Y, Chen Q. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed Res Int. 2016;2016:2476842. doi:10.1155/2016/2476842
25. Song L, Li D, Gu Y, et al. MicroRNA-126 targeting PIK3R2 inhibits NSCLC A549 cell proliferation, migration, and invasion by regulation of PTEN/PI3K/AKT pathway. Clin Lung Cancer. 2016;17:e65–e75. doi:10.1016/j.cllc.2016.03.012
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.