Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Serum MOTS-C Levels are Decreased in Obese Children and Associated with Vascular Endothelial Function
Authors Luo YH, Xie L, Li JY, Xie Y, Li MQ, Zhou L
Received 7 January 2023
Accepted for publication 28 March 2023
Published 12 April 2023 Volume 2023:16 Pages 1013—1020
DOI https://doi.org/10.2147/DMSO.S403934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Yan-Hua Luo,1 Li Xie,2 Jiao-Yang Li,3 Yuan Xie,4 Man-Qin Li,1 Li Zhou1
1Department of Metabolism and Endocrinology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421001, People’s Republic of China; 2Pediatric Medical Center, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421001, People’s Republic of China; 3Department of Preventive Medicine, School of Public Health, Wuhan University, Wuhan, Hubei, 430071, People’s Republic of China; 4Department of Radiology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421001, People’s Republic of China
Correspondence: Li Zhou, Department of Metabolism and Endocrinology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, 421001, People’s Republic of China, Tel +86 18573477875, Email [email protected]
Purpose: The increasing prevalence of obesity in children and its associated risk with cardiovascular diseases demand more discovery of the novel biomarkers for developing new treatment options for this complex disease. This study aimed to investigate the association of serum MOTS-C (a peptide encoded in the mitochondrial genome) levels and vascular endothelial function in obese children.
Patients and Methods: A total of 225 obese children (aged 8.1 ± 2.6 years) and 218 healthy children (aged 7.9 ± 2.2 years) were enrolled. Related anthropometric assessment and biochemical evaluation were done in all subjects. Reactive hyperemia index (RHI), as assessed by the peripheral arterial tonometry, was used for evaluation of peripheral endothelial function. Enzyme-linked immunosorbent assay (ELISA) was used to measure the level of serum MOTS-C.
Results: Levels of serum MOTS-C and RHI were lower in the obese children compared with the healthy children (P < 0.01). The RHI level was independently associated with body mass index, high-density lipoprotein cholesterol, and MOTS-C in linear regression analysis. Further analysis showed a significant mediating effect of MOTS-C on the correlation between body mass index and RHI in children, with the ratio of mediating effect value of 9.12%.
Conclusion: These data identify that MOTS-C is a previously unknown regulator in the development process of obesity-induced vascular changes.
Keywords: obese, children, mitochondrial-derived peptides, MOTS-C, vascular endothelial function
Introduction
Childhood obesity is still a serious global health problem although many efforts have been done to decrease the rate of obesity in children. It has been shown that 40 million children under the age of 5 years and more than 330 million children and adolescents aged 5–19 years were overweight or obese in 2016.1 This worldwide epidemic has serious consequences, including psychiatric, psychological, and psychosocial disorders in childhood and increases the risk of dyslipidemia, insulin resistance, and long-term vascular complications.2 In addition, the children with excess weight are likely to become adults with obesity which harder the treatment of obesity. Therefore, the member states of the World Health Organization have been encouraged by these trends to endorse a target of no increase in obesity in childhood by 2025.
Importantly, research in children with obesity suggest that children may exhibit early signs of cardiovascular dysfunction, primarily atherosclerotic, as a result of their excess adiposity.3 In fact, in 2007, it was predicted that in the United States, the number of additional cardiovascular events attributable to excess weight in adolescence is expected to be >100,000 by 2035.4 These concerns suggest the problem is not only one of future or long-term cardiovascular disease risk but rather, one requiring immediate attention to prevent progressive cardiovascular damage in childhood. Endothelial cells are sentinels of cardiovascular health, and are essential for hemostasis, maintenance of vascular tone and redox homeostasis. There is substantial evidence that endothelial dysfunction is an essential element in the progression of atherosclerosis and plaque, and is the earliest detectable change in the life history of atherosclerotic lesions.5 Stimulation of the proinflammatory state, insulin resistance and high production of free fatty acids have been shown to be the most important factors involved in the mechanism leading to endothelium damage.6 Fortunately, endothelial dysfunction during childhood appears to be a reversible process.7 Therefore, it is imperative to clarify the related potential molecular mechanisms and intervention targets.
MOTS-C is a 16-amino acid polypeptide encoded by mitochondrial 12S rRNA, found in various organs and tissues such as skeletal muscle, myocardium, kidney, fat, and circulating plasma in rodents and humans. MOTS-C could directly target skeletal muscle and result in inhibition of the folate cycle and restriction of de novo purine biosynthesis, leading to AMP-activated protein kinase (AMPK) activation.8 MOTS-C can translocate to the nucleus during metabolic stress (such as glucose restriction or oxidative stress), directly regulating the expression of adaptive nuclear genes to promote cell homeostasis.9,10 Studies have confirmed that the improvement of insulin resistance, inhibition of weight gain and liver fat accumulation could be obtained by MOTS-C through activating the AMPK pathway to regulate metabolic homeostasis.8 In addition, mitochondrial respiration could be inhibited by MOTS-C, resulting in the suppressive occurrence and development of aging-related diseases.11 Recent clinical studies showed that a significant decrease of plasma MOTS-C levels could be found in obese diabetic patients, obese children and adolescents, and chronic kidney disease patients.12–15 In addition, the serum MOTS-C levels in adult patients with coronary heart disease or coronary endothelial dysfunction also decreased significantly.16,17 The reason may be that endothelial dysfunction is reduced by inhibiting the mitogen-activated protein kinase (MAPK) / nuclear factor-κB (NF-κB) pathway, thereby exerting the role of vascular endothelial function protective factor.18 However, it is not yet clear whether MOTS-C is involved in the injury of vascular endothelial function in obese children. Therefore, this study aimed to investigate the association of serum MOTS-C levels in obese children and endothelial function damage to provide new strategies for early prevention of obesity-derived cardiovascular diseases.
Materials and Methods
Study Design
The study was carried out at the endocrinology outpatient clinic of the First Affiliated Hospital of University of South China (Hengyang, China). All participants completed a uniform questionnaire containing medical history, taking medications history, and factors of lifestyle, and received a comprehensive physical examination. Besides, biochemical examinations, including serum cortisol and thyroid function tests, were examined. The exclusion criteria were: Children with potential endocrine disorders, or any chronic systemic diseases (respiratory, neurologic, cardiovascular, and gastrointestinal), history of drug use (antiepileptics, anti-psychotic sand steroids), or suspected obesity-related syndromes (Prader-Willi, Bardet-Biedl and Alstrom syndrome). Finally, according to the Chinese National standard,19 a total of 225 obese children with body mass index (BMI) at or above the 95th percentile and 218 healthy children with normal BMI were included in this study.
Anthropometric and Biochemical Evaluation
Measurement parameters, including height, weight, waist circumference, and blood pressure containing systolic blood pressure (SBP) and diastolic blood pressure (DBP), were measured using a standardized protocol. Blood samples were collected after fasting overnight for at least 10 hours. The related biochemical tests, including total cholesterol, triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), and fasting insulin (FINS) were also determined as previously published.20 The homeostasis model assessment of the insulin resistance (HOMA-IR) index was calculated as follows: FPG (mmol/L) × FIns (μU/mL)/22.5. Serum MOTS-C levels were measured using the ELISA kits (Catalog No. CEX132Hu; Cloud-clone corp., Wuhan, China) according to the manufacturer’s recommended protocol. All samples were assayed in duplicate and random order.
Vascular Endothelial Function Test
The use of ultrasound to measure brachial artery flow-mediated dilation (FMD) has become the most applied technique for noninvasive detection of endothelial function.21,22 Many laboratories have shown robust test–retest reliability for FMD when conditions are rigorously standardized.21,23 However, the technique requires specialized training and high-resolution sonography equipment. Thus, it is expensive and highly operator-dependent. Pulse amplitude tonometry (PAT), a Food and Drug Administration-approved method which is relatively inexpensive and operator-independent, is increasingly being used as an alternative measure of endothelium-dependent dilation in response to reactive hyperemia. Feasibility and reproducibility of PAT have been demonstrated to be excellent.24
Assessment of endothelial function in this study was performed by digital reactive hyperemia (EndoPAT 2000; Israel). Firstly, a blood pressure cuff was placed on the upper forearm and the index fingers were placed into probes after having 10 min of quiet rest in the supine position. Secondly, the pulse amplitudes obtained from both fingers were recorded. Thirdly, the cuff in the test arm was inflated to suprasystolic pressure for 5 minutes after the collection of 5 min of baseline data. Fourthly, the pulse wave was recorded for another 5 minutes after cuff release. Finally, the reactive hyperemic index (RHI), the ratio of the hyperemic pulse amplitude to the baseline, was obtained. The greater the vasodilation, the higher the RHI.
Statistical Analysis
All analyses were performed by SPSS version 24.0 (Chicago, IL, USA) and R version 4.1.2 (Foundation, Vienna, Austria). Normal distribution of the data was determined by the Kolmogorov–Smirnov test. Parametric tests, while usually more stringent than non-parametric tests, have higher statistical power (more likely to show that there is a difference when a difference truly exists).25 The variables which were not normal distribution were transformed to near normality by logarithmic transformation before analysis. Comparisons of categorical and continuous variables were performed by the chi-squared and student’s t-tests, respectively. Pearson correlation analysis and stepwise multiple linear regression were used for evaluating the associations among variables. The potential effect modification by gender was explored through testing the significance of interaction term in multivariable models. The mediation effect was tested by the Baron and Kenny method with MOTS-C as the intermediate variable, BMI as the independent variable and RHI as the dependent variable. The Sobel test was used for significance testing. P-values < 0.05 (two-sided) were considered statistically significant.
Results
Subjects’ Physical and Metabolic Characteristics
Anthropometric and metabolic parameters of the study subjects are showed in Table 1. As expected, BMI, SBP, TG, LDL-C, FINS, and HOMA-IR were significantly increased, while HDL-C was significantly decreased in the obesity group compared to the healthy group (P < 0.05). However, there were no significant differences in DBP, TC and FBG between the obesity group and the healthy group (P < 0.05).
 |
Table 1 Clinical and Demographic Data for Control and Obese Children |
Serum MOTS-C Levels and Vascular Endothelial Function
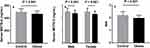
Compared with the healthy group, levels of serum MOTS-C were significantly lower in the obesity group (463.15 ± 50.57 vs 495.12 ± 54.16 ng/mL; P < 0.001) (Figure 1a). In gender-stratified subgroup analysis, both the serum MOTS-C levels of the male and female obese children were lower than the healthy group (male: 469 ± 52.64 vs 497.36 ± 58.27 ng/mL, P < 0.001; female: 456.37 ± 50.73 vs 494.24 ± 60.25 ng/mL, P < 0.001) (Figure 1b). In addition, RHI of vascular endothelial function was significantly lower in the obese group compared to the healthy group (1.95 ± 0.24 vs 2.18 ± 0.30; P < 0.001) (Figure 1c).
Correlation Between Vascular Endothelial Function and Metabolic Characteristics
In all subjects, RHI was positively correlated with MOTS-C and HDL-C levels (P < 0.05), while negatively correlated with BMI, TG, and HOMA-IR (P < 0.05) (Table 2). Multiple linear regression analysis was performed using RHI as the dependent variable and statistically significant indexes in the correlation analysis as independent variables, and age and sex were adjusted. The results showed that there was an independent linear regression correlation between RHI and BMI, HDL-C and MOTS-C (P < 0.05) (Table 2). In addition, there was no significant interaction between MOTS-C and gender (P > 0.05).
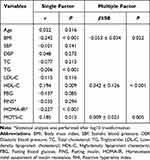 |
Table 2 Linear Regression Analysis of Factors Independently Related with RHI |
The Mediating Effect of Serum MOTS-C Levels in the Association of BMI and RHI
Based on the correlation analysis, the variables were considered to meet the prerequisites for mediating effects. The results showed a significant mediating effect of MOTS-C in the association between BMI and RHI (mediating effect value of −0.0047, P = 0.019), with a mediating effect percentage of 9.12%, and a direct effect of −0.046 for BMI and RHI (P < 0.001) (Table 3).
 |
Table 3 The Mediating Effect of Serum MOTS-C in the Association of BMI and RHI in Children |
Discussion
Although a small number of studies have reported the association of serum MOTS-C levels and obesity or coronary endothelial dysfunction,8,13,16 but this is the first study to investigate the relationship between serum MOTS-C levels and vascular endothelial function in obese children. The results showed MOTS-C is an independent positive correlation factor of RHI (a endothelial function marker). In addition, serum MOTS-C is a mediating variable in the associated between BMI and RHI, indicating that the vascular endothelial function damage may be partially caused by the decrease of serum MOTS-C in obese children.
MOTS-C, a short cytoprotective peptide secreted by mitochondria, has been shown to improve glucose metabolism in skeletal muscle, suggesting a benefit in disease such as diabetes, obesity and aging.26 Previous studies have found that circulating MOTS-C is significantly reduced in obese children and adolescents and is associated with insulin resistance and obesity markers, and the present study showed similar results.13 However, Cataldo et al found that obese and normal-weight adults have similar plasma MOTS-C levels, which may be affected by the study sample (n=10 per group).27 In addition, MOTS-C may be affected by different ages or ethnicities, needing further studies for confirmation.
Endothelial dysfunction is an important risk factor for cardiovascular disease and is a priority for the prevention and treatment of overweight or obese children. The RHI obtained by PAT is considered a well-established metric for quantifying endothelial function and a strong predictor of cardiovascular events.28 This study found that the RHI significantly decreased in obese children, indicating the presence of possible endothelial dysfunction. At present, the mechanism of obesity-related cardiovascular changes is still not fully clear. Another purpose of this study was to evaluate the relationship between circulating MOTS-C and endothelial dysfunction in obese children. The results showed that in addition to the traditional risk factors including obesity, dyslipidemia, elevated blood glucose, and insulin resistance, RHI was also significantly correlated with serum MOTS-C levels. In addition, there was an independent linear regression relationship between the changes in RHI and MOTS-C, showing that MOTS-C may be involved in the development of endothelial dysfunction. Several population studies assessed the predictive role of MOTS-C in cardiovascular disease and found that circulating MOTS-C levels were downregulated in patients with stable coronary artery disease,17 acute coronary syndrome,29 or coronary endothelial dysfunction.16
As we all know, obesity is a state of low-grade chronic inflammation, which is considered the first sign of the development of obesity-related diseases (especially vascular changes). Endothelial dysfunction is characterized by a shift in the role of endothelial cells towards a pro-inflammatory state, pro-thrombosis and reduced vasodilation.30 Notably, MOTS-c could suppress the expression of pro-inflammatory cytokines and adhesion molecules by inhibiting MAPK signaling pathway,18,31,32 suggesting mechanistically a possible role of MOTS-C in the occurrence of vascular endothelial dysfunction. However, more data are warranted to demonstrate this view in the future. In addition, several in vivo and vitro studies have also explored the potential protective mechanisms of MOTS-C against cardiac dysfunction and pathological remodeling. MOTS-C can prevent the development of heart failure via the activation of the AMPK pathway.33 Functional enrichment analysis showed that MOTS-c improved angiogenesis, inflammation and apoptosis in terms of cell function.34 MOTS-C can protect against lipopolysaccharide-induced cardiomyopathy via attenuation of the inflammatory response in cardiomyocytes, inhibition of cardiomyocyte apoptosis, and maintenance of mitochondrial homeostasis.35 An imbalance of nitric oxide and reactive oxygen species, so-called oxidative stress, is a major contributor to endothelial dysfunction. The rat H9c2 cardiomyocytes experiment shown that pretreatment with MOTS-C significantly reversed hydrogen peroxide-induced oxidative damage via the nuclear factor erythroid 2-related Factor 2/ antioxidative response element and NF-κB pathways.10 Altogether, these studies reveal some new potential mechanisms for the effect of MOTS-C on endothelial dysfunction. Obesity usually precedes vascular endothelial function impairment. Therefore, MOTS-C may play a mediating role between childhood obesity and vascular endothelial function, and this was confirmed by the mediating effect in the present study. The contribution of MOTS-C in childhood obesity-induced endothelial dysfunction was found to be 9.12%.
MOTS-c is the first mitochondrial-encoded peptide that has been subjected to clinical trials, and the pharmacological effects in cardiovascular diseases show an incalculable potential. However, MOTS-c has been used less frequently in disease treatment, and no effective method of applying MOTS-c in the clinic has been developed. As Zheng et al suggested, the use of synthetic biology techniques provide the possibility of using MOTS-C for disease treatment,26 but there is still much to understand about MOTS-c, including basic molecular mechanisms, stability in biological systems, and oral bioavailability.
Our study has several limitations. First, this type of study reveals associations but not causality. Any assessments of causality will require specifically designed in vitro and in vivo experimental studies or prospective cohort studies. Second, largely because of the complex pathophysiology of endothelial impairment, a full understanding of this phenomenon will require consideration of a large collection of markers. Finally, our patient cohort was enrolled from a single center. Thus, these findings will need to be replicated in large multicenter trials with patients from a large range of socio-demographic settings.
Conclusion
Our study suggests that the decrease of serum MOTS-C levels in obese children may be related to the damage of vascular endothelial function. A future study of the related mechanism will be of great importance to determine how it exerts its clinical therapeutic effects. As a promising target for treatment development, MOTS-c is expected to be used in the treatment development of various cardiovascular diseases.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors (Li Zhou) upon reasonable request.
Ethics Approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of University of South China with the written informed consent provided by all participants’ parents based on the principles of the Helsinki Declaration. Trial Registration: ChiCTR1800016839.
Acknowledgments
We thank all patients and their families for consenting use their medical documentations and information that lead to our paper.
Funding
This work was supported by the Scientific Research Fund key Project of Hunan Provincial Health Commission (No. A2017011) and the Hunan Province Social Science Project (No. XSP22YBC361).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Development Initiatives. 2018 global nutrition report: shining a light to spur action on nutrition. Bristol: Development Initiatives Poverty Research Ltd; 2018. Available from: https://globalnutritionreport.org/.
2. Daniels SR. Complications of obesity in children and adolescents. Int J Obes. 2009;33(Suppl 1):S60–S65. doi:10.1038/ijo.2009.20
3. Wang H, Sun J, Zhang Z, et al. Waist circumference change and risk of high carotid intima-media thickness in a cohort of Chinese children. J Hypertens. 2021;39(9):1901–1907. doi:10.1097/HJH.0000000000002881
4. Bibbins-Domingo K, Coxson P, Pletcher MJ, et al. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357(23):2371–2379. doi:10.1056/NEJMsa073166
5. Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636. doi:10.1161/CIRCRESAHA.115.306301
6. Alexander Y, Osto E, Schmidt-Trucksäss A, et al. Endothelial function in cardiovascular medicine: a consensus paper of the European society of cardiology working groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc Res. 2021;117(1):29–42. doi:10.1093/cvr/cvaa085
7. Litwin M, Niemirska A, Sladowska-Kozlowska J, et al. Regression of target organ damage in children and adolescents with primary hypertension. Pediatr Nephrol. 2010;25(12):2489–2499. doi:10.1007/s00467-010-1626-7
8. Lee C, Zeng J, Drew BG, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443–454. doi:10.1016/j.cmet.2015.02.009
9. Kim KH, Son JM, Benayoun BA, et al. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 2018;28(3):516–524. doi:10.1016/j.cmet.2018.06.008
10. Shen C, Wang J, Feng M, et al. The mitochondrial-derived peptide MOTS-c attenuates oxidative stress injury and the inflammatory response of H9c2 cells through the Nrf2/ARE and NF-κB pathways. Cardiovasc Eng Technol. 2022;13(5):651–661. doi:10.1007/s13239-021-00589-w
11. Mohtashami Z, Singh MK, Salimiaghdam N, et al. MOTS-c, the most recent mitochondrial derived peptide in human aging and age-related diseases. Int J Mol Sci. 2022;23(19):11991. doi:10.3390/ijms231911991
12. Ramanjaneya M, Bettahi I, Jerobin J, et al. Mitochondrial-derived peptides are down regulated in diabetes subjects. Front Endocrinol. 2019;10:331. doi:10.3389/fendo.2019.00331
13. Du C, Zhang C, Wu W, et al. Circulating MOTS-c levels are decreased in obese male children and adolescents and associated with insulin resistance. Pediatr Diabetes. 2018;19(6):1058–1064. doi:10.1111/pedi.12685
14. Liu C, Gidlund EK, Witasp A, et al. Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease. Am J Physiol Renal Physiol. 2019;317(5):F1122–F1131. doi:10.1152/ajprenal.00202.2019
15. Yin Y, Pan Y, He J, et al. The mitochondrial-derived peptide MOTS-c relieves hyperglycemia and insulin resistance in gestational diabetes mellitus. Pharmacol Res. 2022;175:105987. doi:10.1016/j.phrs.2021.105987
16. Qin Q, Delrio S, Wan J, et al. Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. Int J Cardiol. 2018;254:23–27. doi:10.1016/j.ijcard.2017.12.001
17. Yaşar E, Çakmak T, Bayramoğlu A, et al. MOTS-c as a predictor of coronary lesions and complexity in patients with stable coronary artery disease. Eur Rev Med Pharmacol Sci. 2022;26(16):5676–5682. doi:10.26355/eurrev_202208_29501
18. Li H, Ren K, Jiang T, et al. MOTS-c attenuates endothelial dysfunction via suppressing the MAPK/NF-κB pathway. Int J Cardiol. 2018;268:40. doi:10.1016/j.ijcard.2018.03.031
19. Li H, Zong XN, Ji CY, et al. Body mass index cut-offs for overweight and obesity in Chinese children and adolescents aged 2–18 years. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31(6):616–620.
20. Li JY, Wang YD, Qi XY, et al. Serum CCN3 levels are increased in type 2 diabetes mellitus and associated with obesity, insulin resistance and inflammation. Clin Chim Acta. 2019;494:52–57. doi:10.1016/j.cca.2019.03.006
21. Mućka S, Miodońska M, Jakubiak GK, et al. Endothelial function assessment by flow-mediated dilation method: a valuable tool in the evaluation of the cardiovascular system. Int J Environ Res Public Health. 2022;19(18):11242. doi:10.3390/ijerph191811242
22. Starzak M, Stanek A, Jakubiak GK, et al. Arterial stiffness assessment by pulse wave velocity in patients with metabolic syndrome and its components: is it a useful tool in clinical practice? Int J Environ Res Public Health. 2022;19(16):10368. doi:10.3390/ijerph191610368
23. Donald AE, Halcox JP, Charakida M, et al. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51(20):1959–1964. doi:10.1016/j.jacc.2008.02.044
24. McCrea CE, Skulas-Ray AC, Chow M, et al. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17(1):29–36. doi:10.1177/1358863X11433188
25. Sedgwick P. A comparison of parametric and non-parametric statistical tests. BMJ. 2015;350(apr17 1):h2053. doi:10.1136/bmj.h2053
26. Zheng Y, Wei Z, Wang T. MOTS-c: a promising mitochondrial-derived peptide for therapeutic exploitation. Front Endocrinol. 2023;14:1120533. doi:10.3389/fendo.2023.1120533
27. Cataldo LR, Fernández-Verdejo R, Santos JL, et al. Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals. J Investig Med. 2018;66(6):1019–1022. doi:10.1136/jim-2017-000681
28. Matsuzawa Y, Kwon T-G, Lennon RJ, et al. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4(11):e002270. doi:10.1161/JAHA.115.002270
29. Ikonomidis I, Katogiannis K, Kyriakou E, et al. β-Amyloid and mitochondrial-derived peptide-c are additive predictors of adverse outcome to high-on-treatment platelet reactivity in type 2 diabetics with revascularized coronary artery disease. J Thromb Thrombolysis. 2020;49(3):365–376. doi:10.1007/s11239-020-02060-4
30. Sun HJ, Wu ZY, Nie XW, et al. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. 2020;10:1568. doi:10.3389/fphar.2019.01568
31. Xinqiang Y, Quan C, Yuanyuan J, et al. Protective effect of MOTS-c on acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol. 2020;80:106174. doi:10.1016/j.intimp.2019.106174
32. Yin X, Jing Y, Chen Q, et al. The intraperitoneal administration of MOTS-c produces antinociceptive and anti-inflammatory effects through the activation of AMPK pathway in the mouse formalin test. Eur J Pharmacol. 2020;870:172909. doi:10.1016/j.ejphar.2020.172909
33. Zhong P, Peng J, Hu Y, et al. Mitochondrial derived peptide MOTS -c prevents the development of heart failure under pressure overload conditions in mice. J Cell Mol Med. 2022;26(21):5369–5378. doi:10.1111/jcmm.17551
34. Li S, Wang M, Ma J, et al. MOTS-c and exercise restore cardiac function by activating of NRG1-ErbB signaling in diabetic rats. Front Endocrinol. 2022;13:812032. doi:10.3389/fendo.2022.812032
35. Wu J, Xiao D, Yu K, et al. The protective effect of the mitochondrial-derived peptide MOTS-c on LPS-induced septic cardiomyopathy. Acta Biochim Biophys Sin. 2023;55(2):285–294. doi:10.3724/abbs.2023006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.