Back to Journals » Clinical Ophthalmology » Volume 15
Serum Malondialdehyde as a Biomarker of Oxidative Stress in Patients with Primary Ocular Carcinoma: Impact on Response to Chemotherapy
Authors Maurya RP , Prajapat MK, Singh VP, Roy M, Todi R, Bosak S, Singh SK, Chaudhary S, Kumar A, Morekar SR
Received 24 October 2020
Accepted for publication 31 December 2020
Published 26 February 2021 Volume 2021:15 Pages 871—879
DOI https://doi.org/10.2147/OPTH.S287747
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Rajendra Prakash Maurya,1 Manish Kumar Prajapat,2 Virendra Pratap Singh,1 Meghna Roy,1 Ravina Todi,1 Sanjay Bosak,1 Sunit Kumar Singh,3 Sunil Chaudhary,4 Anil Kumar,5 Sunil R Morekar6
1Regional Institute of Ophthalmology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India; 2Mahamaya Rajkiya Allopathic Medical College, Ambedkar Nagar, UP, India; 3Department of Molecular Biology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India; 4Department of Radiotherapy and Radiation Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India; 5Department of Statistics, Faculty of Science & Technology, Mahatma Gandhi Kashi Vidyapith, Varanasi, Uttar Pradesh, India; 6Apollo Specialty Hospital, Navi Mumbai, India
Correspondence: Rajendra Prakash Maurya Email [email protected]
Purpose: To study the level of serum malondialdehyde (MDA), a biomarker of oxidative stress before and after chemotherapy in various ocular malignancies and to correlate its significance with clinicopathological parameters.
Methods: Thirty two histopathologically confirmed cases of primary ocular malignancies were included in this longitudinal cohort study. Detailed clinicopathological assessment was done. Analysis of serum MDA level in the patient before and after chemotherapy was measured and its prognostic significance was analyzed.
Results: Maximum cases were of eyelid malignancy (n=18, 56.2%) followed by retinoblastoma (18.8%) and OSSN (6, 18.8%). About 43.75% patients were in the advanced-stage. The tumor was histopathologically well-differentiated in 20 (62.5%) cases. Most common malignancy was sebaceous gland carcinoma of the eyelid (n=10,31.25%). Serum MDA level in patients were significantly higher than controls [5.5712± 0.32779 vs 2.5531± 0.08056 nmol/mL, p< 0.001]. Level was significantly reduced after chemotherapy (4.5146± 0.23209 nmol/mL). Serum MDA was maximum in cases of rhabdomyosarcoma (5.9450± 0.23335 nmol/mL) and retinoblastoma (5.7433± 0.14334 nmol/mL). It was minimum in basal cell carcinoma (5.3775± 0.17746 nmol/mL). Pre chemotherapy serum MDA level was significantly higher in patients having larger tumor (> 20mm, p< 0.001) and having lymph node metastasis than those without lymph node metastasis [5.8350± 0.17113 vs 5.4833± 0.32193 nmol/mL, p< 0.006]. No significant difference was observed in post chemotherapy serum MDA level as for as size of tumor (p=0.947) and histopathological differentiation (p=0.109) was concerned.
Conclusion: The serum MDA level is a potential biomarker in primary ocular carcinoma to assess oxidative stress and its impact on response to chemotherapy.
Keywords: malondialdehyde, chemotherapy, malignancy, oxidative stress, biomarker
Introduction
Although ocular malignancies are not common but they can be highly problematic due to their potential threat to both sight and life that they can cause. The ocular malignant tumors can be classified into two types vis., primary ocular malignant tumors in which tumor originates inside the eyeball (intraocular malignancy) and in the structures of ocular adnexa (eyelid, conjunctiva, and orbit) and secondary or metastatic tumors. Common intraocular malignancies are retinoblastoma in children and uveal melanoma in adults. Secondary ocular malignancy originates from the adjacent structures, including the paranasal sinuses, nasal cavity, nasopharynx, cavernous sinus and intracranial structures.1 Metastatic orbital tumors originate elsewhere in the body such as lung, breast, thyroid, testis, liver, prostate, etc., and spread into the eyes via hematogenous route or perineural spread.2,3
The eyelid tumors are the most frequent neoplasm in ophthalmic practice. Approximately 5–9% of skin tumor arises in the eyelid.4,5 Among them, basal cell carcinoma (BCC) is the most common eyelid malignancy found worldwide comprising 85–90% of all eyelid tumors.6 Squamous cell carcinoma (SCC) of the eyelid is quite a rare neoplasm. It accounts for about 10–12% of cutaneous malignancy.7 Sebaceous gland carcinoma (SGC) is relatively rare and slow-growing but aggressive and the most lethal eyelid carcinoma. It represents about 1% of all cutaneous malignancy.8,9 Malignant melanoma of the eyelid is uncommon comprises 1% of all eyelid malignancies which is similar to melanoma of the skin.10,11 Retinoblastoma and uveal melanoma are intraocular malignancies. They are very aggressive and life-threatening if left untreated. Advanced stage ocular malignancy may result in poor prognosis with decreased disease-free survival. Although surgery is the gold standard and only curative treatment for ocular cancer, neoadjuvant chemotherapy is the next best option when surgery is contraindicated due to poor general condition of the patient or extensive surgery leading to disfigurement and functional loss.
Oxidative stress is closely related to carcinogenesis in the form of reactive oxygen species (ROS). It is involved in various physiological and pathological processes which include DNA damage, proliferation, cell adhesion, survival and apoptosis. There are complex interactions among ROS generation, ROS signaling, ROS induced damage and carcinogenesis. ROS are central contributors in tumor occurrence and progression by irreversible DNA damage, modulating the signaling pathways and disrupting the physiological mechanism of apoptosis.11 Accumulation of ROS may cause cancer cells to be more sensitive. It is supposed that increased oxidative stress by exogenous ROS generation therapy affects selectively by killing cancer cells by apoptosis. Likewise, many anticancer chemotherapy drugs (eg Cis-platinum, carboplatin, and oxaliplatin), epi-podophyllotoxins (etoposide and teniposide) and camptothecins (topotecan and irinotecan) generate a high level of ROS and kill tumors cells by apoptosis.12 Malignant cells that are highly resistant to treatment (chemotherapy or radiotherapy) are believed to be cancer stem cells (CSCs). They are defined by their capacity to self-renew and differentiate into heterogeneous cancer cell types and play an important role in tumor progression, development and disease recurrence.13,14 Administration of antioxidants during anticancer chemotherapy may reduce chemotherapy induced side effects and may enhance the effectiveness of anticancer drugs.13
It is well known that primary targets of free radicals are cellular lipids, whose end products are a variety of aldehydes such as ‘Malondialdehyde’ (MDA). Since MDA is highly cytotoxic and carcinogenic agent it is frequently used as a biomarker of oxidative stress during major health problems such as cancer, etc.15 An increase in lipid peroxidation level (MDA) has been demonstrated in various tumors.16 The aim of this study was to estimate the serum expression of Malondialdehyde (MDA), a biomarker of oxidative stress, before and after chemotherapy, and to correlate the same with clinicopathological parameters of primary ocular malignancies and to evaluate the impact of MDA on chemotherapy response.
Patients and Methods
This study was conducted in the Department of Ophthalmology, Department of Molecular Biology and Department of Radiotherapy and Radiation Medicine, Institute of Medical Sciences, Banaras,Hindu University, Varanasi, India from November 2016 to March 2018. The study was conducted following the declaration of Helsinki principle and approved by the ethical committee of the Institute of Medical Sciences, Banaras Hindu University. Prior informed consent was obtained from the study subjects regarding participation in research study and their data (including clinical images) being published. It was an interventional study in which the cases were collected from Ophthalmology Outpatient Department. 32 newly diagnosed and histologically confirmed patients with primary ocular malignancies without earlier exposure to chemotherapy or radiotherapy. These were consecutively recruited from the Ophthalmology out-patients Department. Besides, a group of 16 age and sex-matched healthy controls were recruited from the refraction unit.
Clinical assessment included patients’ demographic profile, clinical presentation, torchlight and slit-lamp examination, visual acuity by Snellen’s chart, ophthalmoscopy, X-Ray, CT scan, MRI and Ultrasonography B scan of both eyes. Also, a histopathological examination of incisional or excisional biopsy was done to confirm the diagnosis and assessed pathological differentiation. Tumors were classified as either well-differentiated or poorly/undifferentiated based on the predominant cell type. Tumor staging was done in accordance with the 8th edition of TNM staging system proposed by the American Joint Committee on Cancer for eyelid carcinomas, ocular surface squamous neoplasia (OSSN), and retinoblastoma.17–19 Blood urea nitrogen (up to 20 mg/100 mL), serum creatinine (up to 15 mg/100 mL), white blood count (≥ 4000 mm3) and platelet count (100,000 mm3) were used as a laboratory screening parameters before and after chemotherapy. The following chemotherapy regimens were used: for SGC and BCC of eyelid Cisplatin 75mg/m2 (Day 1) and 5-fluorouracil 750mg/m2 (Day1 to Day5) were given at interval of 3 weeks for 3–6 cycles. For retinoblastoma, 6–9 cycle of Vincristine 1 to 5 mg/m2, Etoposide 150 mg/m2 and Carboplatin 560 mg/m2 was administered. In cases of orbital rhabdomyosarcoma, intravenous Vincristine 1.3 mg/kg (Day 1), Cyclophosphamide 450 mg/kg and Adriamycin 45 mg/kg on Day 1 to Day 5 was administered. OSSN cases were treated by topical 5FU 1% two drops four times a day for one week then repeated on every one-week interval. The mean follow-up period was of 18 months.
Laboratory investigation was also used for the estimation of the biochemical assay of serum Malondialdehyde.
Peripheral blood samples were collected into a sterile serum separator tube (Vacutainer, Becton Dickinson, Plymouth, UK) and were allowed to coagulate at 4°C for 30 minutes followed by centrifugation at 2500 rpm for 10 minutes to separate the serum which aliquoted. It was stored at −80°C until used. Serum samples from patients were collected on first visit (before chemotherapy) and after the first cycle of chemotherapy. Malondialdehyde (nmol/mL) level was estimated in patients’ serum before and after chemotherapy by same method as used by Yagi.20 Twenty microliters of serum was mixed with 4.0mL of N/12 sulfuric acid and 0.5mL of 10% phosphotungstic acid to deproteinize the plasma. The mixture was kept at room temperature for 5 minutes, then centrifuged at 3000 rpm for 10 minutes. The sediment was again mixed with N/12 sulfuric acid (2.0 mL) and 10% phosphotungstic acid (0.3 mL) and the mixture was centrifuged at 3000 rpm for 10 minutes. The sediment was suspended in 4.0 mL of distilled water and 1.0 mL of TBA reagent (prepared by equal volumes of 0.067% thiobarbituric acid and glacial acetic acid) and heated at 95° C for 60 minutes. After cooling with tap water, 5.0 mL of n-butanol was added and the pink colored mixture was obtained for fluorometric measurement at 553 nm with excitation at 515nm. The serum malondialdehyde level was estimated by reading the fluorescence intensity of n-butanol layer and standard solution (1,1ʹ,3,3ʹ-Tetramethoxypropane).
The statistical analysis of MDA and its different selected parameters for inference was done using statistical package SPSS for Windows version 16.0 (SPSS, Chicago, IL) software. The Chi-squire test was used for comparison of MDA before and after chemotherapy. The difference was considered to be statistically significant at p<0.05 (two-sided).
Results
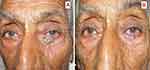
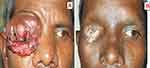
Our cohort of 32 patients consisted of 16 male and 16 females. The age distribution (years) was less than 20 (25%), 40–50 (18.8%), 51–60 (21.9%) and greater than 60 (34.4). Residence was semi-urban/urban (12.5%) and rural (87.5). Location of origin of tumor as Eyelid (56.25%) [Figures 1 and 2], Conjunctiva (18.8%), Orbit (6.2%) and intraocular/eyeball (18.8%).
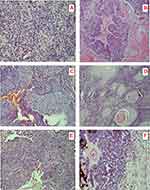
Tumor spread as local invasion (50%), lymph node metastasis (25%) and systemic metastasis (25%). As per TNM classification, in 18 (56.25%) patients tumor were in early stage. However 43.75% patients had advanced stage cancer (18.75% had eyelid tumor, 9.38% were retinoblastoma cases, 9.38% had OSSN and 6.25% were cases of rhabdomyosarcoma). Histopathological types were sebaceous gland carcinoma (31.2%), basal cell carcinoma (25%), OSSN (18.8%), Rhabdomyosarcoma (6.2%) and retinoblastoma (18.8%) whereas histopathological differentiations were distributed as well differentiated (62.5%), moderately differentiated (6.2%) and poorly differentiated respectively [Figure 3]. 12.50% patients of retinoblastoma had histopathologic high-risk features (optic nerve and scleral invasion) however 25.0% patients of sebaceous gland carcinoma eyelid had orbital extension and lymph node metastasis.
Table 1 shows the analysis for serum level of malondialdehyde (MDA) in the healthy controls, and the patients with different types of primary ocular malignancy. The distribution of levels of MDA in patients with ocular malignancy (5.5712± 0.32779) was significantly higher than healthy controls (2.5531±0.08056 nmol/mL) (p<0.001), while the level of MDA was significantly reduced after chemotherapy [5.5712±0.32779 nmol/mL vs 4.5146 ± 0.23209 nmol/mL, p<0.001].
 |
Table 1 Analysis for Serum Malondialdehyde (MDA) Levels in Controls (N=16) and Cancer Patients (N=32, Before and After Chemotherapy) |
Table 2 shows the level of serum malondialdehyde according to age and tumor advancement in patients before and after chemotherapy. Serum MDA level in < 20 years age group and 40–60 years age group was significantly reduced after chemotherapy (p-value < 0.001) while in > 60 years age group the levels reduced after chemotherapy and was statistically not significant (p-value 0.969).
 |
Table 2 Analysis for Serum Malondialdehyde (MDA) Levels According to Age of Patient and Tumor Advancement (Before and After Chemotherapy) |
Pre-chemotherapy level of Serum MDA was significantly higher in large size tumor (>20mm) as compared to small size tumour (<20mm) [5.6617±0.29951nmol/mL vs 5.2933±0.12662 nmol/mL] and significantly reduced after chemotherapy [5.6617±0.29951 nmol/mL vs 4.5135 ± 0.22534 nmol/mL, p<0.001] while no significant difference was observed after chemotherapy in small size (<20mm) tumor [5.2933± 0.29951 nmol/mL vs 4.5233±0.33858, p>0.04]. Pre-chemotherapy serum MDA level was significantly higher in patients having lymph node metastasis in comparison to those without lymph node metastasis [5.8350±0.17113 vs 5.4833± 0.32193 nmol/mL p<0.006]. No statistical significance was observed in MDA level after chemotherapy [4.3938±16,928 vs 4.5683 ± 0.23976 nmol/mL, p=0.076]. Pre-chemotherapy MDA level was higher in advance stage carcinoma (5.8407±0.2021) than early-stage (5.3608± 0.177). However serum MDA level in early and advanced stage carcinoma were significantly decreased after chemotherapy (p< 0.001).
Table 3 analyzes serum Malondialdehyde (MDA) levels according to the histopathological type and differentiation of Tumor. Pre-chemotherapy serum MDA was maximum in the cases of rhabdomyosarcoma (5.9450 ± 0.2335 nmol/mL), retinoblastoma (5.7432 ± 0.143340) and sebaceous gland carcinoma (5.7088 ± 0.26008) as compared to basal cell carcinoma (5.3775 ± 0.17746) and ocular surface squamous neoplasia (5.4253 ± 0.04243). The Serum MDA level was significantly reduced after chemotherapy in all histopathological subtypes. Maximum reduction was observed in cases of rhabdomyosarcoma followed by retinoblastoma and sebaceous gland carcinoma (p<0.001). Serum MDA level before and after chemotherapy does not show any significant difference among histopathological differentiation of tumor.
 |
Table 3 Analysis for Serum Malondialdehyde (MDA) Levels According to Histopathological Type and Differentiation. (Before and After Chemotherapy) |
Discussion
The eyelid tumors are the most frequently seen neoplasm in ophthalmic practice. The common malignant eyelid tumors are basal cell carcinoma, sebaceous gland carcinoma and squamous cell carcinoma.21–25 Though multimodal treatments are available for ocular and periocular carcinoma, surgery is the gold standard. Systemic neoadjuvant chemotherapy is indicated if surgery is contraindicated due to poor general condition of the patient or advanced stage of tumor.26 The response of systemic chemotherapy depends on several factors including oxidative stress in tumor and host cell.
Oxidative stress is the disequilibrium between free radicals/reactive oxygen species (ROS) and endogenous antioxidant defense mechanisms. ROS promotes many aspects of tumor development and progression including (a) cellular proliferation eg, extracellular-regulated kinase ½ (ERK1/2) activation; (b) evasion of apoptosis eg Src, NF-B and phosphatidylinositol-3 kinase (PI3K)/Akt activation; (c) tissue invasion and metastasis eg metalloproteinase (MMP) secretion into the extracellular matrix (ECM); and (d) angiogenesis eg release of vascular endothelial growth factor and angiopoietin.27 Excessive oxidative stress inhibits drug-induced apoptosis, thereby reducing the ability of chemotherapeutic drugs to kill cancer cells.28 Polyunsaturated lipids are more susceptible to free radical attack, causing lipid peroxidation. Malondialdehyde (MDA) is an unstable end product of lipid peroxidation.29 It is an index of lipid peroxidation, is highly cytotoxic, and acts as tumor promoter and co-carcinogen. Increased level of serum MDA is markedly associated with decrease in antioxidant parameters as demonstrated in many cancers (colorectal, oral, prostate, gastric, lung and breast etc.).30–36 It was not studied earlier in ocular malignancy but has been studied in melanoma is where levels reduced after surgery also.37 Increased lipid peroxidation is attributed to deficiency in antioxidant defenses. Serum antioxidant deficiency may be due to sequestration by tumor cells as well as scavenging of lipid peroxides.
In this study, pre-chemotherapy levels of serum MDA in primary ocular malignancy was significantly higher (p-value <0.001) than controls. Furthermore, the levels of MDA were reduced following chemotherapy. Similar findings of significantly higher serum MDA levels than healthy controls were reported in colorectal carcinoma,29,30 lung cancer,34,36 oropharyngeal carcinoma28 and breast cancer.35 Boyd and McGuire found urinary MDA level two-fold higher in breast cancer patients than healthy controls.38 Peluso found a higher MDA level in tissues obtained by FNAC from breast cancer.39
We observed that post-chemotherapy serum MDA level was significantly reduced in < 20 years age group and 40–60 years age group (p-value < 0.001) while in > 60 years age group level also reduced after chemotherapy although statistically not significant (p-value= 0.969). This may be because of lower antioxidant defence in older age. Franca et al 2011 described the reason for the non-significant change in MDA after radiotherapy in advanced age (>60 years) breast cancer patients as a low level of selenium like trace elements.40
In our study, Pre-chemotherapy serum MDA level was higher in advance stage carcinoma than early-stage and serum MDA level was significantly decreased after chemotherapy (p< 0.001). Sener et al,2007 reported a trend of increasing MDA concentration in malignant tissue as the TNM stage increases.35 Bakan observed that increase in oxidative stress as proportionate to the stage of gastric cancer.33 However, Liehr reported a decreased level of MDA in patients with advanced-stage compared to an early-stage disease because of reduced physical activity and weakened immune system in advanced-stage cancer.41 We noticed that the pre-chemotherapy level of Serum MDA was significantly higher in larger tumors (>20mm) as compared to smaller ones (<20mm). The level was significantly reduced after chemotherapy in larger size tumors. Our results suggest that there is high oxidative stress with a large size tumor. Hristozov and colleagues reported a marked reduction of MDA level after complete surgical excision of solid tumors that might be attributed to decreased oxidative burden due to the removal of tumors.42
In this study, we have demonstrated increased lipid peroxidation resembling high oxidative stress in aggressive types of malignant tumors like rhabdomyosarcoma, retinoblastoma, and sebaceous gland carcinoma and tumors with lymph node metastasis. Increased oxidative stress may interfere with drug-induced apoptosis thus the response of chemotherapy. This connection is supported by other studies.43,44 Sener DE observed a trend toward higher MDA concentration in the malignant tumor as the TNM stage increased.35
Significant limitations of the present study are the small sample size for internal subgroup comparison and short follow-up period. However, a larger patient cohort and a longer follow-up period may yield more significant data. It is unfortunate that estimation of primary antioxidant enzymes such as glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) etc. which are involved in the direct elimination of ROS was not done in the study.
Conclusion
To conclude, the present study is the first study in literature to report the oxidative stress by assessing lipid peroxidation activity in various ocular malignancies. Our results suggest that there is an increased serum MDA level in eye cancer patients, particularly in advanced-stage disease. Hence additional research is needed to substantiate these findings.
Ethical Clearance
The study was approved by “Ethical Committee” of Institute of medical Sciences, Banaras Hindu University (EC Registration No. ECR/526/Inst/UP/2014 Dt. 31.1.14) with the reference No. Dean/2015-16/EC/557 dated 19.01.2017.
Informed Consent
Informed consent was obtained from the patients/parents of the patients regarding participation in research study, review of their medical records and reports and to have their data including clinical images published.
Author Contributions
All authors have made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published and agree to take responsibility for and accountable for all aspects of the work.
Disclosure
The authors declare no potential conflicts of interest.
References
1. Maurya RP, Bhatia RP, Maurya OPS, Jain RK. Ocular manifestations of the tumors of the paranasal sinuses and nasopharynx. Annals Ophthalmol Glauco. 1996;28(4):254–257.
2. Shields CL, Shield JA, Peggs M. Tumors metastatic to the orbit. Ophthal Plast Reconstr Surg. 1988;4:73–80. doi:10.1097/00002341-198804020-00003
3. Gunalp I, Gunduz K. Metastatic orbital tumors. Jpn J Ophthalmol. 1995;39:65–70.
4. Scotto J, Fears T, Fraumeni JJ. The Incidence of Non-Melanma Skin Cancer in the United States, Public Health Service. Bethesda, MD: National Institute of Health (NIH Publication No.82-2433); 1981.
5. Cook BE, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108(11):2088–2098. doi:10.1016/S0161-6420(01)00796-5
6. Nerad JA, Whitaker DC. Periocular basal cell carcinoma in adults 35 years of age and younger. Am J Ophthalmol. 1988;106:723–729. doi:10.1016/0002-9394(88)90708-8
7. Mehta M, Fay A. Squamous cell carcinoma of the eyelid and conjunctiva. Int Ophthalmol Clin. 2009;49(1):111–121.
8. Ghosh SK, Bandyopadhyay D, Gupta S, Chatterjee G, Ghosh A. Rapidly growing extraocular sebaceous carcinoma occurring during pregnancy: a case report. Dermatol Online. 2008;14(8):8.
9. Maurya RP, Singh VP, Singh MK, Srivastava T, Dwivedi M. Ocular sebaceous gland carcinoma in northern India: clinico-pathological features and treatment outcome. Int J Ocular Oncol Oculopalsty. 2016;2(3):168–174.
10. Grossniklaus HE, McLean IW. Cutaneous melanoma of the eyelid. Clinicopathologic Features Ophthalmol. 1991;98:1867–1873.
11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
12. Conklin KA. Chemotherapy-associated oxidative stress. Impact Chemotherapeutic Effectiveness. 2004;3(4):294–300.
13. Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26(17):2839–2845. doi:10.1200/JCO.2007.15.1829
14. Wicha MS, Liu S, Dont G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:4. doi:10.1158/0008-5472.CAN-05-3153.
15. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi:10.1016/0003-2697(79)90738-3
16. Cheeseman KH, Emery S, Maddix SP, et al. Studies on lipid peroxidation in normal and tumour tissues, The Yoshida rat liver tumour. Bioche J. 1988;250:247–252. doi:10.1042/bj2500247
17. AJCC Cancer ma. AJCC Cancer Staging Manual.
18. Coupland S. TNM staging system for conjunctival tumors. Acta Ophthalmol. 2016;94(S56):. doi:10.1111/j.1755-3768.2016.0138
19. Mallipatna AC, Gallie BL, Chevez-Barrios P, et al. Retinoblastoma. In: Admin MB, Edge S, Green F, et al, editors. AJCC Cancer Staging Manual. 8th ed. Chicago: International Publishing. 2016:819-831.
20. Yagi K. Lipid peroxide and human disease. Chem Phys Lipids. 1987;45:337–351. doi:10.1016/0009-3084(87)90071-5
21. Shields JA, Demirci H, Marr BP, Eagle RC, Shields CL. Sebaceous carcinoma of the ocular region: a review. Survey Ophthalmol. 2005;50:103–122. doi:10.1016/j.survophthal.2004.12.008
22. Allali J, Hermies DF, Renard G. Basal cell carcinomas of the eyelids. Ophthalmologica. 2005;219(2):57–71. doi:10.1159/000083263
23. Margo CE, Waltz K. Basal cell carcinoma of the eyelid and periocular skin. Surv Ophthalmol. 1993;38(2):169–192.
24. Cook BE, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County. Minnesota Ophthalmol. 1999;106(4):746–750. doi:10.1016/S0161-6420(99)90161-6
25. Hiroshi T, Hidetoshi Y. Clinicopathological analysis of malignant eyelid tumor cases at yamagata university hospital: statistical comparison of tumor incidence in Japan and in other countries. Jpn J Ophthalmol. 2005;49:349–354. doi:10.1007/s10384-005-0229-5
26. Maurya RP, Bhushan P, Singh VP, Singh MK, Pandey M. Clinico-pathological response of neoadjuvant chemotherapy in advance ocular carcinoma. World J Surg Medi Radiational Oncolol. 2012;1:25–31.
27. Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Leonart ME L. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376–390. doi:10.1016/j.arr.2012.10.004
28. Shacter E, Williams JA, Hinson RM. Oxidative stress interferes with cancer chemotherapy: inhibition of lymphoma cell apoptosis and phagocytosis. Blood. 2000;96(1):307–313. doi:10.1182/blood.V96.1.307
29. Otamiri T, Sjodahl R. Increased lipid peroxidation in malignant tissues of patients with colorectal cancer. Cancer. 1989;61:122–125.
30. Leuratti C, Watson MA, Deag EJ, Welch A, Singh R, Gottschalg E. Detection of malondialdehyde DNA adducts in human colorectal mucosa: relationship with diet and the presence of adenomas. Cancer Epidemiol Biomarkers Prev. 2002;11:267–273.
31. Chole RH, Patil RN, Basak A, Palandurkar K, Bhowate R. Estimation of serum malondialdehyde in oral cancer and precancer and its association with healthy individuals, gender, alcohol, and tobacco abuse. J Cancer Res Ther. 2010;4:487–491. doi:10.4103/0973-1482.77106
32. Zhang S, Qi L, Li M. Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J Exp Clin Cancer Res. 2008;27(1):62. doi:10.1186/1756-9966-27-62.
33. Bakan E, Taysi S, Polat MF, Dalga S, Umudum Z, Bakan N. Nitric oxide levels and lipid peroxidation in plasma of patients with gastric cancer. Jpn J Clin Oncol. 2002;32:162–166. doi:10.1093/jjco/hyf035
34. Gupta A, Srivastava S, Prasad R, Natu SM, Mittal B, Negi MP. Oxidative stress in non-small cell lung cancer patients after chemotherapy: association with treatment response. Respirology. 2010;15:349–356. doi:10.1111/j.1440-1843.2009.01703.x
35. Sener DE, Gönenç A, Akinci M, Torun M. Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem Funct. 2007;25:377–382. doi:10.1002/cbf.1308
36. Nowak D, Janczak M. Effect of chemotherapy on serum end products of lipid peroxidation in patients with small cell lung cancer: association with treatment results. Respir Med. 2006;100:157–166. doi:10.1016/j.rmed.2005.04.002
37. Gadjeva V, Dimov A, Georgieva NJ. Influence of therapy on the antioxidant status in patients with melanoma. Clin Pharm Ther. 2008;33(2):179–185. doi:10.1111/j.1365-2710.2008.00909.x
38. Boyd NF, McGuire V. The possible role of lipid peroxidation in breast cancer risk. Free Rad Biol Med. 1991;10:185–190. doi:10.1016/0891-5849(91)90074-D
39. Peluso M, Munnia A, Risso G, Catarzi S, Piro S. Breast fine-needle aspiration malondialdehyde deoxyguanosine adduct in breast cancer. Free Radic Res. 2011;45:477–482. doi:10.3109/10715762.2010.549485
40. Franca CAS, Nogueira CR, Ramalho A, Carvalho ACP, Viera SL, Penna ABRC. Serum levels of selenium in patients with breast cancer before and after treatment of external beam radiotherapy. Ann Oncol. 2011;22:1109–1112. doi:10.1093/annonc/mdq547
41. Liehr JG. Dual role of oestrogens as hormones and pro-carcinogens: tumour initiation by metabolic activation of oestrogens. European J Cancer Prevention. 1997;6(1):3–10. doi:10.1097/00008469-199702000-00002
42. Hristozov D, Gadjeva V, Vlaykova T, Dimitrov G. Evaluation of oxidative stress in patients with cancer. Arch Physiol Biochem. 2001;109(4):331–336. doi:10.1076/apab.109.4.331.4248
43. Conklin KA. Dietary antioxidents during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Can. 2000;37:1–18. doi:10.1207/S15327914NC3701_1
44. Lamson DW, Brignall MS. Antioxidents in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev. 1999;4:304–329.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.