Back to Journals » Journal of Inflammation Research » Volume 14
Serum Level of Complement C1q is Associated with Contrast-Associated Acute Kidney Injury in Patients Undergoing Emergency Percutaneous Coronary Intervention
Authors Tao J, Ye C, Dai W, Li D, Zhou M, Li Y
Received 10 October 2021
Accepted for publication 22 November 2021
Published 24 December 2021 Volume 2021:14 Pages 7331—7339
DOI https://doi.org/10.2147/JIR.S343715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Jun Tao,1,* Chenglin Ye,2,* Wen Dai,1 Di Li,1 Man Zhou,1 Yan Li1
1Department of Clinical Laboratory, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China; 2Department of Urology, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Li; Wen Dai Tel +8613886079252
; +8613476029919
Email [email protected]; [email protected]
Background: As an inflammatory factor, complement C1q is related to the prevalence and progression of atherosclerosis; however, in patients undergoing emergency percutaneous coronary intervention (PCI), it is unclear whether C1q is related to the prevalence of contrast-associated acute kidney injury (CA-AKI).
Methods: From November 2018 to March 2021, 1182 patients who underwent emergency PCI were continuously recruited. Patients were divided into CA-AKI group (n = 234) and non-CA-AKI group (n = 948). CA-AKI was defined as an increase in serum creatinine from the baseline level (≥ 25% or ≥ 0.5 mg/dL) 48– 72 hours after contrast exposure. All subjects were tested for serum C1q levels when they were admitted to the hospital.
Results: Among the 1182 patients undergoing emergency PCI, 234 patients (19.80%) developed CA-AKI. The level of preoperative serum complement C1q in the CA-AKI group was significantly higher than that in the non-CA-AKI group. Logistic regression and restricted cubic spline analyses showed that the incidence of CA-AKI was positively associated with the serum C1q level pre-PCI. Univariate and multivariate logistic regression analyses showed that C1q was an independent predictor of whether CA-AKI occurred after emergency PCI. The area under the curve (AUC) of the C1q was 0.703 [95% confidence interval (CI) 0.667– 0.739] in patients receiving emergency PCI. CA-AKI model included the following three predictors: C1q, eGFR, and IABP use. The AUC of forecast probability was 0.718 [95% CI 0.682– 0.754].
Conclusion: In patients receiving emergency PCI procedure, a high C1q level before PCI is associated with the increased risk of CA-AKI.
Keywords: complement C1q, contrast-associated acute kidney injury, percutaneous coronary intervention, coronary heart disease, inflammation
Introduction
Contrast-associated acute kidney injury (CA-AKI) refers to the acute renal damage caused by the use of contrast agents during angiography or other medical procedures. As the common and serious complications of coronary angiography (CAG) and percutaneous coronary intervention (PCI), it is the third leading cause of acute kidney injury in hospital after hypoperfusion and drug-induced kidney injury.1–3
CA-AKI is closely related to long-term hospitalization, end-stage renal injury, adverse cardiovascular events, hospital death, and long-term prognosis.4,5 Since there is no standard effective treatment for CA-AKI, preventing the occurrence of the disease is still the focus of treatment. Hydration and statins have been found to be effective in preventing CA-AKI.6 Therefore, an important part of reducing the risk of CA-AKI is to be able to identify patients with high risk of CA-AKI, so that closer monitoring and rapid intervention can be implemented.
Complement C1q is the initiator of the classical pathway of complement, and it participates in the occurrence and development of renal impairment by regulating the inflammatory response.7,8 C1q promotes the infiltration of monocytes and T lymphocytes, which causes vascular wall inflammation and plays a major role in the development of renal impairment.9,10 At the same time, C1q can induce the production of reactive oxygen species (ROS), lead to the activation of the complement system, cause renal vascular endothelial cell dysfunction, and cause renal medulla ischemia and hypoxia, which may play an important role in the incidence of CA-AKI.11–13 The association between serum C1q levels and CA-AKI has not been reported. We assumed, a priori, that elevated preprocedural C1q could be associated with increased risk of CA-AKI. This study aimed to evaluate the association between serum C1q levels and the risk of subsequent CA-AKI among patients undergoing emergency PCI.
Materials and Methods
Study Population
We used a prospective observational study design to evaluate the association between serum C1q levels and the risk of subsequent CA-AKI among patients undergoing emergency PCI. All participants were recruited consecutively between November 2018 and March 2021 at Renmin Hospital of Wuhan University. All patients who underwent an emergency PCI were included, and the exclusion criteria were those patients (1) who have incomplete medical history; (2) who were allergic to iodinated contrast medium; (3) who had been exposed to contrast agents within 14 days before the operation; (4) who presented with severe heart failure, severe cardiac stroke, serious valvular heart disease, and out-of-hospital cardiac arrest; (5) who presented with uremia or have had kidney transplantation; and (6) those who suffer from severe liver disease, cancer, autoimmune diseases, or infectious disease (Figure 1).
 |
Figure 1 Diagram of patient selection. Abbreviation: CA-AKI, contrast-associated acute kidney injury. |
The research protocol has been approved by the Ethics Committee of the Renmin Hospital of Wuhan University, and the research has been carried out in accordance with the Helsinki statement. All patients or their relatives signed written informed consent before the operation.
Clinical Assessments
Detailed medical histories and physical examination results were obtained from all included patients. The following baseline data from blood sample examinations were recorded from the first visit of the patients: age (years), weight, history of hypertension, diabetes mellitus, anemia, chronic kidney disease, heart failure, and coronary heart disease as well as history of alcohol drinking, cigarette smoking, and drug use.
Blood Sampling and Analysis
When the patients were admitted to hospital, medical staff collected 5 mL of procoagulant blood and 2 mL of ethylenediaminetetraacetic acid (EDTA) anticoagulated venous whole blood from the cubital vein. Serum was separated from the procoagulant tube and stored in the refrigerator at −80°C for later use. Procoagulant blood was used to detect serum C1q, TP (total protein), ALB (albumin), urea, Cr (creatinine), eGFR (estimated glomerular filtration rate), UA (uric acid), Glu (glucose), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and hsCRP (high-sensitivity C-reactive protein). Anticoagulant blood was used for the detection of red blood cell count (RBC) and hemoglobin. Among them, the immunoturbidimetric method was used to detect complement C1q, and the C1q reagent was purchased from Shanghai Beijia Biochemical Reagent Co., Ltd. The serum Cr level was measured at the time of admission, and daily post-procedure until the patient was discharged. All subjects were tested for serum C1q levels when they were admitted to the hospital. The eGFR was calculated using a CKD-EPI 2009SCr equation:14
Female with the concentration of serum creatinine ≤0.7 mg/dL,
eGFR (mL/min/1.73 m2) = 144 × (Scr/0.7) −0.329× (0.993)age;
Female with the concentration of serum creatinine >0.7 mg/dL,
eGFR (mL/min/1.73 m2) = 144 × (Scr/0.7) −1.209× (0.993)age;
Male with the concentration of serum creatinine ≤0.9 mg/dL,
eGFR (mL/min/1.73 m2) = 141 × (Scr/0.9) −0.411× (0.993)age;
Male with the concentration of serum creatinine >0.9 mg/dL,
eGFR (mL/min/1.73 m2) = 141 × (Scr/0.9) −1.209× (0.993)age
Percutaneous Coronary Intervention
PCI involves balloon expansion and/or stent placement of blood vessels related to infarction. Stents include drug-eluting stents and drug-coated stents. The operation was performed by the surgeon according to the intervention procedure, and the amount of contrast agent and the operation time during the operation were recorded. The procedure was guided by optical coherence tomography (OCT), intravascular ultrasound (IVUS) or angiography. Coronary angiography was performed by injection of iopamidol as a contrast agent. CA-AKI was defined as acute kidney injury that occurred within 48–72 h after administration of the contrast agent, with a 25% increase in serum Cr or 0.5 mg/dL as the diagnostic criteria.15 After exposure to the contrast agent, normal saline (0.9%) was injected intravenously at a rate of 1 mL/kg/h for 12 hours, and the hydration rate (and hence the total volume administered) was adjusted to the left ventricular end-diastolic pressure, central venous pressure, or bioimpedance vector analysis appropriately.
Statistical Analysis
Analyses were performed using SPSS 23.0 and R 3.5.2. According to the Kolmogorov–Smirnov test, age, TP and ALB are continuous variables that obey normal distribution and are expressed as mean ± standard deviation. RBC, Hb, urea, Cr, UA, eGFR, C1q, Glu, TC, TG, LDL-C, HDL-C, hsCRP, contrast volume and contrast exposure time do not obey normal distribution and are expressed as the median with the interquartile range. Categorical variables are expressed in quantity (percentage). The overall mean was compared by the two-independent-sample t test or the Mann–Whitney U-test. The chi-square t test was used to compare groups on the percentages of categorical variables. Logistic regression was used to explore the relationship between serum C1q levels and the prevalence of CA-AKI. To further investigate the relationship between serum C1q levels and the incidence of CA-AKI, restricted cubic spline analysis was conducted for all risk factors included in this study. The receiver operating characteristic (ROC) curve analysis was performed to evaluate the area under the curve (AUC) of C1q and CA-AKI model, to establish optimal cutoff values, and to calculate the sensitivity and specificity.
Results
Clinical Characteristics
Among the 1182 patients undergoing emergency PCI, 234 (19.80%) patients developed CA-AKI. Compared with patients without CA-AKI, patients with CA-AKI were older and more likely to suffer from hypertension, anemia, chronic kidney disease, heart failure, preoperative hypotension and cardiogenic shock, receive diuretic treatment, and use of IABP. In addition, the levels of C1q, urea, Cr, UA and hsCRP before PCI in the CA-AKI group were higher than those in the non-CA-AKI group, while the Hb concentration and eGFR before PCI were lower than those in the non-CA-AKI group. Compared with other indicators, there were no statistical differences (Table 1).
 |
Table 1 Baseline Clinical Features of the CA-AKI and Non-CA-AKI Groups |
Logistic Regression Between Serum C1q Level and the Prevalence of CA-AKI
Univariate and multivariate logistic regression analyses were performed to assess the effects of variables on CA-AKI development. Variables with P< 0.1 in univariate analyses were included in the multivariate model. The results of multivariate logistic regression analyses showed that the C1q level (OR: 1.016, 95% CI: 1.011–1.020, P < 0.001), eGFR level (OR: 0.978, 95% CI: 0.960–0.996, P = 0.018) and IABP use (OR: 1.801, 95% CI: 1.025–3.163, P = 0.041) were significant predictors of CA-AKI in patients who underwent emergency PCI (Table 2).
 |
Table 2 Univariate and Multivariate Analyses for the Risk Factors of CA-AKI in Patients Who Underwent an Emergency PCI |
According to the baseline C1q level, the quartile cut-off values are Q1 (≤155.50 mg/L), Q2 (155.51–176.15 mg/L), Q3 (176.16–199.30 mg/L) and Q4 (>199.30 mg/L). Univariate analysis showed that patients with higher serum C1q concentrations were more likely to develop CA-AKI: there were 21 patients (7.09%) in Q1, 43 (14.53%) in Q2, 65 (22.03%) in Q3, and 105 (35.59%) in Q4 (P < 0.001).
To analyze the relationship between the prevalence of CA-AKI and the serum C1q level, we divided patients into C1q quartiles and calculated the odds ratios (ORs) of their CA-AKI risk, taking patients in the Q1 as a reference (Table 3). In the unadjusted model 1, the serum C1q concentration was positively correlated with the prevalence of CA-AKI. After adjustment for eGFR and IABP use in Model 2, the results were similar to those of model 1. This association remained statistically significant and changed little after controlling for age, hypertension, anemia, chronic kidney disease, heart failure, Hb, urea, Cr, UA, eGFR, TC, hsCRP, diuretics, hypotension, cardiogenic shock, and IABP use in Model 3.
 |
Table 3 Association of the Prevalence of CA-AKI with the Serum C1q Concentration Before PCI |
Incidence of CA-AKI at Different C1q Levels
Figure 2 depicts the association between prevalence of CA-AKI and preprocedural C1q levels in patients with emergency PCI using restricted cubic spline analyses. We found a suggestion of S-shaped associations. The prevalence of CA-AKI slightly increased with increasing pre-procedural C1q concentrations, and in the range of C1q concentration from 150mg/L to 200mg/L, the incidence of CA-AKI increased fastest with the preoperative C1q concentration.
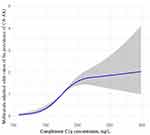 |
Figure 2 Nonlinear associations between serum C1q concentration and the prevalence of CA-AKI. |
The restricted cubic spline model of the ORs of the prevalence of CA-AKI with serum C1q level was built in patients before PCI. The grey area represented the 95% confidence interval. It was adjusted for age, sex, smoking, drinking, hypertension, diabetes, anemia, chronic kidney disease, heart failure, coronary heart disease, acute coronary syndrome (ACS) types, RBC, Hb, TP, ALB, urea, Cr, UA, eGFR, Glu, TC, TG, HDL-C, LDL-C, hsCRP, statins, ACEI/ARB, β-blockers, calcium channel blockers, diuretics, preoperative hypotension, preoperative LVEF <40%, cardiogenic shock, contrast volume, contrast exposure time and multivessel disease, radial approach, drug-eluting stent use, IABP use, OCT use, and IVUS use.
ROC Curve Analysis
As shown in Figure 3, ROC curve showed that the AUC of C1q in predicting the occurrence of CA-AKI after emergency PCI was 0.703 [95% CI 0.667–0.739], the optimal cut-off value was 180.25, the Youden Index was 0.344, the sensitivity was 72.4%, and the specificity was 61.7%. Additionally, according to multivariate logistic regression analyses, CA-AKI model included C1q, eGFR and IABP use, in which AUC of forecast probability was 0.718 [95% CI 0.682–0.754].
 |
Figure 3 ROC curve of C1q level and CA-AKI model in predicting the occurrence of CA-AKI after emergency PCI. |
In-Hospital Clinical Outcomes
Patients with CA-AKI had a significantly higher rate of the presence of cardiac arrest (8.12% vs 2.22%, P < 0.001) and in-hospital mortality (5.98% vs 0.95%, P < 0.001) compared with patients without CA-AKI.
Discussion
To the best of our knowledge, this is the first article on the association between serum C1q levels and the prevalence of CA-AKI after PCI. In this study, we found that patients with CA-AKI had higher serum C1q levels than those without CA-AKI, and with the increase of C1q levels, the prevalence of CA-AKI also increased. After adjusting for confounding factors, the level of C1q is still related to the prevalence of CA-AKI, indicating that the preoperative C1q level of patients is a potential risk factor for the prevalence of CA-AKI after PCI.
The pathogenesis of CA-AKI is not entirely clear. The nephrotoxicity of contrast media, renal medullary ischaemia and hypoxia, as well as oxidative stress are thought to be correlated with the progression of CA-AKI.16,17 Immune system was also considered to be related to the prevalence of the disease. Previous studies have found that elevated levels of CRP and WBC are related to the increase in the prevalence of CA-AKI, which suggests that immune response may be an important factor in the development of CA-AKI.18 As the main components of innate immune system, whether complement system is related to CA-AKI has not been reported. This study recruited 1182 subjects to explore the relationship between C1q and CA-AKI and analyze the clinical value of preoperative C1q level in CA-AKI.
C1q is the initiator molecule of the classical pathway of complement. When activated, it leads to the formation of the membrane attack complex (MAC), which in turn activates the classical pathway of complement.19 MAC can stimulate endothelial cells to secrete tissue factor and plasminogen activating enzyme inhibitor. They not only promote the binding of fibrinogen to endothelial cells but also promote platelet aggregation and thrombosis, and it also stimulates endothelial cells to express cell adhesion molecules, membrane cofactor protein and other cell adhesion molecules and chemokines and promotes the infiltration of monocytes and T lymphocytes, thereby promoting the occurrence and development of atherosclerosis.20–22 In addition, C1q levels are also related to the occurrence of kidney disease. The deposition of C1q and other complements in the renal mesangial area and vascular loops can lead to glomerulonephritis.23–25
Although the pathophysiological mechanisms of high C1q levels and CA-AKI are still unclear, they may be related to the activation of the complement system and the generation of ROS. Contrast agents can accelerate the activation of adenosine A1 receptors, increase the production of endothelin, induce renal vasoconstriction, cause renal medulla ischemia and hypoxia, and cause renal ischemia-reperfusion injury.2,26 The ischemia-reperfusion injury of the kidney will cause the activation of C1q and then the activation of the complement system, leading to the recruitment of inflammatory cells and the damage to the renal medulla.27,28 At the same time, C1q can induce the production of ROS, and ROS can further aggravate renal damage by regulating the levels of angiotensin II, thromboxane A2, endothelin and adenosine and further regulating vasoconstriction.11,12,29 Ischemia, hypoxia and exposure to contrast agents can cause cellular damage to renal tubular epithelial cells. Damaged renal tubular epithelial cells will increase the binding of C1q to gC1qR expressed by epithelial cells, thereby directly activating the classical pathway of complement, leading to kidney deficiency.13,30
This study has several limitations. First, due to the small sample size, there is no stratification for other risk factors of CA-AKI. Second, since the lack of follow-up, this study did not evaluate the correlation between C1q levels and the long-term renal function or long-term prognosis of patients. Third, we only tested the level of serum C1q in patients and did not test the levels of other components of the complement system at the time of patient admission and assess whether they have a suggestive effect on the pathogenesis of CA-AKI. Therefore, it cannot be explained that the disease of CA-AKI is related to the activation of the complement system. To explain this problem, we need to test other complement components in the patient’s serum and urine, such as C4d, C3a, C5a, etc., and perform kidney biopsy.
Conclusion
All in all, in patients receiving emergency PCI treatment, elevated serum C1q levels are a potential risk factor for CA-AKI. According to the C1q level before PCI, it is helpful to identify high-risk patients with CA-AKI.
Acknowledgments
We are grateful to all the people who participated in our study.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rundback JH, Nahl D, Yoo V. Contrast-induced nephropathy. J Vasc Surg. 2011;54:575–579. doi:10.1016/j.jvs.2011.04.047
2. Zhang F, Lu Z, Wang F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. 2020;259:118379. doi:10.1016/j.lfs.2020.118379
3. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380:2146–2155. doi:10.1056/NEJMra1805256
4. Wi J, Ko YG, Kim JS, et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97:1753–1757. doi:10.1136/hrt.2010.218677
5. Efe SC, Karagöz A, Doğan C, et al. Prognostic significance of malnutrition scores in elderly patients for the prediction of contrast-induced acute kidney injury. Int J Clin Pract. 2021;75:e14274. doi:10.1111/ijcp.14274
6. Putzu A, Boscolo Berto M, Belletti A, et al. Prevention of contrast-induced acute kidney injury by furosemide with matched hydration in patients undergoing interventional procedures: a systematic review and meta-analysis of randomized trials. JACC Cardiovasc Interv. 2017;10:355–363. doi:10.1016/j.jcin.2016.11.006
7. Ma H, Sandor DG, Beck LH
8. Thurman JM. Complement and the kidney: an overview. Adv Chronic Kidney Dis. 2020;27:86–94. doi:10.1053/j.ackd.2019.10.003
9. Willows J, Brown M, Sheerin NS. The role of complement in kidney disease. Clin Med (Lond). 2020;20:156–160. doi:10.7861/clinmed.2019-0452
10. Teh BK, Yeo JG, Chern LM, et al. C1q regulation of dendritic cell development from monocytes with distinct cytokine production and T cell stimulation. Mol Immunol. 2011;48:1128–1138. doi:10.1016/j.molimm.2011.02.006
11. Skoglund C, Wetterö J, Bengtsson T. C1q regulates collagen-dependent production of reactive oxygen species, aggregation and levels of soluble P-selectin in whole blood. Immunol Lett. 2012;142:28–33. doi:10.1016/j.imlet.2011.11.003
12. Tenner AJ, Cooper NR. Stimulation of a human polymorphonuclear leukocyte oxidative response by the C1q subunit of the first complement component. J Immunol. 1982;128:2547–2552.
13. Yin W, Ghebrehiwet B, Weksler B, et al. Classical pathway complement activation on human endothelial cells. Mol Immunol. 2007;44:2228–2234. doi:10.1016/j.molimm.2006.11.012
14. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011 Sep 20;155(6):408]. Ann Intern Med. 2009;150:604–612. doi:10.7326/0003-4819-150-9-200905050-00006
15. Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. doi:10.1007/s00330-017-5247-4
16. Toprak O, Cirit M. Risk factors for contrast-induced nephropathy. Kidney Blood Press Res. 2006;29(2):84–93. doi:10.1159/000093381
17. Okoye O, Ojogwu L, Unuigbe E, Oviasu E. Frequency and risk factors of contrast-induced nephropathy after contrast procedures in a Nigerian tertiary centre. West Afr J Med. 2013;32(1):19–25.
18. Yuan Y, Qiu H, Hu X, et al. Predictive value of inflammatory factors on contrast-induced acute kidney injury in patients who underwent an emergency percutaneous coronary intervention. Clin Cardiol. 2017;40(9):719–725. doi:10.1002/clc.22722
19. Dalmasso AP. Complement in the pathophysiology and diagnosis of human diseases. Crit Rev Clin Lab Sci. 1986;24:123–183. doi:10.3109/10408368609110272
20. Tedesco F, Pausa M, Nardon E, et al. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–1627. doi:10.1084/jem.185.9.1619
21. Eriksson O, Mohlin C, Nilsson B, et al. The human platelet as an innate immune cell: interactions between activated platelets and Eriksson O, Mohlin C, Nilsson B, Ekdahl KN. The complement system. Front Immunol. 2019;10:1590. doi:10.3389/fimmu.2019.01590
22. Kilgore KS, Ward PA, Warren JS. Neutrophil adhesion to human endothelial cells is induced by the membrane attack complex: the roles of P-selectin and platelet activating factor. Inflammation. 1998;22:583–598. doi:10.1023/a:1022362413939
23. Sun ZJ, Li XQ, Chang DY, et al. Complement deposition on renal histopathology of patients with diabetic nephropathy. Diabetes Metab. 2019;45:363–368. doi:10.1016/j.diabet.2018.08.011
24. Imai H, Yasuda T, Satoh K, et al. Pan-nephritis (glomerulonephritis, arteriolitis, and tubulointerstitial nephritis) associated with predominant mesangial C1q deposition and hypocomplementemia: a variant type of C1q nephropathy? Am J Kidney Dis. 1996;27:583–587. doi:10.1016/s0272-6386(96)90171-7
25. Rossen RD, Reisberg MA, Singer DB, et al. Soluble immune complexes in sera of patients with nephritis. Kidney Int. 1976;10:256–263. doi:10.1038/ki.1976.105
26. Wong PC, Li Z, Guo J, et al. Pathophysiology of contrast-induced nephropathy. Int J Cardiol. 2012;158:186–192. doi:10.1016/j.ijcard.2011.06.115
27. Castellano G, Melchiorre R, Loverre A, et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am J Pathol. 2010;176:1648–1659. doi:10.2353/ajpath.2010.090276
28. Li K, Sacks SH, Zhou W. The relative importance of local and systemic complement production in ischaemia, transplantation and other pathologies. Mol Immunol. 2007;44:3866–3874. doi:10.1016/j.molimm.2007.06.006
29. Heyman SN, Rosen S, Khamaisi M, et al. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45:188–195. doi:10.1097/RLI.0b013e3181d2eed8
30. Ghebrehiwet B, CebadaMora C, Tantral L, et al. gC1qR/p33 serves as a molecular bridge between the complement and contact activation systems and is an important catalyst in inflammation. Adv Exp Med Biol. 2006;586:95–105. doi:10.1007/0-387-34134-X_7
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
