Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Serum Level of Brain-Derived Neurotrophic Factor (BDNF) Associated with Depression in Patients with Rosacea: A Candidate Predictive Biomarker
Authors Wang T, Liu F, Jia X, Tan J, Qi B, Guo J, Mu Q , Zhang H
Received 25 March 2022
Accepted for publication 18 May 2022
Published 2 June 2022 Volume 2022:15 Pages 1029—1036
DOI https://doi.org/10.2147/CCID.S367545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Tegexibaiyin Wang,1,* Fen Liu,2,* Xiujuan Jia,3 Jing Tan,3 Baopeng Qi,4 Jingxue Guo,4 Qiri Mu,3 Hong Zhang5
1Laboratory of Pharmacy, International Mongolian Hospital of Inner Mongolia, Hohhot, 010065, People’s Republic of China; 2Department of Intensive Care Medicine, The First Affiliated Hospital of Shandong First Medical University, Jinan, 250014, People’s Republic of China; 3Department of Dermatology, International Mongolian Hospital of Inner Mongolia, Hohhot, 010020, People’s Republic of China; 4Inner Mongolia Medical University, Hohhot, 010010, People’s Republic of China; 5Department of Pediatrics, Qilu Hospital, Shandong University, Jinan, 250012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiri Mu, Department of Dermatology, International Mongolian Hospital of Inner Mongolia, 83 University East Street, Hohhot, Inner Mongolia, 010020, People’s Republic of China, Email [email protected] Hong Zhang, Department of Pediatrics, Qilu Hospital, Shandong University, 107 Wenhua West Road, Jinan, 250012, People’s Republic of China, Email [email protected]
Background: The biomarker to predict the depression in patients with rosacea was absent.
Objective: We aimed to explore the potential association between BDNF and depression in patients with rosacea, and also to determine whether serum BDNF level is a potential biomarker for identifying depression in patients with rosacea.
Methods: The patients with rosacea, rosacea with depression and healthy control were included, clinical evaluation (DLQI, RSSs, BDI-II) and serum BDNF levels detection were performed on subjects, the comparisons and correlation analysis of the obtained data were performed.
Results: In clinical evaluation, whether DLQI or RSSs, rosacea with depression group was significantly higher compared to rosacea group. Besides, we found the serum BDNF levels were lower in patients with rosacea and rosacea with depression compared to healthy controls, also in the rosacea with depression group, serum BDNF levels were lower than in rosacea patients. Whatever in rosacea or rosacea with depression group, the statistical significance of serum BDNF levels between the different subtypes like the ETR and PPR was not found. In further correlation analysis, we found no correlation between serum BDNF and RSSs in patients with rosacea whatever the subtype of ETR or PPR. Interestingly, we found a negative correlation between serum BDNF levels and BDI-II in rosacea with depression group, the decreased serum BDNF levels were associated with the increased BDI-II, also the ROC confirmed it can evaluate the depression in patients with rosacea.
Conclusion: Serum BDNF level is a potential biomarker for identifying depression in patients with rosacea.
Keywords: rosacea, biomarker, brain-derived neurotrophic factor, depression
Introduction
Rosacea, which is one of the common, chronic and inflammatory cutaneous conditions with pathogenesis complicatedly and not fully understands, it involved the interaction of multiple factors like immune and neurovascular dysregulation, genetic and environmental factors, presence of microorganisms, and others.1–3 Clinically, the flushing, transient or persistent erythema, papules/pustules, telangiectasia, and phymatous changes in patients with rosacea can be presented, the ocular may also be involved and some secondary symptoms including itching, burning, or stinging, are often observed.2,4,5 Rosacea with varying phenotypic features and facial localization resulting the patients with a negative and substantial impact on quality of life, it also affects the mental health and socialization bringing severe psychosocial burden and implications in patients with rosacea.6,7 In recent years, the conception of rosacea should be considered as the systemic disease was highlighted, also associated with multiple systemic comorbidities, such as cardiovascular diseases, autoimmune conditions, migraines, Parkinson’s disease, gastrointestinal disorders, and others, however, the argued was presented that comorbidity should not be overemphasized.8–11 The higher incidences of embarrassment, social anxiety, depression, decreased quality of life, and an increased risk of developing depression and anxiety were showed to a great extent and tend to avoid social situations in patients with rosacea.12,13 The relationship between the rosacea and its comorbidity with psychiatric disorders remains controversial.14
With the increasing risk of developing psychiatric disorders such as depression and anxiety in patients with rosacea, for clinicians, on the one hand, the better treatment outcomes can bring significant improvement in psychological symptoms, on the other hand, it is necessary to identify the psychological aspects of patients with rosacea as soon as possible, which may play an important role in clinical decision-making.13–15 However, identifying comorbidities of psychological symptoms including depression and anxiety in patients with rosacea faces challenges due to the biomarker to predict the depression in patients with rosacea was absent. Brain-derived neurotrophic factors (BDNF), which is one member of the neurotrophin and contribute to the pathophysiology of depression, some dermatological diseases like acne vulgaris and psoriasis may result in the depression, previous evidence indicated the decreased serum levels of BDNF associated with depressive symptoms in patients with acne vulgaris, which can as a good predictor to evaluate chronic stress in such patients, also in patients with psoriasis, decreased levels of BDNF are not only associated with the severity of depression but also closely associated with the severity of psoriasis vulgaris.16–18 To date, no study to evaluate the serum BDNF levels in patients with rosacea, thus our study aimed to demonstrate the potential association between BDNF and depression in patients with rosacea, and also to determine whether serum BDNF level is a potential biomarker for identifying depression in patients with rosacea.
Methods
Subjects
All subjects were enrolled from the Department of Dermatology in International Mongolian Hospital of Inner Mongolia during February 2021 to December 2021. The inclusion and exclusion criteria of subjects mainly include rosacea patients older than or equal to 18 years old as well obtain the patient’s written informed consent and the diagnosis of rosacea patients was according to the diagnostic criteria with the 2017 update by the National Rosacea Society Expert Committee.19 The exclusion criteria are as follows, the subject had severe heart, liver, and kidney disease or malignant tumor; the medication history of isotretinoin, systemic corticosteroids, antidepressants, and antianxiety within four weeks; the medical history of nervous system diseases such as epilepsy, migraine and so on in the past; the female during pregnancy and breastfeeding stage. Also, the rosacea patients who were diagnosed as depression that according to the diagnostic criteria with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) in the Department of psychology were included, and the number-, and aged- and sex-matched healthy control volunteers were also included.20 The demographic information was recorded to obtain the peripheral venous blood from patients with rosacea, patients with rosacea with depression, and healthy control subjects, and the clinical parameters scoring was performed. This study was approved by the Ethics Committee of the Institutional Review Board of International Mongolian Hospital of Inner Mongolia. Informed written consent was obtained from all subjects before inclusion in the study. The study complies with the Declaration of Helsinki.
Clinical Parameters
The evaluation of clinical parameters was performed for all patients, including Dermatology Life Quality Index (DLQI), Rosacea Severity scores (RSSs), and evaluation of Beck Depression Inventory-II (BDI-II) for patients with rosacea with depression.21–23 The evaluation details of all clinical parameters are as follows.
Dermatology Life Quality Index (DLQI)
DLQI, which is a simple practical questionnaire technique for evaluating the patients’ life quality with the dermatological disease in routine clinical practice, scores according to the patient’s answers to ten questions.21 The score ranged from 0 to 30 and the higher the score, the more serious the impact on the quality of life of patients. Specifically, 0 to 1 means no effect at all on patient’s life, 2 to 5 means the small effect on patient’s life, 6 to 10 means a moderate effect on the patient’s life, 11 to 20 means a very large effect on patient’s life and 21 to 30 means extremely large effect on patient’s life.
Rosacea Severity Scores (RSSs)
The severity evaluation according to the RSSs proposed by the National Rosacea Society Expert Committee.22 Four subtypes consist of erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR) phymatous rosacea (PhR), and ocular rosacea (OR) with rosacea were classified, and each subtype was scored 0 to 3 according to none, mild, moderate and severe.
Beck Depression Inventory-II (BDI-II)
BDI-II, is a widely used psychometric instrument in clinical practice for the capacity to discriminate between depressed and non-depressed subjects and measure the severity of depression with high reliability.24,25 A total of 21 questions in the inventory, and each question has 4 options, these 4 options represent 0–3 points respectively, and the score range is 0–63. The higher the score, the higher the possibility of indicating depression or severity, and depression severity is mainly based on the score range, 0 to 9 indicates minimal depression, 10 to 18 indicates mild depression, 19 to 29 indicates moderate depression, and 30 to 63 indicates severe depression.
Detection of Serum BDNF Level
The 5 milliliters venous blood sample from all patients was obtained in serum separator tubes, the samples were left for 30 minutes at room temperature to allow clotting then centrifuged (15 minutes/3000 rpm). The serum samples treated as above were separated and stored at −20 °C biological storage.17 Serum BDNF levels with all samples were measured by enzyme-linked immunosorbent assay (ELISA) and using commercial ELISA kits (RX104552H) from Quanzhou Ruixin Biotechnology Company (Quanzhou, Fujian Province, China) according to the manufacturer’s instructions and the concentration of BDNF was expressed as ng/mL.
Statistics Analysis
All the obtained data are transferred to Excel, SPSS windows (Version 21, SPSS Inc., Chicago, Illinois, USA), and GraphPad Prism 7.04 software (GraphPad Software, Inc., San Diego, CA, USA) were used for statistical analysis and chart design. The comparisons of the two groups were computed using Mann–Whitney U-test. Correlations were analyzed by Pearson’s correlation test. Also, the receiver operating characteristic curves (ROCs) were used to test the overall diagnostic accuracy. In all tests, P<0.05 was considered statistically significant.
Results
Subjects
A total of 260 subjects including rosacea (n=118), rosacea with depression (n=42), and healthy controls (n=100) was included in this study, all subjects were in strict accordance with the inclusion and exclusion criteria. The included patients were classified according to different subtypes, 78 erythematotelangiectatic rosacea (ETR) and papulopustular rosacea (PPR) in the rosacea group also the 30 ETR and 12 PPR in rosacea with depression group, no phymatous rosacea (PhR) and ocular rosacea (OR) were included. Demography, clinical, and laboratory data of subjects are shown in Table 1. All patients completed the DLQI and RSSs evaluation, patients with rosacea with depression also performed the BDI-II score, obtained the peripheral blood of all subjects, and obtained the written informed consent of all subjects. There was no significant difference in mean age among the three groups, but for gender ratio, female with rosacea with depression accounted for more. In clinical evaluation, a very significant difference in DLQI between rosacea and rosacea with depression was presented, the score of rosacea with depression group is significantly higher than that of the rosacea group (P<0.001), also in the rosacea with depression group the RSSs has presented the higher level compared to rosacea group (P<0.001).
 |
Table 1 Demography, Clinical, and Laboratory Data |
Serum BDNF Levels Analysis
Serum BDNF levels were obtained after ELISA detection and statistical analysis between the groups was performed. There were significant differences in serum BDNF levels among the healthy control group, rosacea, and rosacea with depression, especially between the healthy control group and rosacea with depression group (P<0.001), and between rosacea and rosacea with depression group (P<0.001), similarly, the differences were also found between the healthy control group and the rosacea group (P<0.01). The serum BDNF levels in patients with rosacea, the rosacea with depression compared to rosacea without depression, were significantly lower than healthy controls (Figure 1A). According to the significant difference test of serum BDNF level among different subtypes (ETR and PPR) of rosacea, there was no statistical significance in either the rosacea group or rosacea with depression group (Figure 1B and C).
Correlation Analysis
Correlation analysis between the serum BDNF levels and RSSs in the rosacea group also between serum BDNF levels and BDI-II in rosacea with depression group were performed. In rosacea group, the correlation between serum BDNF levels and RSSs was not found (r=0.03563, P=0.7017), whatever in subtype of ETR (r=0.03157, P=0.7838) or PPR (r=0.03721, P=0.8197) (Figure 2A–C). In the group of rosacea with depression, the serum BDNF levels and BDI-II showed a significant negative correlation, the lower the serum BDNF levels, the higher the level of BDI-II was presented (r=−0.5489, ***P=0.0002) (Figure 2D).
Receiver Operating Characteristic (ROC) Curve Analysis
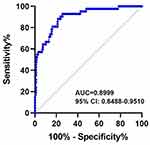
ROC curve analysis confirmed the decreased serum BDNF levels, which may be a potential predictive biomarker for identifying and predicting depression in patients with rosacea (AUC = 0.8999, 95% CI: 0.8488–0.9510) (Figure 3).
Discussion
In the present study, we included the patients with rosacea and rosacea with depression and aimed to evaluate the serum BDNF levels in patients with rosacea and their correlation with clinical parameters. In clinical evaluation, whether DLQI or RSSs, rosacea with depression group was significantly higher compared to rosacea group. Besides, we found the serum BDNF levels were lower in patients with rosacea and rosacea with depression compared to healthy controls, also in the rosacea with depression group, serum BDNF levels were lower than in rosacea patients. Whatever in rosacea or rosacea with depression group, the statistical significance of serum BDNF levels between the different subtypes like the ETR and PPR was not found. In further correlation analysis, we found no correlation between serum BDNF and RSSs in patients with rosacea whatever the subtype of ETR or PPR. Interestingly, we found a negative correlation between serum BDNF levels and BDI-II in rosacea with depression group, the decreased serum BDNF levels were associated with the increased BDI-II, also the ROC confirmed it can evaluate the depression in patients with rosacea. In brief, our study revealed the potential significance and value of rosacea, especially in rosacea with depression.
Rosacea is a common, chronic and inflammatory cutaneous disorder worldwide that almost 5.46% of the adult population is affected, also the pathogenesis remained unclear and involves the complex interplay of multiple environmental and endogenous factors, especially the neurovascular and immunologic mechanisms like abnormalities of cutaneous vasculature and dysregulation of the inflammatory response are involved.1,8,11,26,27 In recent years, some treatment options were widely used, like skincare and cosmetic treatments, topical, oral, light-based also injection therapies.5 Although a variety of treatment options can be considered, unmet clinical needs are still presented, also, the chronic course of rosacea, leads to a huge psychosocial burden and negative quality of life for patients.6 Despite the current diagnostic and classification criteria and treatment options were proposed, with a further understanding of rosacea, which is considered associated with several systemic disorders, like a metabolic disease, cardiovascular diseases, gastrointestinal diseases, tumors, autoimmune diseases, and others.11,28–30 Also, the burden of psychiatric comorbidity was highlighted in rosacea patients, such as anxiety, depression, Parkinson’s and Alzheimer’s disease, and dementia.14,31–33 The psychosocial impact in patients with rosacea can result in severe and debilitating, but it is underestimated in clinical practice.34 The results from a systematic review and meta-analysis which included a total of 14 studies involving 14,134,021 patients with rosacea were revealed and indicated that patients with rosacea are at a higher risk of experiencing depression and anxiety, the pooled prevalence of depression was 19.6% (95% confidence interval [CI] 15.0–24.3%) and that of anxiety was 15.6% (95% CI 11.8–19.3%).15 In China, the high prevalence of comorbidities with anxiety and depression was showed from a 754 rosacea patients’ study, even higher to 53.9% (95% CI: 50.4–57.4%) and 58.1% (95% CI: 54.7–61.6%) respectively.35 The mechanism of comorbidity between rosacea and neuropsychiatric diseases is not completely clear, but it may be considered to be associated with the Gut-Brain-Skin axis at present.36 Up to date, although some biomarkers are used to diagnose depression and detect its treatment response, the reliable biomarker for rosacea with depression was absent, which is almost based on subjective evaluation. BDNF, which is a member of the neurotrophin family of closely related proteins that were first identified as survival factors for sympathetic and sensory neurons, also contributed survival, development, and function of neurons in both the central and peripheral nervous systems, also contributed to the pathophysiology of major depressive disorders.37–39 BDNF also contributed to the pathophysiology of multiple skin diseases.
To date, no study has evaluated the potential link between serum BDNF levels and rosacea. Interestingly, a previous study indicated the potential link between serum BDNF levels and patients with acne, the results showed that serum BDNF levels were lower in consecutive acne vulgaris patients when compared with healthy control as well as a negative correlation between levels of BDNF and the PHQ-9 scores were found, it considered as serum BDNF levels were decreased and negatively associated with depressive symptoms in young Chinese adults with acne vulgaris, receiver operating characteristic also confirmed it.16 In patients with acne, who are at a high risk to develop chronic stress, anxiety, and depression, BDNF was considered a good predictor for assessment of chronic stress in acne patients.17 Similarly, in patients with psoriasis vulgaris, the low level of serum BDNF may increase depression severity and psoriasis vulgaris severity.18 These findings are consistent with our study results the decreased serum BDNF levels were associated with depression severity in rosacea patients, we also found that serum BDNF levels were not associated with the subtype and severity of rosacea, although the mechanism is not completely clear, it may be related to chronic stress. The reduced serum BDNF levels might be used as an early risk evaluation marker for patients with major depression.39 Interestingly, the serum BDNF levels are lower in skin diseases with depression than depression, but the mechanism is still not completely clear. Our study also has several limitations, the rosacea patients with anxiety and PhR, OR subtype were not included, further large sample clinical study is still needed in the future, and only evaluated BDNF that one of members of neurotrophic family in present study, we still need to enrich the potential significance of other neurotrophic family members. In short, serum BDNF level is a potential biomarker for identifying depression in patients with rosacea.
Conclusion
Our study data suggested and enriched the evidence of BDNF contributed to the pathogenesis of rosacea, BDNF as one of neurotrophic members may mediate the pathophysiological process of the psychiatric disorder’s comorbidity of rosacea, serum BDNF level is a potential biomarker for identifying depression in patients with rosacea.
Patient Informed Consent
The written consent form from all subjects was obtained in this study.
Ethics Statement
Ethical approval was obtained from Institutional Review Board (IRB) of International Mongolian Hospital of Inner Mongolia.
Funding
This work was supported by the Natural Science Foundation of Inner Mongolia Autonomous Region (2021ZD15).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36(2):81–86. doi:10.1016/j.det.2017.11.001
2. van Zuuren EJ, Solomon CG. Rosacea. N Engl J Med. 2017;377(18):1754–1764. doi:10.1056/NEJMcp1506630
3. Two AM, Wu W, Gallo RL, et al. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72(5):
4. van Zuuren EJ, Arents BWM, van der Linden MMD, et al. Rosacea: new concepts in classification and Treatment. Am J Clin Dermatol. 2021;22(4):457–465. doi:10.1007/s40257-021-00595-7
5. Zhang H, Tang K, Wang Y, et al. Rosacea treatment: review and update. Dermatol Ther (Heidelb). 2021;11(1):13–24. doi:10.1007/s13555-020-00461-0
6. Oussedik E, Bourcier M, Tan J. Psychosocial burden and other impacts of rosacea on patients’ quality of life. Dermatol Clin. 2018;36(2):103–113. doi:10.1016/j.det.2017.11.005
7. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6(6):348–354.
8. Wollina U. Is rosacea a systemic disease? Clin Dermatol. 2019;37(6):629–635. doi:10.1016/j.clindermatol.2019.07.032
9. Yi JZ, Chen SX, Lukac D, et al. Systemic comorbidities of rosacea: practice gaps among dermatologists. Arch Dermatol Res. 2021. doi:10.1007/s00403-021-02279-y
10. Zhang H, Tang K, Wang Y, et al. Rosacea and its comorbidities: should be emphasized but should not be overemphasized. J Cosmet Dermatol. 2020;19(12):3414–3415. doi:10.1111/jocd.13793
11. Haber R, El Gemayel M. Comorbidities in rosacea: a systematic review and update. J Am Acad Dermatol. 2018;78(4):786–792.e8. doi:10.1016/j.jaad.2017.09.016
12. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71(5):973–980. doi:10.1016/j.jaad.2014.05.036
13. Heisig M, Reich A. Psychosocial aspects of rosacea with a focus on anxiety and depression. Clin Cosmet Investig Dermatol. 2018;11:103–107. doi:10.2147/CCID.S126850
14. Chang H-C, Huang Y-C, Lien Y-J, et al. Association of rosacea with depression and anxiety: a systematic review and meta-analysis. J Affect Disord. 2022;299:239–245. doi:10.1016/j.jad.2021.12.008
15. Dai R, Lin B, Zhang X, et al. Depression and anxiety in rosacea patients: a systematic review and meta-analysis. Dermatol Ther (Heidelb). 2021;11(6):2089–2105. doi:10.1007/s13555-021-00613-w
16. He H-Y, Tian J-L, Deng Y-Q, et al. Association of brain-derived neurotrophic factor levels and depressive symptoms in young adults with acne vulgaris. BMC Psychiatr. 2019;19(1):193. doi:10.1186/s12888-019-2182-8
17. Mikhael NW, Hamed AM, Mansour AI, et al. Serum levels of brain-derived neurotrophic factor in patients with acne vulgaris. J Cosmet Dermatol. 2019;18(6):1998–2003. doi:10.1111/jocd.12940
18. Sjahrir M, Roesyanto-Mahadi ID, Effendy E. Correlation between serum brain-derived neurotrophic factor level and depression severity in psoriasis vulgaris patients. Open Access Maced J Med Sci. 2019;7(4):583–586. doi:10.3889/oamjms.2019.142
19. Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155. doi:10.1016/j.jaad.2017.08.037
20. Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: classification and criteria changes. World Psychiatr. 2013;12(2):92–98. doi:10.1002/wps.20050
21. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
22. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–912. doi:10.1016/j.jaad.2004.01.048
23. Richter P, Werner J, Heerlein A, et al. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31(3):160–168. doi:10.1159/000066239
24. Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Braz J Psychiatr. 2013;35(4):416–431. doi:10.1590/1516-4446-2012-1048
25. Beck AT, Steer RA, Brown GK, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi:10.1001/archpsyc.1961.01710120031004
26. Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–289. doi:10.1111/bjd.16481
27. Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(1):e1361574. doi:10.1080/19381980.2017.1361574
28. Vera N, Patel NU, Seminario-Vidal L. Rosacea Comorbidities. Dermatol Clin. 2018;36(2):115–122. doi:10.1016/j.det.2017.11.006
29. Holmes AD, Spoendlin J, Chien AL, et al. Evidence-based update on rosacea comorbidities and their common physiologic pathways. J Am Acad Dermatol. 2018;78(1):156–166. doi:10.1016/j.jaad.2017.07.055
30. Aksoy B, Ekiz Ö, Unal E, et al. Systemic comorbidities associated with rosacea: a multicentric retrospective observational study. Int J Dermatol. 2019;58(6):722–728. doi:10.1111/ijd.14353
31. Clinical Digest. Evidence suggests rosacea may be linked to Parkinson’s and Alzheimer’s disease. Nurs Stand. 2016;30(39):14.
32. Egeberg A, Hansen PR, Gislason GH, et al. Patients with rosacea have increased risk of dementia. Ann Neurol. 2016;79(6):921–928. doi:10.1002/ana.24645
33. Christensen CE, Andersen FS, Wienholtz N, et al. The relationship between migraine and rosacea: systematic review and meta-analysis. Cephalalgia. 2018;38(7):1387–1398. doi:10.1177/0333102417731777
34. Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102(1):36–38.
35. Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China. Front Psychiatry. 2021;12:659171. doi:10.3389/fpsyt.2021.659171
36. Woo YR, Han YJ, Kim HS, et al. Updates on the risk of neuropsychiatric and gastrointestinal comorbidities in rosacea and its possible relationship with the gut-brain-skin axis. Int J Mol Sci. 2020;21(22):8427. doi:10.3390/ijms21228427
37. Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1–12.
38. Rafieva LM, Gasanov EV. Neurotrophin propeptides: biological functions and molecular mechanisms. Curr Protein Pept Sci. 2016;17(4):298–305. doi:10.2174/1389203716666150623104145
39. Emon MPZ, Das R, Nishuty NL, et al. Reduced serum BDNF levels are associated with the increased risk for developing MDD: a case-control study with or without antidepressant therapy. BMC Res Notes. 2020;13(1):83. doi:10.1186/s13104-020-04952-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.