Back to Journals » International Journal of General Medicine » Volume 13
Serum IgG as a Marker for Opisthorchis viverrini-Associated Cholangiocarcinoma Correlated with HER2 Overexpression
Authors Titapun A , Techasen A, Sa-Ngiamwibool P, Sithithaworn P, Luvira V, Srisuk T , Jareanrat A, Dokduang H , Loilome W, Thinkhamrop B, Khuntikeo N
Received 24 September 2020
Accepted for publication 5 November 2020
Published 26 November 2020 Volume 2020:13 Pages 1271—1283
DOI https://doi.org/10.2147/IJGM.S282519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Attapol Titapun,1,2 Anchalee Techasen,2,3 Prakasit Sa-Ngiamwibool,2,4 Paiboon Sithithaworn,2,5 Vor Luvira,1,2 Tharatip Srisuk,1,2 Apiwat Jareanrat,1,2 Hasaya Dokduang,2,6 Watcharin Loilome,2,6 Bandit Thinkhamrop,2,7 Narong Khuntikeo1,2
1Department of Surgery, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; 2Cholangiocarcinoma Research Institute (CARI), Khon Kaen University, Khon Kaen, Thailand; 3Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand; 4Department of Pathology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; 5Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; 6Department of Biochemistry, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; 7Department of Epidemiology and Biostatistics, Faculty of Public Health, Khon Kaen University, Khon Kaen, Thailand
Correspondence: Attapol Titapun
Department of Surgery, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Tel +66-43-363252
Email [email protected]
Background: Serum antibody for Opisthorchis viverrini (OV) is strong evidence for a history of OV infection in people. Currently, no studies have examined whether varying cholangiocarcinoma (CCA) prevalence levels are linked to previous OV infection nor have they provided comprehensive assessment and characterization of OV-associated CCA.
Objective: Our study examined the prevalence of serum IgG antibodies for OV-positive CCA cases and determined whether there were correlations of IgG antibodies with histopathologic features, HER2, PD-L1, and FGFR2 expression, as well as their roles on the patients’ survival.
Methods: The study involved 221 CCA surgical patients at Khon Kaen University Hospital, Thailand, from 2005 to 2017. Serum specimens were tested for OV IgG by ELISA. CCA tissue microarrays were used to examined for HER2, PD-L1, and FGFR2 expression. Logistic regression was used to investigate an association between factors and IgG. Cox regression was used to determine factors that affected CCA patient survival.
Results: IgG for OV were positive in 162 cases, and the prevalence was 73.3% (95% CI=68.0– 78.7). About three quarters (78.3%) had large duct type tumors and concomitant intraductal papillary neoplasm of bile ducts (IPNB) occurred in 92 (50%) cases. HER2 expression was positive in 94 (61.4%) cases. Positive PD-L1 and FGFR2 expression occurred in 125 (83.9%) and 100 (67.1%) cases. IgG for OV had no significant correlation to any histological feature but had significant correlation with HER2 overexpression with adjusted OR=2.32 (95% CI=1.09– 4.96, P=0.03). Cases of CCA with OV IgG positive had a significantly poor prognosis with adjusted HR=1.66 (95% CI=1.13– 2.43, P=0.01).
Conclusion: We found a high prevalence of serum IgG for OV-positive CCA patients and a correlation with overexpression of HER2. Moreover, IgG for OV and HER2 expression indicated poor survival of CCA. Therefore, future clinical studies for anti-HER2 treatments should focus on OV-associated CCA.
Keywords: survival, intraductal type, FGFR2, PD-L1, immunoglobulin G, fluke-associated CCA
Background
Cholangiocarcinoma (CCA) has a poor prognosis, even in curative cases where the 5-year survival rates are only 20–30%1–4 and palliative cases have median survival between 4–12 months.5,6 This devastating disease has a high incidence in South East Asia, with increasing incidence in western countries.
The liver fluke, Opisthorchis viverrini (OV) is the known common cause of CCA in Thailand. OV causes bile duct injury by several mechanisms such as feeding, suckling, direct toxic excretory substance(s) and host immune response. Epithelial hyperplasia and fibrosis of bile ducts occur in response to repetitive injury progressing to premalignant and invasive cancer even if fluke infection has been cured with drug treatment, specifically praziquantel. Stool examination is a gold standard for diagnosis of OV infection by detecting OV eggs in feces. This technique can diagnose patients with heavy active infection, but false negatives occur in latent infections and the technique is unable to identify past infections. Serum IgG for OV has been shown to be sensitivity for diagnosis of OV infection up to 99.2%, with a specificity of 93%7 and IgG can persist in infected hosts after being cured.8 Therefore, serum IgG for OV can be useful for the diagnosis of OV-associated CCA.
CCA has two histological subtypes according to different postulated cells origins. First, perihilar large duct type originates from peribiliary gland in the large bile duct and is associated with stones, choledochal cysts, and PSC. Second, peripheral small duct types can originate from cells in the canal of Hering, which is associated with chronic hepatitis and cirrhosis.9 Small duct type CCA is associated with mass forming morphology consisting of low columnar or cuboidal cells with no identifiable premalignant lesions. Large duct type CCA has a variety of gross tumors from periductal infiltrate, intraductal, mass forming or mixed features consisting of tall columnar cells with associated premalignant lesions in biliary BilIN and IPNB. Large duct type CCA has mechanism(s) of carcinogenesis compatible with pathology in OV infection, therefore, OV-associated CCA may have histology similar to large duct type CCA.
The genetic landscape comparing between OV and non-OV-associated CCA has been recently reported. A genomic sequencing study found that CCA from high OV infection countries had high amplification for ERBB2/HER2 and TP53 mutations, while CCA from non-OV endemic countries had high expression of PD1, PDL2, and FGFR.10 Studies from non-OV endemic countries have reported low alteration of HER2, for instance, a study in Germany found CCA had only 5% HER2 amplification, and a study from Japan reported HER2 expression in extrahepatic CCA of only 8.5%.11,12 Moreover, the recent study in OV related CCA cases found the high expression of HER2 (55%) and the expression of HER2 was correlated with late recurrence state after undergoing surgical resection.13 These findings suggested ERBB2/HER2 implicated in OV-associated CCA and can be a promising target for CCA treatment. However, the criteria currently used to describe the characterization of OV-associated CCA are not clearly defined.
Therefore, our study aims to explore the prevalence of CCA with serum OV IgG antibodies for positive OV cases as well as to determine whether there is a correlation of IgG antibodies with histopathology and targeted gene expression in CCA.
Materials and Methods
Patients and Serums
The excess formalin-fixed paraffin embedded tissues and serum specimens were obtained from patients who were diagnosed with CCA and had undergone surgery at Srinagarind Hospital, Khon Kaen University during January 1, 2005 to December 31, 2017, and were kept in the biobank of the Cholangiocarcinoma Research Institute (CARI), Khon Kaen University. The clinicopathological data of each patient were provided by the CARI. Written informed consent for usage of excessive tissue specimens and serum samples for the study were obtained from each subject before surgery and this research protocol was carried out by approval and supervision of the Human Research Ethics Committee of Khon Kaen University based on the Declaration of Helsinki and the ICH Good Clinical Practice Guidelines (HE621147). H&E stained slides were examined by a pathologist (PS), and patients were excluded from the study if the result was not CCA and if serum specimens were unavailable. Tumor growth type, cell type, background liver, concomitant premalignant lesions, and bile duct types were recorded.
Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
An indirect ELISA technique was performed to determine immunoglobulin G (IgG) antibodies against OV antigen in a total of 221 serum specimens from patients. The plates were coated with whole adult OV antigen using the following protocols. Whole adult OV antigen was diluted to a concentration of 1,500 µg/mL with 1x PBS pH7.4, then added into 96-well (100 µL/well) flat bottom microtiter plates and incubated at 4°C overnight. Subsequently, plates were washed with 0.05% Tween 20 in PBS, blocked with 250 μL of 3% skimmed milk in PBS, and incubated at 37°C for 1 hour. Tested serum (dilution 1:6,000 in 3% skimmed milk) was added at 100 μL/well in duplicates and incubated at 4°C overnight. After incubation, the plates were washed five times with PBST20, and 100 μL of a conjugate of horseradish peroxidase with goat anti-human IgG (1:3,000 in 3% skimmed milk) was added to each well and incubated for 2 hours at 37°C. The plates were washed again with PBST20, and a 100 μL orthophenylene diamine hydrochloride (OPD) (Zymed, CA, USA) substrate was added for 30 minutes. The reaction was stopped by adding 4N sulfuric acid and measured optical density (OD) at 492 nm by using an ELISA reader (Tecan, Austria). The results were analyzed as described in a previous study.14
CCA Tissue Microarray
Of the total 221 cases, 153 available serum matched CCA tissue specimens were archived and reviewed to select tumor area and normal liver tissue for negative controls. Tissue microarray (TMA) blocks were prepared using a manual tissue microarrayer with a 2.0 mm diameter needle with 70 cores per block. TMA blocks were sliced into 4 µm thick sections, and mounted on glass slides in preparation for immunohistochemistry (IHC) staining.
Antibodies
Primary antibodies in this study are as follows: mouse anti-FGFR2 (ab58201, Abcam, Cambridge, MA), and rabbit anti-PD-L1 (#13684) and rabbit anti-HER2/ErbB2 (#4290) (Cell Signaling Technology, Denver, MA).
Immunohistochemistry (IHC)
Immunohistochemistry (IHC) was performed to determine the expressions of the target proteins in CCA TMA. Briefly, paraffin embedded CCA TMAs were deparaffinized in xylene and rehydrated in a series of concentrations of ethanol. Antigen retrieval was performed by microwaving tissue sections in 1x Sodium citrate plus 0.05% tween 20 for 10 minutes (for FGFR2 or HER2 retrieval) and by Tris EDTA at 110°C for 10 minutes by autoclave (for PD-L1 retrieval). Endogenous peroxidase activity was blocked by using 0.3% (v/v) hydrogen peroxide in PBS and non-specific proteins were blocked with 10% skim milk in PBS. Primary antibodies for FGFR2 (1:20,000), PD-L1 (1:25), and HER2 (1:100) were added to tissue microarrays and incubated at 4°C overnight. Subsequently, sections were washed in 0.1% Tween 20 in PBS for 5 minutes (3-times) and incubated with horseradish peroxidase conjugated EnvisionTM secondary antibody (Dako, USA). After washing three times in PBS plus 0.1% Tween 20 the color was developed with 3,3ʹ diaminobenzidine tetrahydrochloride (DAB) substrate kit (Vector Laboratories, Inc., CA) for 5 minutes, then counterstained with Mayer’s hematoxylin. The sections were rehydrated with stepwise increasing concentration of ethanol and mounted with per mount solution. The sections were observed under a light microscope (Nikon Ni-U, Japan).
IHC Scoring System
The result of immunohistochemistry staining of tumors in human tissue arrays were recorded as frequency and intensity scores. Frequency scores were classified as 1+ if the positive staining area was less than 25%, 2+ for 25–50%, and 3+ if >50% area was stained. Intensity scores were classified as 0 for negative stain, 1 for weakly stained, 2 for moderately stained, and 3 for strongly stained. Total scores were calculated by multiplying the frequency and intensity score in each case. The results were recorded as low expression if the total score was less than the median, high expression if the total score was more than the median, and negative expression for negative staining. Demonstrations of IHC staining in CCA tissue are shown in Figure 1.
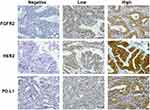 |
Figure 1 Immunohistochemistry of CCA for HER2, PD-L1, and FGFR2 expression, magnification 40x. |
Statistical Analysis
Determining the sample size for a prevalence in one population was calculated by Daniel’s method formula with precision ±0.05 and expected prevalence 0.89. This provided a sample size of 165 cases for an expected data loss of 10%. Continuous data were reported as mean and standard deviation (SD). Categorical data were presented as percentages. Prevalence of IgG for OV were reported as percentages with 95% confidence interval (CI). Bivariate analysis by logistic regression were used for screening for candidate variables to be included in the initial model. All variables with a P-values of less than 0.2 were included in the full model, followed by a backward elimination process as a model fitting strategy using a likelihood ratio test. The odds ratio (OR) with its 95% CI and P-value were obtained. Survival analysis by Kaplan-Meier methods were recorded as median survival time with 95% CI. Cox proportional hazard was used to estimate the use for estimating the median survival of CCA patients. Effect of factors on overall survival were estimated as hazard ratios (HR), it’s 95% CI and P-values were analyzed using the Cox-proportional hazards model. Statistical analyses were performed by using STATA version 14 (Stata Corp, College Station, TX)
Results
Patients Characteristic and Histopathology
A total of 221 serum specimens from CCA patients who received treatment in the CARI from March 2005 to November 2017 were studied for OV IgG antibodies. Of these, 162 (73.3%, 95% CI=68.0–78.7) were positive for OV IgG antibodies. The mean age of patients was 59.8±8.9 years, and 138 (62.4%) were male. Histologically confirmed CCA was found in 98 (44.3%) specimens who had papillary carcinoma, 76 (34.4%) were tubular carcinoma, 13 (5.9%) were combined papillotubular carcinoma, and 34 (15.4%) were non-specific adenocarcinoma and rare variants.
A total of 184 cases had available specimens for pathological examination of tumor growth types, background liver, concomitant premalignant lesion, and histologic subtypes (Table 1).
 |
Table 1 Patient Characteristic and Histopathology |
Mass forming tumor growth (MF) was found in 56 (30.4%) cases, three (1.6%) cases had periductal infiltrate (PI), 44 (23.9%) intraductal (ID), and 81 (44.1%) had mix morphology which consisted of MF with ID (41, (22.3%), MF with PI (25, 13.6%), ID with PI (7, 3.8%), and eight (4.4%) had a combination of all growth types. Most of the patients had normal liver background, 28 (15.2%) had periductal fibrosis background, and only two patients had cirrhotic liver. IPNB were concomitant in 92 (50%) cases, while biliary intraepithelial neoplasia was found in only seven (3.8%) cases. One hundred and forty-four (78.3%) were large duct type, 38 (20.7%) were small duct type, and two (1%) cases had combined hepatocellular-cholangiocarcinoma. There was no statistically significant difference in the proportion of histopathology characters between OV IgG positive and negative except for a greater proportion of ID growth type in the OV IgG negative group (Table 1).
HER2, PD-L1, and FGFR2 Expression
From all patients there were 153 cases that had tumor tissue available for IHC study, four cases were excluded to examine for FGFR2 and PD-L1 because of scanty tumor cells in the arrays. HER2 expression was negative in 59 (38.6%) cases, low expression was found in 37 (24.2%), and high expression was found in 57 (37.2%) cases. PD-L1 expression was negative in 24 (16.1%) cases, low expression in 69 (46.3%), and high expression was found in 56 (37.6%) cases. FGFR2 expression was negative in 49 (32.9%), low expression in 56 (37.6%), and high expression in 44 (29.5%) cases. There was a higher proportion of HER2 expression in the OV IgG positive group, but no difference was found in PD-L1 and FGFR2 expression between the OV IgG positive and negative groups (Table 2).
 |
Table 2 HER2, PD-L1, and FGFR2 Expression with IgG for OV |
The Correlation of Serum OV IgG Positive with Histopathologic Factors, HER2, PD-L1, and FGFR2 Expression in CCA
Bivariable analysis showed OV IgG positive cases were less likely to have pure intraductal growth type CCA with OR=0.42 (95% CI=0.20–0.84, P-value=0.01). Intraductal growth type, papillary type, and concomitant IPNB were included in multivariable logistic analysis due to P-value less than 0.2 from bivariable analysis. Intraductal growth type had a significant correlation with OV IgG with adjusted OR=0.42 (95% CI=0.18–0.95, P-value=0.04) (Table 3). OV IgG positive CCA had a higher chance of HER2 positive outcome from the IHC study with crude OR=2.24 (95% CI=1.10–4.54, P-value=0.02) When adjusted with PD-L1 and FGFR2, HER2 there was a significant correlation with OV IgG positive cases with adjusted OR=2.32 (95% CI=1.09–4.96, P-value=0.03) and HER2 high expression had a significant correlation with OV IgG with adjusted OR=2.50 (95% CI=1.06–5.89, P-value=0.04) (Table 4).
 |
Table 3 Histopathologic Factors Correlated with IgG for OV |
 |
Table 4 HER2, PD-L1 and FGFR2 Expression Correlated to IgG for OV |
Survival by Serum OV IgG, Histopathologic Factors, HER2, PD-L1, and FGFR2 Expression in CCA
Bivariable analysis showed CCA with OV IgG positive cases had a significantly poor prognosis with median survival time 12.7 months (95% CI=9.2–16.0) with HR=1.73 (95% CI=1.23–2.43, P-value<0.01) compared to OV IgG negative (Figure 2). Histopathologic factors which had significant outcomes for survival were mass forming growth type (HR=2.50, 95% CI=1.77–3.51, P-value<0.01), intraductal growth type (HR=0.40, 95% CI=0.26–0.60, P-value<0.01), papillary type (HR=0.52, 95% CI=0.39–0.69, P-value<0.01), concomitant IPNB (HR=0.37, 95% CI=0.27–0.52, P-value<0.01), and large duct type (HR=0.40, 95% CI=0.28–0.58, P-value<0.01). HER2, PD-L1, and FGFR2 expression had no significant effect on survival outcome. Multivariable analysis showed only OV IgG positive and concomitant IPNB were significant prognostic factors with adjusted HR=1.66 (95% CI=1.13–2.43, P-value=0.01) and HR=0.48 (95% CI=0.28–0.82, P-value=0.01), respectively (Table 5).
 |
Table 5 Survival Analysis by OV IgG, Histologic Features, and Targeted Gene Expression |
 |
Figure 2 Survival of CCA by serum IgG for OV. |
To assess the clinical significance of OV-associated CCA, OV IgG status were grouped according to correlated factors, intraductal growth type tumor, and HER2 expression. CCA with both HER2 and OV IgG positive cases had a median survival time of 13.4 months (95% CI=11.5–18.6), only one HER2 or OV IgG positive case had a survival time of 19.2 months (95% CI=13.2–29.2), while both HER2 and OV IgG were negative at 29.4 months. Both HER2 and OV IgG positive cases had significantly poor prognostic factors with HR=2.0 (95% CI=1.13–3.5, P-value=0.01) (Figure 3A and B). Pure intraductal type CCA with OV IgG negative cases showed the best median survival time of 102.8 months (95% CI could not be calculated due to the too low event rate), intraductal type with OV IgG positive and other types with OV IgG negative had median survival time of 22.3 months (95% CI=13.0–29.5). Non-intraductal type with OV IgG positive cases had a median survival time of 13.4 months (95% CI=9.2−6.8) and significantly poor prognostic factors with HR=3.65 (95% CI=1.89–7.04, P-value<0.01, Figure 4A and B).
 |
Figure 3 Survival of serum OV IgG positive with HER2 expression. (A) Survival by HER2 expression and (B) Survival by OV IgG combined with HER2 expression. |
 |
Figure 4 Survival of serum OV IgG positive with intraductal growth type. (A) Survival by intraductal type and (B) Survival by OV IgG combined with intraductal type. |
Discussion
OV-associated CCA has been examined in many previous studies based on no clearly defined characterization criteria. Results from our study propose criteria for OV-associated CCA based on CCA with positive IgG for OV. The majority of CCA in Thailand is associated with OV infection with Ov prevalence in some localities of more than 70%. A previous study of the prevalence of serum OV IgG in CCA patients was reported in 1994 in a study of serum IgG against crude somatic OV antigen in four groups of patients, namely, people from Thailand with CCA, or cholecystitis or accident trauma, and the control group who were healthy volunteers from Japan. Serum OV IgG levels were positive in 89% of patients in the CCA group, 69% in the cholecystitis group, and 42% in the accident victim group. No volunteers from the control group had serum OV IgG.15
The reduction of prevalence of OV-associated CCA can be explained by the decreasing levels of prevalence of OV infection. During the past 30 years in Thailand, especially in the northeast region, there have been extremely high levels of OV infection because of “raw attitudes”, that is the eating of raw, partially cooked or fermented fresh water fish culture.16 In 1984, a study of 1651 people in Khon Kaen province, northeast Thailand, showed that the prevalence of OV infection by stool analyses was extremely high, with prevalence as high as 89.5% in all age groups and, alarmingly, 32% in children under 5 years.17 Since then, there have been many studies and specific projects to control OV infection in Thailand which have resulted in the decline of prevalence of OV infection in northeast Thailand to 15.7% in 200918 and 10% in 2019, from a population based study.19
The decline in OV infection in northeast Thailand has resulted in a corresponding decrease of the incidence of CCA, especially in Khon Kaen province, as shown from a database study from the Khon Kaen cancer registry from 1989 to 2013 showing a decreasing incidence rate of CCA since 2002 and a projected stabilization by 2025.20 This study provided the basis foundation for the hypothesis that OV-associated CCA is preventable and a curable cancer by preventing OV infection, appropriate treatment of infected patients, and early screening for CCA. Unfortunately, however, OV infection and OV-associated CCA still remain important health problems in other areas in Thailand as well as other countries of Southeast Asia. For instance, the prevalence of OV infection in many provinces in northeast Thailand remain very high, with some provinces having up to 60% prevalence, and prevalence in Laos PDR for example, has been recorded as 60–90%.18
Histological features of OV-associated CCA have been described in several studies. For instance, a study compared CCA specimens from Khon Kaen, which were all labeled fluke-associated CCA, and archived CCA specimens from Australia in 1993–1996 reported no difference in histological type or morphology, but liver fluke-associated CCA had more P53 over-expression.21 Indirect evidence of the correlation between OV and histological features was studied in 2018, where results showed a strong correlation of the history of frequently used praziquantel and intrahepatic type CCA and a correlation of papillary type CCA with a history of praziquantel usage were also reported.22
Chronic inflammation in large and medium sized bile ducts is a risk factor for CCA arising from peribiliary gland which is classified as large duct type CCA. Large duct type is composed of mucin producing tall columnar cells in a glandular pattern or mucin producing papillary of tall columnar epithelium. Small duct type on the other hand, is composed of non-mucin low cuboidal columnar tumor and shared characters with hepatocellular carcinoma.23 Large duct type CCA is developed from two types of premalignant lesions consisting of IPNB and BilIN, whereas no detectable premalignant lesions have been found to be associated with small duct type tumors.9,24 In our study, large duct type CCA was identified by mucin producing papillary or tall columnar cells and the presence of concomitant premalignant lesions without an IHC marker. The results showed that there was no difference in histological features or bile duct type between CCA with IgG for OV positive and negative groups. The exception being for IgG for OV positive groups which were significantly less likely to be pure intraductal growth type CCA. This, however, was not significant if intraductal growth combined with other types were included. Morphological and histological features alone are unable to distinguish OV-associated CCA. Targeted IHC marker(s) should be used in combination with histological features for duct type, premalignant lesion to comprehensively examine the correlation of OV IgG status and CCA.
HER2 has been shown to have a significant role in the cell proliferation process, therefore, cancer with high expression of HER2 has a poor prognosis such as in breast cancer and gastric cancer. A study has found that global HER2 expression in biliary tract cancer was 26.5%, and that HER2 amplification rate in the HER2 overexpression group was found to be 57.6%.25 Results from our study found that a high overall HER2 expression rate of 61.4%, especially in IgG for OV positive groups resulted in poor prognosis in OV-associated CCA. A limitation in our study, however, was that a test for HER2 amplification could not be performed. We suggest that therapeutic screening for HER2 expression and clinical prospective studies for targeted therapy should be conducted for OV-associated CCA because of a high rate of HER2 amplification in the HER2 overexpression group.
PD-L1 ligands in tumor tissue have roles in the immune evading process and tumor expressed PD-L1 has a correlation with good responses in anti-PD-L1 immunotherapy. Results in our study found high overall PD-L1 expression of 84% which corresponds with other studies that reported PD-L1 expression in CCA of between 70–94%,26,27 whereas many other studies have reported low expressions of PD-L1 in CCA of around 10%.28–30 These variations of PD-L1 expression between studies may result from tumor biology and different antibody clones used in each study. Therefore, no standard protocol for diagnosis of PD-L1 expression and clinical study for immunotherapy in CCA exists and needs to be evaluated further. The FGFR inhibitor is a novel targeted therapy for CCA and, hence, FGFR expression screening may prove beneficial. Previous studies have reported overall FGFR2 expression of 65%31 which corresponds to level we found herein. However, it has been reported that the prevalence of high FGFR2 expression is low and there is extremely low FGFR2 fusion in fluke-associated CCA.32 Nonetheless, FGFR inhibitor therapy in OV-associated CCA requires more comprehensive studies to evaluate its potential usefulness as a targeted therapy.
The results in our study showed good prognosis in CCA with concomitant IPNB, which is consistent with previous studies that reported outcomes after resection of malignant IPNB which had 5 year survival rates of 47–68.8%.33,34 We found that the overall survival of IgG for OV-positive CCA cases was significantly poor compared to the negative group. This finding supports a previous study which reported that fluke-positive CCA had a different mutation pathways and poor prognosis in nature.10 The aggressiveness of CCA in IgG for OV positive cases may be explained by a chronic inflammatory response that induces cellular adaptation to oxidative stress, immune evasion and cellular proliferation. This hypothesis requires further detailed studies. A previous study has reported that HER2 overexpression is an independent prognostic factor in CCA.35 However, in our study HER2 expression alone did not affect survival outcome but, when combined with IgG for OV, HER2 expression in the OV IgG positive group had a significantly poor outcome. In the same way, combined non-intraductal type with IgG for OV positive cases also has poor outcomes, As OV-associated CCA is a very aggressive type of bile duct cancer with extremely poor survival outcomes, further comprehensive studies should be undertaken to determine which treatment strategies are effective against this type of CCA.
Conclusion
In summary, our study showed high prevalence of CCA with IgG for OV positive cases in Thailand which was associated with overexpression of HER2. Moreover, IgG for OV indicated poor survival of CCA patients. Therefore, prevention and eradication of OV infection is mandatory to enable a reduction of the current high levels of prevalence of OV-associated CCA, and clinical studies should be conducted on anti-HER2 and should focus on OV-associated CCA patients.
Acknowledgments
The authors thank Professor Ross H. Andrews for manuscript editing and language correction, Dr. Malinee Thanee and Dr. Manida Suksawat for laboratory consultation, and Miss Supakan Amontailak for general assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Titapun A, Pugkhem A, Luvira V, et al. Outcome of curative resection for perihilar cholangiocarcinoma in Northeast Thailand. World J Gastrointest Oncol. 2015;7(12):503–512. doi:10.4251/wjgo.v7.i12.503
2. Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258(1):129–140. doi:10.1097/SLA.0b013e3182708b57
3. Hirano S, Kondo S, Tanaka E, et al. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci. 2010;17(4):455–462. doi:10.1007/s00534-009-0208-1
4. DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–762. doi:10.1097/01.sla.0000251366.62632.d3
5. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi:10.1056/NEJMoa0908721
6. Luvira V, Nilprapha K, Bhudhisawasdi V, Pugkhem A, Chamadol N, Kamsa-ard S. Cholangiocarcinoma patient outcome in Northeastern Thailand: single-center prospective study. Asian Pac J Cancer Prev. 2016;17(1):401–406. doi:10.7314/APJCP.2016.17.1.401
7. Tesana S, Srisawangwong T, Sithithaworn P, Itoh M, Phumchaiyothin R. The ELISA-based detection of anti-Opisthorchis viverrini IgG and IgG4 in samples of human urine and serum from an endemic area of north-eastern Thailand. Ann Trop Med Parasitol. 2007;101(7):585–591. doi:10.1179/136485907X229068
8. Akai PS, Pungpak S, Chaicumpa W, et al. Serum antibody responses in opisthorchiasis. Int J Parasitol. 1995;25(8):971–973. doi:10.1016/0020-7519(94)00212-7
9. Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22(2):94–100. doi:10.1002/jhbp.154
10. Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017.
11. Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98(2):418–425. doi:10.1038/sj.bjc.6604129
12. Harder J, Waiz O, Otto F, et al. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol. 2009;15(36):4511–4517. doi:10.3748/wjg.15.4511
13. Padthaisong S, Thanee M, Namwat N, et al. A panel of protein kinase high expression is associated with postoperative recurrence in cholangiocarcinoma. BMC Cancer. 2020;20(1):154. doi:10.1186/s12885-020-6655-4
14. Jariwala AR, Oliveira LM, Diemert DJ, et al. Potency testing for the experimental Na-GST-1 hookworm vaccine. Expert Rev Vaccines. 2010;9(10):1219–1230. doi:10.1586/erv.10.107
15. Itoh M, Pairojkul C, Thamawit W, et al. Association of antibodies to Opisthorchis viverrini with hepatobiliary disease in northeastern Thailand. Am J Trop Med Hyg. 1994;51(4):424–429. doi:10.4269/ajtmh.1994.51.424
16. Grundy-Warr C, Andrews RH, Sithithaworn P, et al. Raw attitudes, wetland cultures, life-cycles: socio-cultural dynamics relating to Opisthorchis viverrini in the Mekong Basin. Parasitol Int. 2012;61(1):65–70. doi:10.1016/j.parint.2011.06.015
17. Upatham ES, Viyanant V, Kurathong S, et al. Relationship between prevalence and intensity of Opisthorchis viverrini infection, and clinical symptoms and signs in a rural community in north-east Thailand. Bull World Health Organ. 1984;62(3):451–461.
18. Sithithaworn P, Andrews RH, Nguyen VD, et al. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012;61(1):10–16. doi:10.1016/j.parint.2011.08.014
19. Sornlorm K, Loahasiriwong W, Sithithaworn P, Thinkhamrop W. Influence of geographic, knowledge and behavioral factors on Opisthorchis viverrini infection in the Northeast of Thailand. Asian Pac J Trop Med. 2019;12(11):499–506. doi:10.4103/1995-7645.271289
20. Kamsa-Ard S, Luvira V, Suwanrungruang K, et al. Cholangiocarcinoma trends, incidence, and relative survival in Khon Kaen, Thailand From 1989 through 2013: a population-based cancer registry study [J Epidemiol 29 (5) (2019) 197-204]. J Epidemiol. 2020;30(2):108–109. doi:10.2188/jea.JE20190251
21. Hughes NR, Pairojkul C, Royce SG, Clouston A, Bhathal PS. Liver fluke-associated and sporadic cholangiocarcinoma: an immunohistochemical study of bile duct, peribiliary gland and tumour cell phenotypes. J Clin Pathol. 2006;59(10):1073–1078. doi:10.1136/jcp.2005.033712
22. Luvira V, Kamsa-Ard S, Kamsa-Ard S, et al. Association between repeated praziquantel treatment and papillary, and intrahepatic cholangiocarcinoma. Ann Hepatol. 2018;17(5):802–809. doi:10.5604/01.3001.0012.3140
23. Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27(8):1163–1173. doi:10.1038/modpathol.2013.241
24. Nakanuma Y, Sasaki M, Sato Y, Ren X, Ikeda H, Harada K. Multistep carcinogenesis of perihilar cholangiocarcinoma arising in the intrahepatic large bile ducts. World J Hepatol. 2009;1(1):35–42. doi:10.4254/wjh.v1.i1.35
25. Galdy S, Lamarca A, McNamara MG, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017;36(1):141–157. doi:10.1007/s10555-016-9645-x
26. Sangkhamanon S, Jongpairat P, Sookprasert A, et al. Programmed Death-Ligand 1 (PD-L1) expression associated with a high neutrophil/lymphocyte ratio in cholangiocarcinoma. Asian Pac j Cancer Prev. 2017;18(6):1671–1674.
27. Suleiman Y, Coppola D, Zibadi S, et al. Prognostic value of tumor-infiltrating lymphocytes (TILs) and expression of PD-L1 in cholangiocarcinoma. J Clin Oncol. 2015;33(3_suppl):294. doi:10.1200/jco.2015.33.3_suppl.294
28. Ueno T, Tsuchikawa T, Hatanaka KC, et al. Prognostic impact of programmed cell death ligand 1 (PD-L1) expression and its association with epithelial-mesenchymal transition in extrahepatic cholangiocarcinoma. Oncotarget. 2018;9(28):20034–20047. doi:10.18632/oncotarget.25050
29. Walter D, Herrmann E, Schnitzbauer AA, et al. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology. 2017;71(3):383–392. doi:10.1111/his.13238
30. Zhu Y, Wang XY, Zhang Y, et al. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8(+) T-cell immune responses. Cancer Manag Res. 2018;10:4113–4123. doi:10.2147/CMAR.S172719
31. Raggi C, Fiaccadori K, Pastore M, et al. Antitumor activity of a novel fibroblast growth factor receptor inhibitor for intrahepatic cholangiocarcinoma. Am J Pathol. 2019;189(10):2090–2101. doi:10.1016/j.ajpath.2019.06.007
32. Kongpetch S, Jusakul A, Lim JQ, et al. Lack of targetable FGFR2 fusions in endemic fluke-associated cholangiocarcinoma. JCO Glob Oncol. 2020;6:628–638. doi:10.1200/GO.20.00030
33. Luvira V, Pugkhem A, Bhudhisawasdi V, et al. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. J Gastroenterol Hepatol. 2017;32(2):527–533. doi:10.1111/jgh.13481
34. Kim WJ, Hwang S, Lee YJ, et al. Clinicopathological features and long-term outcomes of intraductal papillary neoplasms of the intrahepatic bile duct. J Gastrointestinal Surg. 2016;20(7):1368–1375. doi:10.1007/s11605-016-3103-5
35. Fernandes VTO, Silva MJDBE, Begnami MD, Saito A. Prognosis of HER2 expression in cholangiocarcinoma when evaluated using gastric cancer methodology of immunohistochemistry. J Clin Oncol. 2015;33(15_suppl):e15203. doi:10.1200/jco.2015.33.15_suppl.e15203
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
