Back to Journals » Journal of Inflammation Research » Volume 14
Serum C1q Levels Have Prognostic Value for Sepsis and are Related to the Severity of Sepsis and Organ Damage
Authors Li H, Chen J, Hu Y, Cai X, Tang D , Zhang P
Received 28 May 2021
Accepted for publication 3 September 2021
Published 10 September 2021 Volume 2021:14 Pages 4589—4600
DOI https://doi.org/10.2147/JIR.S322391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Huan Li, Juanjuan Chen, Yuanhui Hu, Xin Cai, Dongling Tang, Pingan Zhang
Department of Clinical Laboratory, Renmin Hospital of Wuhan University, Wuhan, 430060, People’s Republic of China
Correspondence: Pingan Zhang Email [email protected]
Objective: To explore the clinical application value of serum complement component C1q levels in sepsis.
Methods: The clinical data and laboratory examination data of 320 research subjects (including 132 cases as sepsis group, 93 cases as nonsepsis group and 95 cases as control group) who were diagnosed and treated in Renmin Hospital of Wuhan University from July 2020 to March 2021 were collected. We compared the levels of each index among the three groups and further analyzed the C1q levels of different severity subgroups and different outcome subgroups of sepsis. Afterwards, we explored the correlation between C1q levels and SOFA score, organ damage indexes and coagulation indexes. Finally, the receiver operating characteristic curve (ROC) was used to analyze the prognostic value of C1q in patients with sepsis.
Results: C1q levels were significantly reduced in the serum of patients with sepsis; the level of C1q in the death group was lower than that in the survival group (127.1 mg/L vs 153.2 mg/L, P < 0.05), and the mortality in the C1q decreased group was higher when compared with C1q normal group; in addition, serum C1q levels were correlated with SOFA score, organ damage indexes and coagulation indexes; C1q had a high area under the curve (AUC) for the prognosis of sepsis, and the combination of other indexes can further improve the prognostic value.
Conclusion: Serum C1q levels have potential clinical value for the condition and prognosis of sepsis.
Keywords: C1q, sepsis, infection, organ damage, prognosis
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.1 It poses a serious threat to the health of people all over the world.2–4 Therefore, it is of great significance for us to find laboratory indicators so as to identify sepsis and to predict the development of the disease. In this way we can intervene earlier to increase the survival rate of patients with sepsis. At present, the laboratory index which is most closely related to sepsis is procalcitonin (PCT), which is often used to guide clinical medication.5–7 However, to predict the development of sepsis more accurately, we may need to integrate multiple indicators.
Immune system imbalance plays an important role in the occurrence and development of sepsis.8 One of its notable features is the uncontrolled activation of the complement system. The activation products of complement can induce pro-inflammatory effects, causing inflammatory response disorders. They can also aggravate inflammatory and cause organ failure.9 After complement activation occurs in patients with sepsis, a large amount of complement protein can be activated and consumed. Among them, complement protein is significantly reduced in patients with poor prognosis.10 Complement is an important defense mechanism against bacterial infections. In recent years, there have been more and more studies on the complement system in sepsis. Xie et al found that injection of complement inhibitors released from platelets could reduce complement attack and attenuate liver dysfunction in septic mice.11 And a study from Charité-University Medicine Berlin found that systemic C5a level was elevated in pneumonia patients. Neutralizing C5a can protect against lung and liver injury in mice with pneumococcal pneumonia. Early neutralization of C5a might be a promising adjunctive treatment strategy to improve outcome in community-acquired pneumonia.12
Although there are many studies on the role of complement system in sepsis in recent years, there is only one simple report on the role of C1q in children with sepsis.13 C1q is also an important component of complement system, unlike most other complement proteins derived mainly from liver, C1q is mainly synthesized by macrophages.14 It can regulate a variety of immune cells and plays an important role in maintaining autoimmune tolerance and regulating inflammatory response.15,16 Therefore, we speculate that C1q is also involved in the occurrence and development of sepsis. This article mainly studied the changes of serum C1q levels in sepsis, then further analyzed its correlation with the condition, organ damage and coagulation function, and finally preliminarily assessed the value of C1q on the prognosis of patients with sepsis.
Patients and Methods
Study Population
A total of 320 subjects (including 132 patients in sepsis group, 93 patients in nonsepsis group and 95 cases in control group) were recruited from Renmin Hospital of Wuhan University from July 2020 to March 2021. Referring to the third edition of sepsis expert consensus,1 the inclusion criteria of sepsis group were the Sequential Organ Failure Assessment (SOFA) score ≥ 2 points with definite infection. The sepsis patients were further divided into sepsis group and septic shock group with the specific standards as follows. Patients with septic shock can be identified with a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain MAP≥65 mm Hg and having a serum lactate level >2 mmol/L (18 mg/dL) despite adequate volume resuscitation.1 Then we divided the patients with sepsis into survival group and death group according to the outcome of 28 days after admission. Patients under 18 years old and those who were pregnant and with autoimmune diseases and tumors were excluded. The nonsepsis group was the patients with inflammatory infection but not diagnosed as sepsis, and the exclusion criteria were the same as those in sepsis group. The control group was the subjects who came to our hospital for physical examination during the same period. Those who were under 18 years old, had signs of infection in the previous month or had received antibacterial drugs or other non-preventive drugs for other reasons were excluded. Figure 1 showed the specific research object inclusion and exclusion details.
 |
Figure 1 Diagram of patient selection. SOFA, sequential organ failure assessment. |
This study was reviewed and approved by the Ethics Committee of the Renmin Hospital of Wuhan University (No. WDRY2020-K223), and approved to exempt patients from informed consent.
Data Collection
This study was a retrospective analysis. The WBC, RBC, hemoglobin (Hb), PLT, PCT, C-reactive protein (CRP), serum amyloid A protein (SAA), C1q, AST, ALT, total bilirubin (TBIL), direct bilirubin (DBIL), Urea, N-terminal pro-brain natriuretic peptide (NT-proBNP), creatine kinase MB (CK-MB), cardiac troponin I (cTnI), prothrombin time (PT), thrombin time (TT) and fibrinogen (FIB) levels of the subjects were the first laboratory test results after admission. In laboratory tests, WBC, RBC, Hb and PLT were analyzed by Sysmex XE-2100 automatic blood cell analyzer; serum PCT was detected by cobas 8000 e 801 automatic chemiluminescence immunoassay analyzer produced by Roche; CRP and SAA were detected with the automatic protein analyzer H780-3 produced by Shenzhen Xilaiheng Company; serum C1q, AST, ALT, TBIL, DBIL and Urea were detected with Siemens ADVIA 2400 biochemical analyzer; NT-proBNP, CK-MB and cTnI were detected by Siemens Luminescence Immunoassay Analyzer Centaur XP; PT, TT and FIB are detected by CA7000 automatic coagulation analyzer of Sysmex company. Among them, complement C1q was detected by immunoturbidimetric method. The normal reference value of serum complement C1q level was 197.00±40.00 mg/l, and there was no missing value in serum C1q detection. The detection method of infected pathogens was implemented in strict accordance with laboratory standards, in which the most appropriate identification method was adopted for the identification of bacteria and fungi according to samples from different sources, and the virus was identified by RT-PCR. Table S1 showed pathogen distribution of specimens from different sources.
Statistical Analysis
SPSS 24.0 (IBM, Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA) were used to analyze the experimental data. The single sample Kolmogorov Smirnov method was used to test whether the data of each group conformed to the normal distribution. The data of normal distribution included age, SOFA and C1q; and WBC, RBC, Hb, PLT, PCT, CRP and SAA did not conform to normal distribution. The normal distribution data was expressed by the mean ± standard deviation, the comparison between multiple groups was performed by analysis of variance (ANOVA), and the further pairwise comparison was performed by LSD-t test, Pearson correlation coefficient was used to express the correlation between two groups of data; non-normal distribution data was expressed by median (p25, p75), Kruskal–Wallis H-test was used for comparison between multiple groups, and Mann–Whitney U-test was used for pairwise comparison, using Spearman correlation coefficient to express the correlation between two sets of data. The enumeration data used χ2 test. Take the receiver operating characteristic (ROC) curve for measurement data. For the ROC curve of multiple indicators, binary logistic regression analysis in SPSS 24.0 software was used to get the regression model P of multiple indicators, at the same time, SPSS 24.0 software would calculate the prediction probability of the combined index, and the ROC curve of the joint index can be drawn by using the probability, and finally the ROC curve area under the curve (AUC) could be calculated. AUC between 0.5 and 0.7 indicates poor diagnostic value, while 0.7 to 0.9 for moderate diagnostic value and over 0.9 for high diagnostic value. P < 0.05 indicated that the difference is statistically significant.
Results
Characteristics of Study Population
The characteristics of all subjects were shown in Table 1. The three groups had no significant differences in the distribution of age and gender (P > 0.05). The levels of inflammation indicators like WBC, CRP, SAA and PCT in the sepsis group were higher than those in nonsepsis group and control group (P < 0.05), and the levels of the above indicators in nonsepsis group were also higher than those in control group. On the contrary, the levels of RBC, Hb, PLT and C1q in sepsis group and nonsepsis group were lower than those in control group (P < 0.05), and the levels of PLT and C1q in sepsis group were also lower than those in nonsepsis group. Sepsis patients had significantly higher SOFA scores and the SOFA score in the death group was high when compared with the survival group (P < 0.05). The results showed that Escherichia coli and Candida albicans were the most common pathogens in patients with sepsis. We further analyzed the C1q levels in sepsis group with different pathogen infections (fungi, gram-positive bacteria, gram-negative bacteria, virus, co-infection and undefined). The results are shown in Figure 2A. There was no significant difference in the levels of C1q among patients infected with different pathogens, but they were all lower than those in the control group, and except for virus infection, the C1q level of patients infected with other pathogens was lower than that of the nonsepsis group. In addition, we also grouped and compared C1q levels according to the primary infection sites of sepsis patients. As shown in Figure 2B, the C1q levels of sepsis patients with different primary infection sites were lower than those of control group and nonsepsis group. The distribution of C1q levels sepsis patients with different primary infection sites had no statistical difference.
 |
Table 1 Characteristics of the Study Population |
Analysis of C1q and Septic Shock
Results indicated that the levels of serum C1q in sepsis group and septic shock group were 148.28±35.45 mg/L and 139.34±33.77 mg/L, but there was no statistical difference between the two groups (Figure 3A, P > 0.05). Figure 3B showed the incidence of septic shock in the C1q decreased group and the C1q normal group. We observed that the incidence of septic shock in the C1q decreased group was higher than that in the C1q normal group, but the difference was not statistically significant (45.45% vs 29.55%, 95% CI = 0.233–1.089, P = 0.079, Figure 3B).
Analysis of C1q and Prognosis of Sepsis
We further divided sepsis patients into survival group and death group according to the outcome of 28-day hospitalization. The results showed that the serum C1q levels of the death group were significantly lower than those of the survival group contrarily (Table 1, Figure 4A, P < 0.05). Similarly, we found that the death rate in the C1q decreased group was 42.05%, while the death rate in the C1q normal group was 13.64%, which was significantly lower than the C1q decreased group (95% CI = 0.083–0.568, P = 0.001, Figure 4B).
Correlation Between Serum C1q Levels and SOFA
Then we divided patients with sepsis into two groups according to the levels of SOFA (the grouping cutoff is median value). The levels of C1q in SOFA < 5 group and SOFA ≥ 5 group were 151.20±37.42 mg/L and 138.95±31.74 mg/L, respectively. As the SOFA score increased, the level of C1q showed a decreasing trend. The levels of C1q in SOFA ≥ 5 group were lower than those of SOFA < 5 group (P < 0.05, Figure 5A). Meanwhile, the Pearson correlation analysis found that C1q was negatively correlated with SOFA, and the correlation coefficient is −0.3111 (P < 0.001, Figure 5B).
Correlation Analysis of Serum C1q with Organ Damage and Coagulation Indicators
The levels of serum C1q in patients with sepsis were related to the changes of organ damage and coagulation indicators. Liver function indicators: The C1q levels of the AST elevated group were lower than those of the AST normal group (Figure 6A), and the difference was statistically significant. The results of ALT (Figure 6B), TBIL (Figure 6C) and DBIL (Figure 6D) were the same as above. Renal function: C1q levels of Urea elevated group were lower than those of the Urea normal group (Figure 6E). Cardiac function: The levels of C1q in increased group of NT-proBNP, CK-MB and cTnI were lower than those in normal group of NT-proBNP, CK-MB and cTnI (Figure 6F–H). Coagulation function: The levels of C1q in PLT decreased group were lower than those in PLT normal group (Figure 6I), and the levels of C1q in PT increased group were lower than those in PT normal group (Figure 6J). The trend of C1q level in TT (Figure 6K) and FIB (Figure 6L) was the same.
The Predictive Value of C1q on the 28-Day Outcome of Patients with Sepsis
We have drawn a ROC curve to further analyze the clinical value of serum C1q level in predicting 28-day outcome of sepsis patients (Figure 7). Table 2 showed the analysis results of ROC curve of C1q combined with SOFA score and PCT. AUC comparison: Combination (0.871) > SOFA (0.791) > C1q (0.703) > PCT (0.606). Sensitivity comparison: C1q (83.72%) > SOFA (72.09%) > Combination (58.14%) > PCT (32.56%). Specificity comparison: Combination (93.26%) > PCT (88.76%) > SOFA (75.28%) > C1q (51.69%). Positive likelihood ratio (+LR) comparison: Combination (8.62) > SOFA (2.92) > PCT (2.90) > C1q (1.73). Negative likelihood ratio (- LR) comparison: PCT (0.76) > Combination (0.45) > SOFA (0.37) > C1q (0.31). Youden index comparison: Combination (0.5140) > SOFA (0.4737) > C1q (0.3541) > PCT (0.2532). The cut-off values of C1q, PCT and SOFA were 152.6 mg/L, 90.7 ng/mL and 6 points, respectively.
 |
Table 2 The ROC Analysis of C1q for Predicting the Development of 28-Day Mortality in Sepsis |
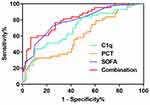 |
Figure 7 ROC curve of 28-day prognostic value of C1q in patients with sepsis. |
Discussion
With the deepening of sepsis research, more and more attention has been paid to the role of immune dysfunction in the pathogenesis of sepsis.17 Complement system is one of the main components of innate immune system against pathogens.18 Further study on the changes of complement system in sepsis will greatly promote the understanding of the pathogenesis of sepsis and open up new ideas for the prevention and treatment of sepsis. In this study, 320 subjects were recruited to analyze the clinical value of important complement component C1q in sepsis. We found that the levels of serum C1q in patients with sepsis were significantly decreased, and C1q was related to the severity of sepsis, organ damage and coagulation function. At the same time, C1q also had potential clinical value in predicting the 28-day outcome of sepsis patients.
The innate immune response, especially neutrophils and macrophages, plays an important role in early anti-bacterial infection.19 C1q is synthesized by macrophages, which suggests that C1q plays an important role in anti-infection. The results of this study showed that the serum C1q levels in patients with sepsis were significantly decreased, which was the same as other complement components C3,20 C4 in patients with sepsis.21 We considered that the complement system was activated in a large amount in order to fight against inflammatory damage in uncontrolled inflammatory state, and serum C1q, C2 and C4 participate in the activation of classical complement pathway, then finally the formation of C3 converting enzyme (C4bC2a) led to the massive consumption of C1q,22 which made the serum levels of C1q decreased. In a previous study, nevertheless, an increase of serum C1q in children with sepsis was observed.13 We believed that there were several reasons: Firstly, the immune system of children was very different from that of adults, which would cause the response and regulation of inflammation to be different; Secondly, we observed that the main pathogen of infection in children with sepsis was mycoplasma in previous studies, and there were reports in the literature indicated that children with mycoplasma infection had elevated other complement components,23 we guessed that C1q was also be elevated. The main pathogens in adult patients with sepsis in this study were bacteria and fungi. The difference of pathogen types may also lead to different responses to external stimuli, which further affected the synthesis and secretion of C1q by macrophages; Thirdly, the inclusion criteria of children with sepsis in the previous study were not exactly the same as this study, that was, there may be differences in the severity of the disease between the two research objects, and the severity of the disease will also lead to different stress reactions of the body.24 In the analysis of pathogens, we found that serum C1q levels in patients with sepsis caused by bacteria, viruses and fungi were all decreased, suggesting that different kinds of pathogens will not affect the consumption of complement components after complement system activation. C1q can combine with bacterial lipopolysaccharide components to activate the complement system, in the case of immune dysfunction, the complement system causes a large consumption of C1q in order to resist inflammatory damage, and finally leads to the reduction of its level.25 Studies suggest that the classical activation pathway of complement induced by fungi plays only a minor role in the mixed infection of multiple pathogens, but if it is a simple fungal infection, such as Candida albicans, the classical activation pathway of fungi by combining with yeast and mycelium cannot be ignored.26 Viral infection also causes a similar depletion effect on complement components of classical activation pathways. Studies have found that C1q levels in serum of HBV infected patients are lower than those in the control group, and often indicate poor prognosis, which is similar to our results.27 It has also been reported that the serum C1q level of COVID-19 patients has been reduced, which also further suggests that viral infection plays an important role in the classical complement activation pathway.28 Our results showed that C1q levels were not significantly related to septic shock. However, previous studies have reported that other complement components are related to the occurrence of shock.29 Our results were not obvious, perhaps that was because we only observed the patients in only one hospital in a certain area. Studies have shown that the serum complement C3 and C4 levels of sepsis patients in the death group are lower than those in the survival group, which suggests that the consumption of complement in sepsis may lead to a poor prognosis.30 Similarly, in this study, we found that the sepsis patients in the death group had lower serum C1q levels than the survival group, and the patients in the C1q decreased group had a higher mortality rate. This may also suggest that we could target complement C1q to formulate a treatment plan for patients with sepsis. Up to now, the US Food and Drug Administration (FDA) and the European Medicines Agency have approved complement-targeted drugs for four diseases that can be used in routine clinical,31 one of which was a mAb-blocking cleavage of C5, named Eculizumab (Soliris), used to prevent PNH hemolysis,32 was also used to treat atypical hemolytic uremic syndrome (aHUS) later,33 the other three were ravulizumab (Ultomiris) and Zilucoplan and Eculizumab.34–36 In sepsis, there were also animal experiments for complement targeted therapy. Studies have shown that injecting C5a neutralizing antibodies into sepsis mice can greatly reduce the organ damage of mice and improve the survival rate of mice,37 the original principle of this scheme was to block a large number of activated C5a from exerting biological functions, so whether this idea can be used for reference in C1q, this still needs more exploration.
SOFA is one of the diagnostic criteria of sepsis in the latest version of sepsis definition, which reflects the severity of sepsis. The higher SOFA score is, the more serious the patient’s condition is. The results showed that a higher SOFA score was always accompanied by a lower C1q level, which suggested that C1q levels may evaluate the severity of sepsis patients to a certain extent. In this study, we found that the serum levels of C1q were correlated with the laboratory indexes of multiple organ damage, which indicated that C1q could reflect the severity of organ injury in patients with sepsis. Thrombocytopenia is a common complication of sepsis, 20–50% of patients with sepsis have thrombocytopenia in ICU.38 The decreased blood coagulation function after sepsis has been attributed to a pathogenesis of the disease itself. Recently, literature has shown that complement deficiencies, such as C3 and factor B, were highly associated with coagulopathy in sepsis.39 In fact, the complement system directly enhanced blood coagulants to enhance the inflammatory response, which played a vital role in thrombosis.40 And so far, sepsis was one of the most studied diseases in which coagulation insufficiency and complement-related inflammation coexist. Our research discovered that the C1q levels of patients with sepsis were related to the coagulation function of patients with sepsis, which was also similar to other complement components and conforms to the theoretical basis above. Finally, we also noticed that serum C1q also has potential clinical value in predicting the outcome of patients with sepsis at 28 days of admission. The combination with PCT and SOFA scores can also further improve the prediction accuracy. Since the case group selected in this paper was sepsis patients in ICU, the cut-off values of PCT and SOFA scores obtained in this study were different from those in other studies.41,42 The limitation was that this study only analyzed the research objects of one single hospital in a limited region, so the cut-off value obtained by ROC curve analysis in this paper cannot be used as a clinical reference value. We suggest that the conditional research group can carry out a larger scale study to further explore the clinical value of C1q in sepsis.
Conclusion
In summary, we found that serum C1q had potential clinical value in sepsis. It can not only indicate the severity of sepsis, but also be related to organ damage and coagulation dysfunction. What’s more, it can also be used to judge the prognosis of patients with sepsis. Due to the small sample size, we only analyzed the serum C1q level of the patients, which is only a preliminary exploration. In view of the decrease of C1q in the serum of patients with sepsis, we consider that C1q plays a protective role in the progress of sepsis. Scholars may learn from the methods of predecessors and use C1q to pretreat septic animals to explore the specific mechanism of C1q participation in sepsis.
Ethical Statement
This study was reviewed and approved by the Ethics Committee of the Renmin Hospital of Wuhan University (No. WDRY2020-K223), and approved to exempt patients from informed consent. The reasons are as follows: our study does not interfere with patients, has no contact with patients, does not involve patients’ personal privacy, does not provide research results to patients, does not serve as auxiliary diagnostic basis, and has no risk to patients. It complies with the provisions of articles 33 and 39 of the measures for ethical review of biomedical research involving human beings (implemented since December 1, 2016) issued by our national health and Family Planning Commission, so it is allowed to exempt the patient from informed consent.
This study only analyzed the previous laboratory test data of patients, did not contact with patients, did not pose a threat to the health of patients, and did not harm the interests of patients. On the premise of exempting patients from informed consent, our study is compliance with the Declaration of Helsinki.
All authors declare that we will keep the information of all research objects strictly confidential and will not infringe the privacy of any research object. All authors declare that our study is compliance with the Declaration of Helsinki. Please refer to the supplement for this statement.
Funding
This research was funded by the National Natural Science Foundation of China (81773444) and the Natural Science Foundation of Hubei Province (2019CFC846).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241–1249. doi:10.1001/jama.2017.13836
3. Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15(5):581–614. doi:10.1016/S1473-3099(15)70112-X
4. Paoli CJ, Reynolds MA, Sinha M, et al. Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889–1897. doi:10.1097/CCM.0000000000003342
5. Battaglia F, Baldoneschi V, Meucci V, et al. Detection of canine and equine procalcitonin for sepsis diagnosis in veterinary clinic by the development of novel MIP-based SPR biosensors. Talanta. 2021;230(12):122347. doi:10.1016/j.talanta.2021.122347
6. Gutiérrez-Pizarraya A, León-García MDC, De Juan-idígoras R, et al. Clinical impact of procalcitonin-based algorithms for duration of antibiotic treatment in critically ill adult patients with sepsis: a meta-analysis of randomized clinical trials. Expert Rev Anti Infect Ther. 2021;13(4):1–10. doi:10.1080/14787210.2021.1932462
7. Honore PM, Redant S, Kaefer K, et al. Procalcitonin is useful for antibiotic deescalation in sepsis and septic shock: beware of some confounder! Crit Care Med. 2021;49(6):e659. doi:10.1097/CCM.0000000000004934
8. Qiu P, Zhou J, Zhang J, et al. Exosome: the regulator of the immune system in Sepsis. Front Pharmacol. 2021;12(10):671164. doi:10.3389/fphar.2021.671164
9. Singla S, Machado RF. Death of the endothelium in sepsis: understanding the crime scene. Am J Respir Cell Mol Biol. 2018;59(1):3–4. doi:10.1165/rcmb.2018-0051ED
10. Abe T, Kubo K, Izumoto S, et al. Complement activation in human sepsis is related to sepsis-induced disseminated intravascular coagulation. Shock. 2020;54(2):198–204. doi:10.1097/SHK.0000000000001504
11. Xie Z, Shao B, Hoover C, et al. Monocyte upregulation of podoplanin during early sepsis induces complement inhibitor release to protect liver function. JCI Insight. 2020;5(13):e134749. doi:10.1172/jci.insight.134749
12. Müller-Redetzky H, Kellermann U, Wienhold SM, et al. Neutralizing complement C5a protects mice with pneumococcal pulmonary sepsis. Anesthesiology. 2020;132(4):795–807. doi:10.1097/ALN.0000000000003149
13. Li H, Chen J, Hu Y, et al. Elevated serum C1q levels in children with sepsis. Front Pediatr. 2021;9(3):619899. doi:10.3389/fped.2021.619899
14. Yang J, Lin P, Yang M, et al. Integrated genomic and transcriptomic analysis reveals unique characteristics of hepatic metastases and pro-metastatic role of complement C1q in pancreatic ductal adenocarcinoma. Genome Biol. 2021;22(1):4. doi:10.1186/s13059-020-02222-w
15. Ghebrehiwet B, Hosszu KH, Peerschke EI. C1q as an autocrine and paracrine regulator of cellular functions. Mol Immunol. 2017;84(9):26–33. doi:10.1016/j.molimm.2016.11.003
16. Yin C, Ackermann S, Ma Z, et al. Publisher correction: apoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med. 2019;25(3):529. doi:10.1038/s41591-019-0378-6
17. Yang Y, Ding Y, Fan B, et al. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J Control Release. 2020;321(10):463–474. doi:10.1016/j.jconrel.2020.02.030
18. Rawish E, Sauter M, Sauter R, et al. Complement, inflammation and thrombosis. Br J Pharmacol. 2021. doi:10.1111/bph.15476
19. Chen L, Zhao Y, Lai D, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018;9(6):597. doi:10.1038/s41419-018-0538-5
20. Yuan Y, Yan D, Han G, et al. Complement C3 depletion links to the expansion of regulatory T cells and compromises T-cell immunity in human abdominal sepsis: a prospective pilot study. J Crit Care. 2013;28(6):1032–1038. doi:10.1016/j.jcrc.2013.09.007
21. Miller EC, Chase NM, Densen P, et al. Autoantibody stabilization of the classical pathway C3 convertase leading to C3 deficiency and Neisserial sepsis: C4 nephritic factor revisited. Clin Immunol. 2012;145(3):241–250. doi:10.1016/j.clim.2012.09.007
22. Thielens NM, Tedesco F, Bohlson SS, et al. C1q: a fresh look upon an old molecule. Mol Immunol. 2017;89(12):73–83. doi:10.1016/j.molimm.2017.05.025
23. Jin Y, Xue J, Ruan M, et al. Expression of Serum miR-155 in children with mycoplasma pneumoniae pneumonia and its role in immunity to Mycoplasma pneumoniae. Infect Drug Resist. 2021;14(9):1273–1281. doi:10.2147/IDR.S273423
24. Gupta R, Gant VA, Williams B, et al. Increased Complement Receptor-3 levels in monocytes and granulocytes distinguish COVID-19 patients with pneumonia from those with mild symptoms. Int J Infect Dis. 2020;99(21):381–385. doi:10.1016/j.ijid.2020.08.004
25. Pont S, Fraikin N, Caspar Y, et al. Bacterial behavior in human blood reveals complement evaders with some persister-like features. PLoS Pathog. 2020;16(12):e1008893. doi:10.1371/journal.ppat.1008893
26. Held K, Thiel S, Loos M, et al. Increased susceptibility of complement factor B/C2 double knockout mice and mannan-binding lectin knockout mice to systemic infection with Candida albicans. Mol Immunol. 2008;45(15):3934–3941. doi:10.1016/j.molimm.2008.06.021
27. Li Q, Lu Q, Zhu MQ, et al. Lower level of complement component C3 and C3a in the plasma means poor outcome in the patients with hepatitis B virus related acute-on-chronic liver failure. BMC Gastroenterol. 2020;20(1):106. doi:10.1186/s12876-020-01258-3
28. Tang Y, Sun J, Pan H, et al. Aberrant cytokine expression in COVID-19 patients: associations between cytokines and disease severity. Cytokine. 2021;143(17):155523. doi:10.1016/j.cyto.2021.155523
29. Cheng TH, Puskarich M, Li X, et al. Circulating complement C3-alpha chain levels predict survival of septic shock patients. Shock. 2020;54(2):190–197. doi:10.1097/SHK.0000000000001502
30. Bao D, Zhang C, Li L, et al. Integrative analysis of complement system to prognosis and immune infiltrating in colon cancer and gastric cancer. Front Oncol. 2021;10(21):553297. doi:10.3389/fonc.2020.553297
31. Garred P, Tenner AJ, Mollnes TE. Therapeutic targeting of the complement system: from rare diseases to pandemics. Pharmacol Rev. 2021;73(2):792–827. doi:10.1124/pharmrev.120.000072
32. Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–1037. doi:10.1056/NEJMoa2029073
33. Brodsky RA. Eculizumab and aHUS: to stop or not. Blood. 2021;137(18):2419–2420. doi:10.1182/blood.2020010234
34. Pugh D, O’Sullivan ED, Duthie FA, et al. Interventions for atypical haemolytic uraemic syndrome. Cochrane Database Syst Rev. 2021;3(3):CD012862. doi:10.1002/14651858.CD012862.pub2
35. Howard JF
36. Cognard N, Gautier-Vargas G, Perrin P, et al. COVID-19 in a kidney transplant recipient treated with eculizumab for atypical hemolytic uremic syndrome: a case report. J Nephrol. 2021;13(4):1–4. doi:10.1007/s40620-021-01057-3
37. Gabarin RS, Li M, Zimmel PA, et al. Intracellular and extracellular lipopolysaccharide signaling in sepsis: avenues for novel therapeutic strategies. J Innate Immun. 2021;12(9):1–10. doi:10.1159/000515740
38. Dewitte A, Lepreux S, Villeneuve J, et al. Blood platelets and sepsis pathophysiology: a new therapeutic prospect in critically [corrected] ill patients? Ann Intensive Care. 2017;7(1):115. doi:10.1186/s13613-017-0337-7
39. Ehrnthaller C, Ignatius A, Gebhard F, et al. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol Med. 2011;17(3–4):317–329. doi:10.2119/molmed.2010.00149
40. Oikonomopoulou K, Ricklin D, Ward PA, et al. Interactions between coagulation and complement–their role in inflammation. Semin Immunopathol. 2012;34(1):151–165. doi:10.1007/s00281-011-0280-x
41. Cabral L, Fernandes M, Marques S, et al. PCT kinetics in the first week postburn for sepsis diagnosis and death prognosis-an accuracy study. J Burn Care Res. 2021;42(3):545–554. doi:10.1093/jbcr/iraa199
42. Spoto S, Cella E, de Cesaris M, et al. Procalcitonin and MR-proadrenomedullin combination with SOFA and qSOFA scores for sepsis diagnosis and prognosis: a diagnostic algorithm. Shock. 2018;50(1):44–52. doi:10.1097/SHK.0000000000001023
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.