Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Serum Alpha-1 Antitrypsin Levels and the Clinical Course of Chronic Obstructive Pulmonary Disease
Authors Takei N , Suzuki M, Makita H, Konno S, Shimizu K, Kimura H, Kimura H, Nishimura M
Received 1 August 2019
Accepted for publication 2 December 2019
Published 10 December 2019 Volume 2019:14 Pages 2885—2893
DOI https://doi.org/10.2147/COPD.S225365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Nozomu Takei,1,* Masaru Suzuki,1,* Hironi Makita,2 Satoshi Konno,1 Kaoruko Shimizu,1 Hiroki Kimura,1 Hirokazu Kimura,1 Masaharu Nishimura1,2
1Department of Respiratory Medicine, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan; 2Hokkaido Medical Research Institute for Respiratory Diseases, Sapporo, Japan
*These authors contributed equally to this work
Correspondence: Masaharu Nishimura
Department of Respiratory Medicine, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, North 15 West 7, Kita-ku, Sapporo 060-8638, Japan
Tel +81 11 706 5911
Fax +81 11 706 7899
Email [email protected]
Purpose: Alpha-1 antitrypsin deficiency is associated with the development of chronic obstructive pulmonary disease (COPD), whereas increased levels of serum alpha-1antitrypsin occur in response to inflammation. The effects of alpha-1 antitrypsin levels on the clinical course of COPD had been unclear. We investigated the association of serum alpha-1 antitrypsin levels with the clinical course of COPD patients based on data from a 10-year prospective cohort study.
Patients and methods: We analyzed 278 COPD patients who participated in the Hokkaido COPD cohort study and who did not meet the criteria for alpha-1 antitrypsin deficiency. We divided the subjects into 3 groups according to quartiles of serum alpha-1 antitrypsin levels at baseline: lower group (141 mg/dL, n = 67). The annual change in forced expiratory volume in 1 s (FEV1) and events of COPD exacerbation were monitored during the first 5 years, and mortality was followed-up during the entire 10 years.
Results: At baseline, the higher group showed lower body mass index; higher computed tomography emphysema score; lower diffusing capacity; higher levels of acute-phase proteins; and higher blood neutrophil counts. Longitudinal analyses revealed that in the higher group, the annual decline in FEV1 was rapid and the 10-year mortality was higher, but there was no association between serum alpha-1 antitrypsin levels and time to first exacerbation.
Conclusion: COPD subjects with higher serum alpha-1 antitrypsin levels were associated with a worse systemic inflammation status and higher 10-year mortality.
Keywords: chronic obstructive pulmonary disease, alpha-1 antitrypsin, inflammation, lung function decline, mortality
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation with chronic lung inflammation, which is activated by exposure to cigarette smoking, aerotoxins, infections, and biomass fume.1 An imbalance between proteases derived from inflammatory cells and epithelial cells, such as neutrophil elastase, and anti-proteases, such as alpha-1 antitrypsin, has been thought to be associated with the pathogenesis of COPD, especially pulmonary emphysema.2 Indeed, alpha-1 antitrypsin deficiency is one of the most important causes of COPD all over the world, except in Eastern Asia, including Japan. On the other hand, alpha-1 antitrypsin is also well known as one of the acute-phase proteins. To date, several types of inflammatory markers, including C-reactive protein (CRP), fibrinogen, and blood neutrophil count, have been reported to be associated with the clinical course of patients with COPD, including exacerbation, lung function decline, poor quality of life (QOL), and mortality.3 However, it has been unclear how alpha-1 antitrypsin, which is an anti-protease and inflammatory marker, is related with the clinical course of COPD patients who are not deficient in alpha-1 antitrypsin.
The Hokkaido COPD cohort study was a carefully designed, multicenter, observational cohort study that primarily aimed to examine the natural history and prognosis of Japanese patients with COPD.4–7 In this present study, we aimed to investigate the association of serum alpha-1 antitrypsin levels with the baseline clinical characteristics and longitudinal clinical course of COPD patients who were prospectively followed for over 10 years in the Hokkaido COPD cohort study.
Materials and Methods
Participants
The recruitment of the COPD patients has been described previously.4–7 Briefly, Japanese patients with COPD were recruited at Hokkaido University Hospital, Sapporo, Japan and its 9 affiliated hospitals from May 2003 to May 2005. All were aged over 40 years and were either current or former smokers with a smoking history of at least 10 pack-years. Subjects with clinically diagnosed asthma were carefully excluded by respiratory specialists.7 During the first year of follow-up, the diagnosis was reconfirmed based on the spirometry criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.1 A total of 279 subjects with COPD (GOLD 1, 26%; GOLD 2, 45%; GOLD 3, 24%; and GOLD 4, 5%) were eligible for subsequent follow-up. One patient who met the criteria for alpha-1 antitrypsin deficiency (ie, serum alpha-1 antitrypsin level <90 mg/dL) was excluded from this study. As a result, we analyzed 278 subjects with COPD (GOLD 1, 26%; GOLD 2, 45%; GOLD 3, 24%; and GOLD 4, 5%) in this study. This study was conducted in accordance with the Declaration of Helsinki and received approval from the ethics committee of Hokkaido University School of Medicine (med02-001) and written informed consent was obtained from all participants.
Study Protocol
On the 1st visit, information on demographics, including sex; age; height; weight; smoking history; medical history; and medications and information on respiratory symptoms were collected. Until the 5th year of the study, information on COPD exacerbations was collected every month. Any change in smoking status, medical history, and pharmacotherapy was monitored. Spirometry before and after bronchodilator inhalation was performed every 6 months. Diffusion capacity testing, chest computed tomography (CT) scan, and health-related QOL assessment by St. George’s Respiratory Questionnaire (SGRQ) were performed every year. The severity of emphysema was visually assessed by 3 independent pulmonologists, according to the modified Goddard scoring system.4,5,8 Exacerbation was defined as worsening or new onset of either (1) 2 major symptoms among increased dyspnea, change in sputum purulence, or increased sputum volume or (2) any 1 major symptom plus any minor symptom of fever, increased cough, or wheezing that required a change in prescription.6
After the 5th year, spirometry after bronchodilator inhalation and diffusion capacity testing was performed every year for those who agreed with the extension of a regular follow-up program until the 10th year. Spirometry data after the diagnosis of lung cancer were not used in this study. Majority of the subjects continued to visit the outpatient clinics for appropriate medical care even if they dropped out from the regular follow-up program. Therefore, telephone interview and/or medical chart review to monitor the annual mortality data for 10 years was needed in only a minority of the study population.
Measurement of Blood Biomarkers
We measured the serum alpha-1 antitrypsin levels at enrolment using the nephelometry method (SRL Inc., Tokyo, Japan). The Japanese clinical criterion for alpha-1 antitrypsin was a level below 90 mg/dL. Plasma CRP and haptoglobin levels at enrolment were measured using Human CardiovascularMAP v1.0 (Myriad RBM, Austin, TX, USA).9 Circulating blood cell counts were measured annually until the 5th year of the study.
Statistical Analysis
The annual changes in post-bronchodilator FEV1 during the first 5 years were estimated by a linear mixed-effects model.5 Differences among the groups were analyzed using one-way analysis of variance (ANOVA), Kruskal–Wallis test with the Mann–Whitney test, or Chi-square test, as appropriate. Bivariate correlations were analyzed using the Spearman’s rank correlation coefficient. The trend among the groups was analyzed using the Jonckheere–Terpstra test. Association between serum alpha-1 antitrypsin levels and the annual changes in post-bronchodilator FEV1 was analyzed using the linear regression model. Exacerbation-free survival and mortality were analyzed using the Kaplan–Meier method with the log-rank test and the Cox proportional hazards model. Statistical significance was defined as p <0.05. All analyses were performed using R version 3.1.2 software (The R Foundation; http://www.r-project.org/).
Results
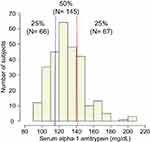
Figure 1 shows the distribution of serum alpha-1 antitrypsin levels. The subjects were classified into 3 groups according to the quartiles of distribution of serum alpha-1 antitrypsin levels: lower group (<116 mg/dL or 25th percentile), middle group (116 to ≤141 mg/dL), and higher group (>141 mg/dL or 75th percentile). Table 1 shows the baseline background characteristics of the 3 groups based on the serum alpha-1 antitrypsin levels. Although age, sex, smoking status, post-bronchodilator FEV1, and QOL score were not significantly different among the groups, the higher group had significantly lower body mass index (BMI) and diffusion capacity and higher emphysema score on CT, compared with those of the other groups. On the other hand, the lower group had milder dyspnea symptoms, which were assessed by the modified Medical Research Council (mMRC) dyspnea scale. Regarding comorbidities, diabetes mellitus was more prevalent in the lower group than in the other groups. The serum alpha-1 antitrypsin levels were weakly correlated with BMI (ρ = −0.21, p < 0.01, Supplementary Figure 1A) and CT emphysema score (ρ = 0.15, p = 0.01, Supplementary Figure 1B).
 |
Table 1 Relationship Between Serum AAT Levels and the Baseline Characteristics of Subjects with COPD |
Since alpha-1 antitrypsin is one of the acute-phase proteins, we analyzed its association with the other inflammatory markers and found that the higher and middle groups had significantly higher plasma CRP and haptoglobin levels compared with those of the lower group (Figure 2A and B), and that the higher group had significantly higher blood neutrophil count compared with that of the other groups (Figure 2C). Furthermore, the serum alpha-1 antitrypsin levels had weak but significant correlations with CRP (ρ = 0.20, p = 0.001, Supplementary Figure 2A); haptoglobin (ρ = 0.18, p = 0.003, Supplementary Figure 2B); and blood neutrophil counts (ρ = 0.16, p = 0.006, Supplementary Figure 2C). In addition, the higher serum alpha-1 antitrypsin group tended to continue to have higher blood neutrophil counts compared to the other groups during the first 5 years of this study (Figure 3).
 |
Figure 3 Line graph of blood neutrophil counts for 5 years according to serum AAT levels. *p <0.05 vs AAT ≤141. Abbreviation: AAT, alpha-1 antitrypsin. |
Table 2 and Figure 4 show that the higher serum alpha-1 antitrypsin group was associated with rapid annual FEV1 decline during the first 5 years (p = 0.047 for ANOVA, p = 0.02 for trend), whereas the linear regression models with or without adjustment for confounders did not show significant association between the serum alpha-1 antitrypsin levels and the annual FEV1 decline (Table 3). Pharmacotherapy with anticholinergics or inhaled corticosteroids was less frequent in the lower group than in the other groups (Table 2). Although there was no association between serum alpha-1 antitrypsin levels and the development of exacerbation (Figure 5A), the higher group clearly had a higher 10-year mortality compared with that of the other groups (p = 0.01, Figure 5B). Multivariate Cox proportional hazards models showed that higher serum alpha-1 antitrypsin levels were significantly associated with 10-year mortality, independent of the BMI or emphysema score on CT at baseline (Table 4). Further, higher serum alpha-1 antitrypsin levels tended to be associated with 10-year mortality, after adjustments for both BMI and emphysema score on CT (Table 4). There were no significant relationships between serum alpha-1 antitrypsin levels and causes of death (Supplementary Table 1).
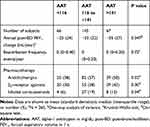 |
Table 2 Relationship Between Serum AAT Levels and the Longitudinal Characteristics of the Subjects with COPD During the First 5 Years |
 |
Table 3 Multiple Regression Analysis for Lung Function Decline During the First 5 Years |
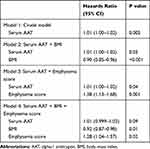 |
Table 4 Multiple Cox Proportional Hazards Models for 10-Year Mortality |
 |
Figure 5 Kaplan–Meier curves according to serum AAT levels: (A) exacerbation-free survival and (B) all-cause mortality. Abbreviations: AAT, alpha-1 antitrypsin; N.S., not significant. |
Discussion
Alpha-1 antitrypsin is a main inhibitor of serine proteases, such as neutrophil elastase. Alpha-1 antitrypsin deficiency is well known to be one of the most important causes of COPD all over the world, except in Eastern Asia where the frequency of functionally deficient SERPINA gene alleles is extremely low.10 On the other hand, alpha1-antitrypsin is also a well-known acute-phase protein whose plasma concentrations increase in response to inflammation. So far, few studies have examined the influence of circulating alpha-1 antitrypsin levels on the longitudinal clinical course of COPD patients without alpha-1 antitrypsin deficiency.11 In this study, we found that higher serum alpha-1 antitrypsin levels were associated with lower BMI, lower diffusion capacity, higher CT emphysema score, and higher systemic inflammatory markers in COPD patients at baseline. In addition, we found that subjects with higher serum alpha-1 antitrypsin groups were associated with rapid lung function decline and higher 10-year mortality. To our best knowledge, this was the first study that investigated the association between serum alpha-1 antitrypsin levels and the longitudinal changes in lung function and mortality in patients with COPD.
Previous studies have reported that inflammatory markers, such as CRP, fibrinogen, tumor necrosis factor-α, interleukin-8, and interleukin-6, were increased in patients with COPD, compared with healthy subjects.3,12,13 In addition, some studies revealed that subjects with elevated inflammatory markers had more frequent exacerbations, worse lung function, and higher mortality, compared with those without inflammation.13–17 Because the serum alpha-1 antitrypsin levels were correlated with the other systemic inflammatory markers in this study, a higher serum alpha-1 antitrypsin level seemed to reflect a worse systemic inflammation status in patients with COPD, which may lead to weight loss and pathologic changes in the COPD lungs, such as pulmonary emphysema. Hepatocytes are the main producers of alpha-1 antitrypsin as well as other acute-phase proteins such as CRP; therefore, the producing activity of hepatocytes supposed to be elevated by chronic systemic inflammatory status. Higashimoto et al reported in a cross-sectional study that serum alpha-1 antitrypsin levels were inversely correlated with BMI in Japanese patients with COPD,18 which was consistent with our results. Furthermore, Senn et al reported that serum alpha-1 antitrypsin levels were inversely correlated with BMI and FEV1 in the general population.19 We have previously shown that lower BMI and more emphysema on chest CT were associated with future rapid lung function decline.5 In this study, we found that subjects with higher serum alpha-1 antitrypsin groups were associated with rapid lung function decline, whereas the linear regression analysis between serum alpha-1 antitrypsin levels and annual FEV1 decline did not show statistical significance, indicating that serum alpha-1 antitrypsin levels do not have strong effect on future lung function decline in COPD patients independent of other cofounders such as BMI and emphysema score on CT. By contrast, subjects with higher serum alpha-1 antitrypsin groups were associated with a higher 10-year mortality, and the serum alpha-1 antitrypsin levels remained significant or exhibited a tendency after adjustment for BMI or emphysema score on CT or both of them (Table 4). Taken together, measurement of serum alpha-1 antitrypsin levels is clinically important in COPD patients even without alpha-1 antitrypsin deficiency because it reflects systemic status and is associated with the clinical course of COPD, including lung function decline and mortality.
On the other hand, it is important to note that although the elevation of serum alpha-1 antitrypsin levels was clearly associated with higher other inflammatory markers, it was not associated with COPD exacerbations in this study. Ingebrigtsen et al reported that elevated plasma alpha-1 antitrypsin levels were associated with increased risk of exacerbations, whereas genetically lower alpha-1 antitrypsin levels (ie, presence of Z-allele in the SERPINA gene) were also associated with increased exacerbation.11 The lack of a relationship between serum alpha-1 antitrypsin levels and exacerbations in this study may have been due to the relatively low frequency of exacerbations in our cohort.6
We also found that subjects with lower serum alpha-1 antitrypsin levels had a higher prevalence of diabetes, despite the absence of a relationship between serum alpha-1 antitrypsin levels and any cardiovascular disease. In fact, several in vivo and in vitro studies have shown that lower alpha-1 antitrypsin levels were involved with the pathogenesis of both type 1 and type 2 diabetes.20,21 Although not yet validated, the mechanism postulated was that alpha-1 antitrypsin protects pancreatic B cells from apoptosis. There were some studies about the efficacy of alpha-1 antitrypsin augmentation therapy for type 1 diabetes.22 Therefore, we should pay attention to the presence of diabetes in patients with low serum alpha-1 antitrypsin levels.
This study had several limitations. First, we did not perform SERPINA gene genotyping. In previous studies from Western countries, serum alpha-1 antitrypsin levels were regulated by the SERPINA gene single-nucleotide polymorphisms, mainly in the Z-allele and S-allele,23 both of which were clearly associated with lower serum alpha-1 antitrypsin levels compared with the normal M-allele. Nevertheless, quite few individuals in a Japanese population had the Z-allele and S-allele.24 Seyama et al reported that among more than 120 million Japanese individuals, the estimated number of patients with alpha-1 antitrypsin deficiency was only 24 (95% confidence interval, 22–27), and that the S- or Z-allele was not observed at all.10 This explains the absence of benefit in genotyping the SERPINA gene in Japanese subjects. Second, we measured the serum alpha-1 antitrypsin levels only once at baseline. Therefore, the intra-individual variations of serum alpha-1 antitrypsin levels were unclear. Since alpha-1 antitrypsin is one of the acute-phase proteins, its serum levels must be affected by acute inflammation. In this study, the blood samples were collected from patients in a clinically stable state which were free from worsening of their symptoms above daily variation or acute febrile illness. Ideally, we should remeasure these inflammatory markers on a later date to minimize the effect of the underlying acute inflammatory situation on our results. However, considering the fact that no subjects had experienced proximate exacerbations, and that higher serum alpha-1 antitrypsin levels at baseline were associated with sustained higher blood neutrophil counts for several years (Figure 3), we suspect that serum alpha-1 antitrypsin levels seem to reflect the chronic inflammatory state in COPD patients instead of an acute inflammatory state. Third, we did not examine alpha-1 antitrypsin levels in lung tissues, including airway epithelial cells; therefore, we could not assess whether a higher serum alpha-1 antitrypsin level was protective for the lung tissues. Finally, the sample size in this study was relatively smaller than that of previous large-scale clinical studies. Nevertheless, this study was carefully designed and performed with very high quality.
Conclusion
In conclusion, higher serum alpha-1 antitrypsin levels were associated with elevations of the other inflammatory markers, lower BMI, more emphysema, rapid lung function decline, and higher mortality in COPD patients who were not alpha-1 antitrypsin-deficient. Serum alpha-1 antitrypsin could be a clinically important for predicting the clinical course of patients with COPD.
Abbreviations
COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; QOL, quality of life; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CT, computed tomography; SGRQ, St. George’s Respiratory Questionnaire; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ANOVA, one-way analysis of variance; BMI, body mass index; mMRC, modified Medical Research Council.
Acknowledgments
The authors would like to thank all of the Hokkaido COPD cohort study investigators (listed below) for patient recruitment and follow-up, as well as Hideka Ashikaga, Ayako Kondo, and Yuko Takagi of the Central Office of the Hokkaido COPD cohort study (Sapporo, Japan) and the staff of Exam Co., Ltd. (Sapporo, Japan) for data management. This study was supported by a scientific research grant from the Ministry of Education, Science, Culture and Sports of Japan; a grant to the Respiratory Failure Research Group from the Ministry of Health, Labor and Welfare, Japan; and from Boehringer Ingelheim, Pfizer, and AstraZeneca.
Author Contributions
NT and MS equally contributed to the acquisition and interpretation of data, statistical analysis, and drafting of the manuscript. SK, KS, Hiroki Kimura, and Hirokazu Kimura acquired and interpreted the data. HM and MN conceived of and designed the study, acquired and interpreted data, and finalized the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Masaru Suzuki has received research funding from GlaxoSmithKline, AstraZeneca, and Novartis. Masaharu Nishimura has received research funding from Boehringer Ingelheim, Pfizer, AstraZeneca, and Novartis. Nozomu Takei reports grants from Boehringer Ingelheim, grants from Pfizer, grants from AstraZeneca, grants from the Ministry of Education, Science, Culture and Sports of Japan, grants from the Respiratory Failure Research Group from the Ministry of Health, Labor and Welfare, Japan, during the conduct of the study. Satoshi Konno reports grants from Astra Zeneca, KYORIN Pharmaceutical Co. Ltd, Novartis, Japan Allergy Foundation, and the Ministry of Education, Culture, Sports, Science and Technology of Japan, during the conduct of the study. Hiroki Kimura report grants from MSD Life Science Foundation, Public Interest Incorporated Foundation, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. From the global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD). 2019. Available from: http://goldcopd.org.
2. Stockley RA. Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med. 1999;160(5 Pt 2):S49–S52. doi:10.1164/ajrccm.160.supplement_1.13
3. Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi:10.1136/thx.2003.019588
4. Makita H, Nasuhara Y, Nagai K, et al. Characterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax. 2007;62(11):932–937. doi:10.1136/thx.2006.072777
5. Nishimura M, Makita H, Nagai K, et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(1):44–52. doi:10.1164/rccm.201106-0992OC
6. Suzuki M, Makita H, Ito YM, et al. Clinical features and determinants of COPD exacerbation in the hokkaido COPD cohort study. Eur Respir J. 2014;43(5):1289–1297. doi:10.1183/09031936.00110213
7. Suzuki M, Makita H, Konno S, et al. Asthma-like features and clinical course of chronic obstructive pulmonary disease. An analysis from the hokkaido COPD cohort study. Am J Respir Crit Care Med. 2016;194(11):1358–1365. doi:10.1164/rccm.201602-0353OC
8. Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol. 1982;33(4):379–387. doi:10.1016/S0009-9260(82)80301-2
9. Suzuki M, Makita H, Ostling J, et al. Lower leptin/adiponectin ratio and risk of rapid lung function decline in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(10):1511–1519. doi:10.1513/AnnalsATS.201408-351OC
10. Seyama K, Hirai T, Mishima M, et al. A nationwide epidemiological survey of alpha1-antitrypsin deficiency in Japan. Respir Investig. 2016;54(3):201–206. doi:10.1016/j.resinv.2015.12.002
11. Ingebrigtsen TS, Marott JL, Rode L, Vestbo J, Lange P, Nordestgaard BG. Fibrinogen and alpha1-antitrypsin in COPD exacerbations. Thorax. 2015;70(11):1014–1021. doi:10.1136/thoraxjnl-2015-207561
12. Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi:10.1371/journal.pone.0037483
13. Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(3):250–255. doi:10.1164/rccm.200605-713OC
14. Duvoix A, Dickens J, Haq I, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68(7):670–676. doi:10.1136/thoraxjnl-2012-201871
15. Groenewegen KH, Postma DS, Hop WC, et al. Increased systemic inflammation is a risk factor for COPD exacerbations. Chest. 2008;133(2):350–357. doi:10.1378/chest.07-1342
16. Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353–2361. doi:10.1001/jama.2013.5732
17. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC
18. Higashimoto Y, Yamagata T, Honda N, et al. Clinical and inflammatory factors associated with body mass index in elderly patients with chronic obstructive pulmonary disease. Geriatr Gerontol Int. 2011;11(1):32–38. doi:10.1111/j.1447-0594.2010.00629.x
19. Senn O, Russi EW, Schindler C, et al. Circulating alpha1-antitrypsin in the general population: determinants and association with lung function. Respir Res. 2008;9:35. doi:10.1186/1465-9921-9-35
20. Fleixo-Lima G, Ventura H, Medini M, Bar L, Strauss P, Lewis EC. Mechanistic evidence in support of alpha1-antitrypsin as a therapeutic approach for type 1 diabetes. J Diabetes Sci Technol. 2014;8(6):1193–1203. doi:10.1177/1932296814547096
21. Sandstrom CS, Ohlsson B, Melander O, Westin U, Mahadeva R, Janciauskiene S. An association between type 2 diabetes and alpha-antitrypsin deficiency. Diabet Med. 2008;25(11):1370–1373. doi:10.1111/j.1464-5491.2008.02584.x
22. Rachmiel M, Strauss P, Dror N, et al. Alpha-1 antitrypsin therapy is safe and well tolerated in children and adolescents with recent onset type 1 diabetes mellitus. Pediatr Diabetes. 2016;17(5):351–359. doi:10.1111/pedi.2016.17.issue-5
23. American Thoracic S, European Respiratory S. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818–900.
24. de Serres FJ, Blanco I, Fernandez-Bustillo E. Estimated numbers and prevalence of PI*S and PI*Z deficiency alleles of alpha1-antitrypsin deficiency in Asia. Eur Respir J. 2006;28(6):1091–1099. doi:10.1183/09031936.00029806
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.