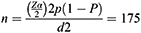Back to Journals » Infection and Drug Resistance » Volume 15
Seroprevalence of Hepatitis B Virus, Hepatitis C Virus, Syphilis and Associated Factors Among Female Sex Workers in Gondar Town, Northwest Ethiopia
Authors Wondmagegn M, Wondimeneh Y , Getaneh A , Ayalew G
Received 2 July 2022
Accepted for publication 5 October 2022
Published 14 October 2022 Volume 2022:15 Pages 5915—5927
DOI https://doi.org/10.2147/IDR.S380952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Mitikie Wondmagegn,1 Yitayih Wondimeneh,2 Alem Getaneh,2 Getnet Ayalew2
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia; 2Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Getnet Ayalew, Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, P.O.Box: 196, Gondar, Ethiopia, Tel +251-918-73-00-13, Email [email protected]
Background: Sexually transmitted infections (STIs) are a public health problem worldwide. Hepatitis B virus infection (HBV), hepatitis C virus infection (HCV), and syphilis are among the STIs. Female sex workers (FSWs) continue to be a high-risk group for STIs due to a variety of factors, including exposure to unsafe sexual practices. Therefore, this study determines the seroprevalence of HBV, HCV, Treponema palladium, and associated factors among FSWs in Gondar town, Northwest Ethiopia.
Methods: A cross-sectional study was conducted on 194 FSWs from March to June 2021 in Gondar town. Socio-demographic and behavioral data were collected using a questionnaire. Five milliliters (5mL) of venous blood was collected and tested for hepatitis B surface antigens, anti-hepatitis C antibodies, and anti-syphilis antibodies using an enzyme-linked immunosorbent assay. Logistic regression, univariate, and multivariate analyses were carried out. A p-value of < 0.05 at a 95% confidence interval was considered statistically significant.
Results: A total of 194 FSWs were included in the study. The seroprevalence rates of HBV infection, HCV infection, and syphilis were 23 (11.9%), 13 (6.7%), and 22 (11.3%), respectively. All three infections were statistically associated with inconsistent condom use (AOR = 1.72, 95% CI: 1.95– 5.07, p = 0.03); (AOR = 10.51, 95% CI: 1.62– 68.26, p = 0.014); and (AOR = 17.3, 95% CI: 4.55– 65.6, p = 0.001). Whereas sex stimulant drug use (AOR = 9.4, 95% CI: 1.002– 88.14), intravenous drug use (AOR = 15.53, 95% CI: 1.9– 127.99, p=0.011), and sex while having a vaginal ulcer (AOR = 5.72, 95% CI: 1.13– 28.9, p=0.035) were all statistically associated with HCV infection.
Conclusion: The prevalence of HBV infection, HCV infection, and syphilis was comparatively higher. Regular screening, health education, and other preventative strategies are advised to lower the STI burden among FSWs.
Keywords: HBV, HCV, syphilis, STIs, FSWs, Gondar town
A Letter to the Editor has been published for this article.
A Response to Letter by Mr Subekti has been published for this article.
Introduction
Sexually transmitted infections (STIs), a group of infections in which the primary mode of transmission is through sexual contact, are public health problems that cause infertility, pelvic inflammatory disease, cancer, and congenital infections.1,2 Because of their work and their behavior, female sex workers (FSWs) are at high risk of acquiring STIs.3 Female sex workers (FSWs) are thought to be at high risk of STI acquisition and transmission due to their sexual behaviors, work, and economic factors.4,5 It is not only that, still, as they are usually in an unfortunate position to negotiate safe sex because of social, economic, cultural, and legal factors, they can transmit STIs to the public through their clients.6
Hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, and syphilis are among the most common STIs, especially among FSWs. Hepatitis B and C viruses cause 96% of viral hepatitis, with a high rate of mortality and morbidity due to viral hepatitis complications.7,8 These viruses can cause acute or chronic hepatitis, which can cause extensive cirrhosis, hepatocellular carcinoma, liver failure, and fatty liver disease. These viruses are major health problems across the globe, particularly in low-income and middle-income countries like Sub-Saharan African countries.9–11
Syphilis is a systemic disease caused by a small organism (spirochetes bacteria) called Treponema palladium (T. palladium), which is a gram-positive bacterium that causes STI and many organ dysfunctions.12,13 It can spread from person to person through unprotected sexual intercourse, skin contact, or mucous membrane contact. It can also be passed from the mother to the fetus.14 The bacteria can remain inactive (dormant) in the body after the initial infection for decades before becoming active. If syphilis is not treated early enough, it can cause severe damage to the heart, brain, or other organs and can be life-threatening. The global burden of syphilis is enormous. In 2016, the WHO estimated 6 million new cases of syphilis. These are mostly seen in Africa.15
The majority of STIs, specifically HBV and HCV, share a common route of transmission. They are mainly transmitted through parenteral routes, unprotected sexual contact, and vertically from mother to child.16 Viral hepatitis is 10 to 100 times more contagious than other STIs, especially HIV.17 The prevalence of STIs in FSWs is higher than the prevalence in the general population.18
The prevalence of some STIs among FSWs in Ethiopia was reported as 0.6% for HCV infection,3 12.5% for Syphilis,3 and 6% to 13.1% for HBV infection.3,5,19 The lack of access to regular screening of STIs among FSWs contributes a lot to the disease’s transmission in resource-limited countries, including Ethiopia.20 Furthermore, age, income, inconsistent condom use, contaminated sharp and piercing materials, condom breakages, use of stimulant drugs, and lack of training on STIs are some of the main factors found in different kinds of literature for STI transmission among FSWs.5,21
Though FSWs are a vital population for the prevention and control of STIs, information about the prevalence of STIs among FSWs is limited in Ethiopia, compared to other groups like pregnant women and blood donors. In addition, little is known about the prevalence and associated determinants of different STIs, particularly HBV, HCV, and T. palladium. Further insight into the characteristics of this population and the associated factors with STI might help target STI control. Therefore, the objective of our study was to determine the seroprevalence of HBV, HCV, T. palladium, and associated factors among FSWs in Gondar town, Northwest Ethiopia.
Methods
Study Area
One of the cities in the Amhara regional state is Gondar Town, where the study was carried out. The town is situated in the Northwest of Ethiopia, some 180 kilometers from Bahir Dar, the seat of the Amhara National Regional State, and 745 kilometers from Addis Ababa, the country’s capital (Figure 1). According to demographic projections made by the Central Statistical Agency of Ethiopia (CSA), Gondar has a total population of 360,600 people, of which 176,593 are men and 184,007 are women.22 The town has more than 60 hotels (level 1, level 2, and level 3), 76 bars and restaurants, and 28 modern cultural nightclubs, according to data from the Gondar Cultural and Tourism Bureau.23
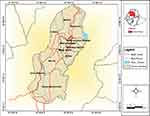 |
Figure 1 Geographical location of Gondar city. Notes: City Profile Gondar (https://www.semanticscholar.org/paper/CITY-PROFILE-GONDAR-Tegegne-Negewo/eaba831e362aacb2b4d25ccee7b9c936a2d8a410. |
Study Design, Period, and Population
A cross-sectional study design was used to conduct this study among FSWs, who have been living in Gondar town working as commercial sex workers for at least 6 months from March 3 to June 30, 2021.
The sample size was estimated using a single population proportion formula, considering the following assumptions: 95% confidence interval (Zα/2=1:96), 13.1% proportion from the previous study,19 and a 5% margin of error.
by adding a 10% nonresponse rate, the final sample size was 194 FSWs.
A total of 194 FSWs were included in the study using respondent-driven sampling from different hotels, bars, and night clubs. They were selected using the snowball method, through a peer recruitment mechanism in which current participants recruit future participants. The initial 3 participants were purposefully chosen on the basis of their strong social network with the FSW population of the town. The initial 3 participants have recruited the next participants by moving from home to home and brought them to a specific site chosen by them for interview and sample collection. This process was repeated until the sample size was achieved.
In this study, FSWs were defined as women who have been living and commercializing sex for the last 6 months in Gondar town. Female sex workers who were over the age of 18 and were willing to participate in this study were included, but those with palpable mental or physical illnesses that prevented them from interviewing and those who were unavailable during the study period were excluded.
Data and Specimen Collection
A standardized questionnaire was utilized to gather data on the sociodemographic, behavioral, and other predisposing characteristics that are linked with the dependent variable after receiving informed and signed consent. Trained data collectors used a serum separator vacationer tube to aseptically collect five milliliters of venous blood. All samples were tagged and delivered in a cool box filled with ice to the Gondar Blood Bank Services laboratory at the University of Gondar Specialized Referral Hospital. Whole blood was centrifuged at 3500 rpm for 5 minutes to separate the serum, which was then kept at −20°C in the fridge until it was tested.
Laboratory Analysis
Using an enzyme-linked immunosorbent test (ELISA), the detection of hepatitis B surface antigen (HBsAg), anti-HCV antibody, and anti-syphilis antibodies was carried out in accordance with the manufacturer’s instructions and standard operating procedures (SOPs). Anti-syphilis antibodies were measured using an Australian DIalab ELISA kit (DIALAB® anti-syphilis, Australian), and HBsAg and HCV antibodies were determined using ELISA assays (AiD HBsAg and anti-HCV antibody, Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.). In the earlier work, the thorough and brief test process and laboratory analysis were provided.24
Quality Control
The process of data gathering was explained to data collectors for one day. By conducting a pre-test on about 10% of the sample size of randomly chosen FSWs in Bahir Dar town, Northwest Ethiopia, the questionnaires were validated and any necessary adjustments were made. The primary investigator assigned a special code to each questionnaire after the completion of data collection and double-checked the information to make sure it was accurate.
Standard Operating Procedure (SOP) was closely followed for the ELISA test, and internal quality control was implemented and carried out in accordance with the manufacturer’s recommendations. In addition to controls supplied by the manufacturers, an internal control was employed for both negative and positive control samples to ensure the regulated performance of our testing procedures. In order to examine the storage conditions of the reagents and the performance capabilities of the method, the reagents and the test method were assessed using those known positive and negative control materials, and all positive samples were repeated.
Ethical Approval
The School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar provided ethical approval under reference number SBMLS/2733. The investigation was also carried out in compliance with the Declaration of Helsinki’s ethical guidelines for using human beings. Verbal information about the study was given to all participants in the study, and their signed agreement was collected. All data were handled with the utmost confidentiality by using codes instead of any personal identifiers and were only utilized for this study. Healthcare providers were informed of the positive findings.
Data Entry and Analysis
Statistical Package for Social Sciences (SPSS) version 21 was used to enter, verify, clean up, and analyse the data. Calculations were made to get the descriptive statistics (means, percentages, or frequencies). To determine the variables connected to HBV, HCV, and T. palladium infections, bivariate logistic regression analysis was made. All sociodemographic, behavioral, and other predisposing characteristic variables with a P-value of 0.2 in the bivariate analysis were considered appropriate for multivariate logistic regression analysis in order to control potential confounding factors. At a 95% confidence level, a p-value of 0.05 or lower was regarded as statistical significance.
Results
Socio-Demographic and Behavioural Characteristics
One hundred ninety-four FSWs were interviewed and have given blood samples for this study. The mean (±SD) age was 26.81 (±5.29) years. Two-fourths (48.5%) of the FSWs were aged between 25 and 34 years. Approximately 6 (3.1%) of the FSWs were previously married, and about 98 (50.5%) of the FSWs were single. Regarding to their educational level, 89 (45.9%) of the FSWs have completed primary school, while 12 (6.2%) of them have completed college and above. Most of the FSWs, 122 (62.9%), were from urban areas and 134 (69.1%) worked as FSWs for about 5 years. More than 30% of the study participants earned within the range of 9001–12, 000 Ethiopian Birr per month. Among the 194 FSWs, 131 (67.5%) used condoms consistently during sexual practice. Also, 180 (98.8%) of them had vaginal sexual practice and 163 (84%) had a routine alcohol consumption.
More than 65% (127) of the FSWs had tattoos, and 12 (6.2%) used intravenous drugs as a sex stimulant. The majority of the study subjects, 173 (89.2%), had no history of blood transfusion and 168 (86.6%) had a history of sexual abuse or harassment. A total of 134 (69.1%) of the participants had up to five sexual partners per day (Table 1).
 |
Table 1 Socio-Demographic, Behavioral Variables Among Female Sex Workers with HBV, HCV, and Syphilis at Gondar Town, Northwest Ethiopia, 2021 |
Prevalence of HBV, HCV, and Syphilis Among Female Sex Workers
In the present study, the overall prevalence of STIs among FSWs was 27.9% (54/194) (95% CI=21.7–34.5). The prevalence of HBV infection, HCV infection, and syphilis infections were 11.9% (23/194) (95% CI=7.2–16.5), 6.7% (13/194) (95% CI=3.6–10.3), and 11.3% (22/194) (95% CI=6.7–16.0), respectively. Three (1.5%) co-infections were found among FSWs. The proportion of both HBV infection and syphilis infections was higher in the age group between 25 and 34 years old (65.2%). Whereas the higher proportion of HCV infection was in the age group between 18 and 24 years old (53.8%). The prevalence of all three infections was high among FSWs from urban areas, with 65.2% having HBV, 53.8% having HCV, and 68.2% having syphilis.
A higher proportion of STIs was observed among FSWs who used condoms inconsistently during sexual intercourse: 56.5% for HBV infection, 84.6% for HCV infection, and 81.8% for syphilis. It was also common among FSWs who had more than five sexual partners each day, with 56.5% infected with HBV, 92.3% infected with HCV, and 54.5% infected with Syphilis. Both HBV, 65.2%, and HCV, 92.3%, infections were higher among FSWs who had used drugs for sexual stimulation.
Factors Associated with HBV, HCV, and Syphilis Among Female Sex Workers
In the multivariate logistic regression model, inconsistent condom utilization during sex was the only variable that had a significant association with HBV infection at a p-value <0.05 (Table 2). Variables like inconsistent condom utilization during sex, intravenous sex stimulant drug use, and sex during genital ulcer had a significant association with HCV infection at a p-value < 0.05 (Table 3). Whereas, inconsistent condom utilization during sex, condom breakage during sex, sex stimulant drug use, and having children had a significant association with syphilis infection at a p-value < 0.05 (Table 4).
 |
Table 2 Bivariate and Multivariate Logistics Analysis of Hepatitis B Virus for Different Possible Risk Factors Among FSWs in Gondar Town, Northwest Ethiopia, 2021 |
 |
Table 3 Bivariate and Multivariate Analysis of Different Possible Risk Factors for Hepatitis C Virus Among FSWs in Gondar Town, Northwest Ethiopia, 2021 |
 |
Table 4 Bivarite and Multivariate Analysis of Syphilis for Different Possible Risk Factors Among FSWs in Gondar Town, Northwest Ethiopia, 2021 |
In the multivariate logistic regression analysis, FSWs who did not always use condoms during sexual practice were more significantly associated with acquiring HBV (AOR = 1.72, 95% CI = 1.95–5.07) than those who did always use condoms during sexual practice (Table 2). Female sex workers who did not always use condoms during sexual practice (AOR = 10.51, 95% CI = 1.62–68.26), used intravenous sex stimulant drugs (AOR = 15.53, 95% CI = 1.9–127.9), and had sex during genital ulcer (AOR = 5.72, 95% CI = 1.13–28.9) were more significantly associated with acquiring HCV than their counterparts (Table 3). Furthermore, female sex workers who did not always use condoms during sexual practice (AOR = 17.3, 95% CI = 4.55–65.6), experienced condom breakage during sex (AOR = 3.30, 95% CI = 0.83–13.3), used sex stimulant drugs (AOR = 0.80, 95% CI = 0.018–0.349), and had children (AOR = 4.58, 95% CI = 1.32–15.88) were more significantly associated with acquiring syphilis than their counterparts (Table 4).
Discussion
In the present study, the seroprevalence of HBV infection, HCV infection, and syphilis was determined among FSWs in Gondar town, and the overall prevalence of STIs was found to be 27.8%. A similar finding was reported from Dessie town, Northeast Ethiopia, at 23.3%.3 This is higher than a report by Ferreira et al from Brazil.25 More specifically, HBV infection, HCV infection, and syphilis prevalence were 11.9% (23/194), 6.7% (13/194), and 11.3% (22/194), respectively. Only three, or 1.5% of the FSWs in our study, had positive results for all STIs (HBV, HCV, and syphilis). The consistency of the results from Dessie town raises the possibility that sociodemographic and socioeconomic parameters, as well as the endemicity of HBV infection, are related.
According to the WHO’s classification of HBV endemicity, Ethiopia is one of the countries having a moderate to high burden. However, studies have shown a variety of findings that have large variations.26 For our 11.9% HBV prevalence among FSWs, a more-or-less comparable finding was published. HBV infection rates were 13.1% in Dessie town, Ethiopia.3 In Hawassa town, 9.2% of the FSWs had HBV, according to Daka et al.5 HBV prevalence was observed to be 12.6% among FSWs in Central Uganda.27 According to earlier studies carried out in Bangui, the Central African Republic, 11.9% of FSWs tested positive for HBsAg.28 Similar HBV prevalence values were recorded in Iran at 11.2%.29 Additionally, Argentina’s HBsAg prevalence was determined to be 14.4%.30 Our HBV prevalence among FSWs, however, was higher than the prevalence in Mekele, Ethiopia (6%),31 the Republic of Congo (4.20%),32 Italy (3.5%),33 South Africa (4%),34 and Rwanda (2.5%).35 Our HBsAg prevalence was lower than that of the finding of the research conducted in Southern Brazil (23.1%)36 and Burkina Faso (18.2%).37
The majority of the disparities in prevalence between studies can be attributed to the endemicity of HBV infection as well as sociodemographic and socioeconomic factors. The prevalence of HBV within the subject is also significantly influenced by the screening, treatment, and prevention programs of the various nations. Additionally, the prevalence of HBV may be influenced by sample size, sampling techniques, and laboratory diagnostic techniques.
In Ethiopia, the seroprevalence of HCV in the community ranged from 0.8 to 2%,7 and among FSWs, 0.6% was recorded.38 In contrast, our research revealed that 13 (6.7%) FSWs had greater anti-HCV antibody values (95% CI; 3.1–10.8). This finding is comparable to the findings of the studies in Burkina Faso, where the rate was 10.6%;36 Iran, where it was 7.4%39 and 8.1%;29 China, where it was 7.1%;40 Brazil, where it was 8.8%;37 Egypt, where it was 3.8%,41 and Argentina, where it was 4.3%.30
The current finding is higher than findings of studies conducted in Dessie, Ethiopia, where the rate was 0.6%;38 Bangui, the Central Republic of Africa, where the rate was 0.8%,28 and Brazil, where the rate was 0.9%.25 Furthermore, it is lower than the South African study, at 16%.34
The number of individuals in our study who tested positive for anti-syphilis antibodies was 22, giving an 11.3% prevalence (95% CI: = 6.7–16). The current prevalence rate was comparable to findings of studies conducted in China, where it was 10.8%40 and 12.54%;42 in Gondar, where it was 10.94%;43 in Dessie, where it was 12.54%;38 and in Brazil, where it was 14.14%.44 It is greater than the finding of the research from the Republic of Congo, where the rate was 2.2%,32 and Burkina Faso, where the rate was 5.6%.45 It was discovered that FSWs from Rwanda had a significantly greater anti-syphilis antibody prevalence, at 51.1%.35 Argentina reported a rate of 45.7%,30 whereas Brazil reported a higher rate of 19.7%.37
Even though the variables that determine the fluctuation of HBV prevalence can be the same as those for syphilis infection, laboratory testing may be the key difference in the prevalence in the Rwandan study. Rapid serological testing was utilized in the Rwandan study to look for syphilis antibodies, however because of the great sensitivity but low specificity of these tests, they were unable to distinguish between true infections and false infections. The real prevalence rate could be impacted by this. In our investigation, the screening test for syphilis infection was carried out using ELISA, which is more precise than a rapid serological test.
This study evaluated the relationships between HBV infection, HCV infection, and syphilis infection among FSWs and independent factors. FSWs who did not always use condoms during sexual activity had a two-fold HBV infection compared to those who did (p=0.03). The prevalence of HCV infection and syphilis was 10 and 17 times higher in female sex workers who did not always use condoms during sexual activity, respectively (p=0.014 and p=0.000, respectively) than those who did. Female sex workers who used sex stimulant drugs had a 15-fold higher HCV infection and a 20% higher syphilis infection, respectively (p=0.011 and p=0.001). Additionally, FSWs who had sex during a genital ulcer were 6 times more likely to contract HCV than FSWs who had no sex during a genital ulcer (p=0.035). Moreover, FSWs with kids had a four-fold higher risk of contracting syphilis than FSWs with no kid (p=0.016).
Our findings, along with findings of those of other studies carried out in Mekele,31 Hawassa,46 and Brazil, support the idea that inconsistent condom usage during sexual activity increases the risk of contracting STIs.44 “Sexualized drug use” is the term for the use of drugs with the goal of increasing sexual encounters. This practice is disproportionately widespread among men, especially those who have sex with other men, and is more prevalent in the United States, Asia, and some European nations, which has an impact on the trend of STI prevalence, notably HIV.47 Meth usage raised HIV positivity by 6 times and syphilis positivity by 2 times, according to a study that looked at the connections between using methamphetamine (meth) and having syphilis and HIV.48
Despite the fact that genital ulcers play a significant role in the spread of STIs, little is known about genital ulcers and how they affect STIs in developing nations, notably in Sub-Saharan Africa, where HIV transmission is widespread. Although genital ulcers, in particular, play a significant role in the facilitation of heterosexual HIV transmission, policymakers have continued to place a strong emphasis on other issues.49 Women were four times more likely to have HCV infection if they had previously experienced genital ulcers, which supports our study.50
In our study, the acquiring of HCV infection and syphilis was positively correlated with FSWs who had used medications for sex stimulation. Similarly, using non-sterile injection equipment, and sharing, and using injectable drugs all raise the risk of STI transmission.36,51,52 In our study, having dependent family members or children was found to be a risk factor for syphilis infection. Contrary to what we discovered, Metaferia et al discovered that FSWs with 1–2 children had a low prevalence of T. pallidum infection.38
Conclusion
The overall prevalence of any STIs, HBV infection, HCV infection, and syphilis was comparatively higher. Female sex workers who were in their thirties showed the highest positivity rate for HBV infection, HCV infection, and syphilis. Inconsistent condom use was a common variable which showed an association with viral hepatitis and syphilis infections. In addition, variables like sex stimulant drug use and having children were significantly associated with one of STIs: HBV infection, HCV infection, and/or syphilis infection. Therefore, screening of FSWs on a regular basis and giving preventive drugs is needed. Giving attention to regular screening for FSWs and preventive measures could be a center to prevent the transmission of STIs to the general population. Indeed, HBV, HCV, and syphilis test and empowered surveillance are mandatory. In this study, there are limitations. Some of the variables are likely to be affected by recall bias. In fact, we have used the ELISA test, one step advanced technique than the routinely used national testing algorithm, but due to inevitability and budget limits, test using molecular methods was not performed.
Abbreviations
Anti- HCV, Anti-Hepatitis C Virus; AOR, Adjusted Odds Ratio; CI, Confidence Interval; COR, Crude Odds Ratio; ELISA, Enzyme-linked Immunosorbent Assay; FSWs, Female Sex Workers; HBsAg, Hepatitis B Surface Antigen; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; SPSS, Statically Package for Social Sciences; STIs, Sexual Transmitted Infections; SOP, Standard Operating Procedure; WHO, World-Health-Organization.
Data Sharing Statement
The manuscript contains all of the data that were produced and analyzed throughout this study.
Acknowledgment
We would like to thank the study participants and the Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. We would also recognize the Central Gondar Health Bureau, Central Gondar Town Police Office, Mahibire-Hiwot Association, Central Gondar female sex worker peer leaders, Central Gondar blood bank, and all data collectors for their unlimited support during data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Newman L, Rowley J, Hoorn SV, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10(12):e0143304. doi:10.1371/journal.pone.0143304
2. Korenromp EL, Rowley J, Alonso M, et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes—estimates for 2016 and progress since. PLoS One. 2012;14(2):e0211720. doi:10.1371/journal.pone.0211720
3. Metaferia Y, Ali A, Eshetu S, Gebretsadik D. Seroprevalence and associated factors of human immunodeficiency virus, treponema pallidum, hepatitis B virus, and hepatitis C virus among female sex workers in Dessie City, Northeast Ethiopia. Biomed Res Int. 2021;2021:1–13. doi:10.1155/2021/6650333
4. Scorgie F, Chersich MF, Ntaganira I, Gerbase A, Lule F, Lo YR. Socio-demographic characteristics and behavioral risk factors of female sex workers in Sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16(4):920–933. doi:10.1007/s10461-011-9985-z
5. Daka D, Hailemeskel G, Fenta DA. Seroprevalence of hepatitis B virus and associated factors among female sex workers using respondent-driven sampling in Hawassa City, Ethiopia. Infect Drug Resist. 2021;14:4301–4311. doi:10.2147/IDR.S332333
6. Remple VP, Patrick DM, Johnston C, Tyndall MW, Jolly AM. Clients of indoor commercial sex workers: heterogeneity in patronage patterns and implications for HIV and STI propagation through sexual networks. Sex Transm Dis. 2007;34(10):754–760. doi:10.1097/01.olq.0000261327.78674.cb
7. Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16(1):761. doi:10.1186/s12879-016-2090-1
8. Cardona-Arias JA, Correa JCC, Higuita-Gutiérrez LF. Prevalence of hepatitis B/C viruses and associated factors in key groups attending a health services institution in Colombia, 2019. PLoS One. 2020;15(9):e0238655. doi:10.1371/journal.pone.0238655
9. Chang CH, Liu CY, Chen SJ, Tsai HC. Hepatitis C virus and hepatitis B virus in patients with schizophrenia. Medicine. 2021;100(22):e26218. doi:10.1097/MD.0000000000026218
10. Razavi H. Global epidemiology of viral hepatitis. Gastroenterol Clin North Am. 2020;49(2):179–189. doi:10.1016/j.gtc.2020.01.001
11. Benzaken AS, Girade R, Catapan E, et al. Hepatitis C disease burden and strategies for elimination by 2030 in Brazil. A mathematical modeling approach. Braz J Infect Dis. 2019;23(3):182–190. doi:10.1016/j.bjid.2019.04.010
12. Kuznik A, Habib AG, Manabe YC, Lamorde M. Estimating the public health burden associated with adverse pregnancy outcomes resulting from syphilis infection across 43 countries in sub-Saharan Africa. Sex Transm Dis. 2015;42(7):369–375. doi:10.1097/OLQ.0000000000000291
13. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97(8):548–562P. doi:10.2471/BLT.18.228486
14. World Health Organization. Prevention and Treatment of HIV and Other Sexually Transmitted Infections for Sex Workers in Low- and Middle-Income Countries: Recommendations for a Public Health Approach. World Health Organization; 2012: 46. Available from: https://apps.who.int/iris/handle/10665/77745.
15. World Health Organization. Report on Global Sexually Transmitted Infection Surveillance 2018. World Health Organization; 2018.
16. Coppola N, De Pascalis S, Onorato L, Calò F, Sagnelli C, Sagnelli E. Hepatitis B virus and hepatitis C virus infection in healthcare workers. World J Hepatol. 2016;8(5):273–281. doi:10.4254/wjh.v8.i5.273
17. Gross G, Tyring SK. Sexually Transmitted Infections and Sexually Transmitted Diseases. Springer Science & Business Media; 2011:896.
18. Cárcamo CP, Campos PE, García PJ, Hughes JP, Garnett GP, Holmes KK. Prevalences of sexually transmitted infections in young adults and female sex workers in Peru: a national population-based survey. Lancet Infect Dis. 2012;12(10):765–773. doi:10.1016/S1473-3099(12)70144-5
19. Bugssa G, Dessalegn B, Dimtsu B, Berhane Y. Prevalence and factors associated with HIV and hepatitis B virus infections among female commercial sex workers in Mekelle, Ethiopia: cross sectional study. Int J Pharm Sci Res. 2015;6(1):135.
20. Mayanja Y, Mukose AD, Nakubulwa S, Omosa-Manyonyi G, Kamali A, Guwatudde D. Acceptance of treatment of sexually transmitted infections for stable sexual partners by female sex workers in Kampala, Uganda. PLoS One. 2016;11(5):e0155383. doi:10.1371/journal.pone.0155383
21. Nasirian M, Kianersi S, Hoseini SG, et al. Prevalence of sexually transmitted infections and their risk factors among female sex workers in Isfahan, Iran: a cross-sectional study. J Int Assoc Provid AIDS Care. 2017;16(6):608–614. doi:10.1177/2325957417732836
22. Abebe A Federal democratic republic of Ethiopia central statistical agency population projection of Ethiopia for all regions at wereda level from 2014–2017; 2021. Available from: https://www.academia.edu/30252151/Federal_Democratic_Republic_of_Ethiopia_Central_Statistical_Agency_Population_Projection_of_Ethiopia_for_All_Regions_At_Wereda_Level_from_2014_2017.
23. Getachew A. Tourism business plan of gondar city; 2021. Available from: https://www.academia.edu/25562622/tourism_business_plan_of_gondar_city.
24. Getie B, Ayalew G, Amsalu A, Ferede G, Yismaw G, Tessema B. Seroprevalence and associated factors of hepatitis B and C virus among pulmonary tuberculosis patients attending health facilities in Gondar Town, Northwest Ethiopia. Infect Drug Resist. 2021;14:3599–3608. doi:10.2147/IDR.S327503
25. da Costa Ferreira-Júnior O, Guimarães MD, Damacena GN, de Almeida WD, de Souza-Júnior PR, Szwarcwald CL. Prevalence estimates of HIV, syphilis, hepatitis B and C among female sex workers (FSW) in Brazil, 2016. Medicine. 2018;97(1 Suppl):S3–S8. doi:10.1097/MD.0000000000009218
26. Yazie TD, Tebeje MG. An updated systematic review and meta-analysis of the prevalence of hepatitis B virus in Ethiopia. BMC Infect Dis. 2019;19(1):1–13. doi:10.1186/s12879-019-4486-1
27. Thokerunga E, Asumprisio A, Dennis S, et al. Hepatitis B infection among commercial sex workers in Lyantonde Town, Central Uganda: prevalence, knowledge and practices. Int Res J Gastroenterol Hepatol. 2020;2020:11–16.
28. Police SMC, Boua-Akélélo NP, Mofini E, et al. Prevalence of HBsAg and antibodies to hepatitis C virus among female sex workers in Bangui. Open J Gastroenterol. 2020;10(06):144. doi:10.4236/ojgas.2020.106015
29. Moayedi-Nia S, Bayat Jozani Z, Esmaeeli Djavid G, et al. HIV, HCV, HBV, HSV, and syphilis prevalence among female sex workers in Tehran, Iran, by using respondent-driven sampling. AIDS Care. 2016;28(4):487–490. doi:10.1080/09540121.2015.1109582
30. Pando MÁ, Berini CA, Bibini M, et al. Prevalence of HIV and other sexually transmitted infections among female commercial sex workers in Argentina. Am J Trop Med Hyg. 2006;74(2):233–238. doi:10.4269/ajtmh.2006.74.233
31. Bugssa G, Dessalegn B, Dimtsu B, Berhane Y. Prevalence and factors associated with HIV and hepatitis B virus infections among female commercial sex workers in Mekelle, Ethiopia: cross sectional study. Int J Pharm Sci Res. 2014;6:135.
32. Niama FR, Loukabou Bongolo NC, Mayengue PI, et al. A study on HIV, syphilis, and hepatitis B and C virus infections among female sex workers in the Republic of Congo. Arch Public Health. 2017;75(1):21. doi:10.1186/s13690-017-0189-5
33. Zermiani M, Mengoli C, Rimondo C, Galvan U, Cruciani M, Serpelloni G. Prevalence of sexually transmitted diseases and hepatitis C in a survey of female sex workers in the north-East of Italy. Open AIDS J. 2012;6(1):60–64. doi:10.2174/1874613601206010060
34. Scheibe A, Young K, Versfeld A, et al. Hepatitis B, hepatitis C and HIV prevalence and related sexual and substance use risk practices among key populations who access HIV prevention, treatment and related services in South Africa: findings from a seven-city cross-sectional survey (2017). BMC Infect Dis. 2020;20(1):1–5. doi:10.1186/s12879-020-05359-y
35. Mutagoma M, Nyirazinyoye L, Sebuhoro D, et al. Syphilis and HIV prevalence and associated factors to their co-infection, hepatitis B and hepatitis C viruses prevalence among female sex workers in Rwanda. BMC Infect Dis. 2017;17(1):1–9. doi:10.1186/s12879-017-2625-0
36. Ouedraogo HG, Kouanda S, Goodman S, et al. Hepatitis B, C and delta viruses’ infections and correlate factors among female sex workers in Burkina Faso, West-Africa. Open Virol J. 2019;13(1):9–17. doi:10.2174/1874357901913010009
37. Schuelter-Trevisol F, Custódio G, da Silva ACB, de Oliveira MB, Wolfart A, Trevisol DJ. HIV, hepatitis B and C, and syphilis prevalence and coinfection among sex workers in Southern Brazil. Rev Soc Bras Med Trop. 2013;10(46):493–497. doi:10.1590/0037-8682-1364-2013
38. Metaferia Y, Ali A, Eshetu S, Gebretsadik D. Seroprevalence and associated factors of human immunodeficiency virus, treponema pallidum, hepatitis B virus, and hepatitis C virus among female sex workers in Dessie City, Northeast Ethiopia. Biomed Res Int. 2021;26(2021):e6650333.
39. Nokhodian Z, Yazdani MR, Yaran M, et al. Prevalence and risk factors of HIV, syphilis, hepatitis B and C among female prisoners in Isfahan, Iran. Hepat Mon. 2012;12(7):442–447. doi:10.5812/hepatmon.6144
40. Fan YG, Liu JJ, Zhang YJ, Dai SY, Li MQ, Ye DQ. HIV, other sexually transmitted infections, and risk behaviors among female sex workers in Liuzhou, China. Int J Gynaecol Obstet. 2015;128(1):18–22. doi:10.1016/j.ijgo.2014.07.024
41. Farghaly AG, Alkassabany YM, El-Ghitany EM. HBV, HCV and HIV among female sex workers; is it a health problem? Sex Relatsh Ther. 2020;35(4):462–477. doi:10.1080/14681994.2020.1778165
42. Dong Y, Zhang H, Wang Y, et al. Multiple abortions and sexually transmitted infections among young migrant women working in entertainment venues in China. Women Health. 2015;55(5):580–594. doi:10.1080/03630242.2015.1022811
43. Moges F, Kebede Y, Kassu A, et al. Seroprevalence of HIV, hepatitis B infections and syphilis among street dwellers in Gondar city, Northwest Ethiopia. Ethiop J Health Dev. 2006;20(3):136–205.
44. de Souza RL, Dos santos madeira LDP, Pereira MVS, et al. Prevalence of syphilis in female sex workers in three countryside cities of the state of Pará, Brazilian Amazon. BMC Infect Dis. 2020;20(1):129. doi:10.1186/s12879-020-4850-1
45. Ouedraogo HG, Meda IB, Zongo I, et al. Syphilis among female sex workers: results of point-of-care screening during a cross-sectional behavioral survey in Burkina Faso, West Africa. Int J Microbiol. 2018;2018:1–10. doi:10.1155/2018/4790560
46. Daka D, Hailemeskel G. Sero-prevalence of hepatitis B surface antigen and associated factors among female sex workers in Hawassa, Ethiopia, 2019. Infect Drug Resist. 2020;14:4301–4311. doi:10.2147/IDR.S332333
47. González-Baeza A, Dolengevich-Segal H, Pérez-Valero I, et al. Sexualized drug use (chemsex) is associated with high-risk sexual behaviors and sexually transmitted infections in HIV-positive men who have sex with men: data from the U-SEX GESIDA 9416 Study. AIDS Patient Care STDS. 2018;32(3):112–118. doi:10.1089/apc.2017.0263
48. Jennings JM, Wagner J, Tilchin C, et al. Methamphetamine use, syphilis, and specific online sex partner meeting venues are associated with HIV status among urban black gay and bisexual men who have sex men. Sex Transm Dis. 2021;48(8S):S32. doi:10.1097/OLQ.0000000000001452
49. O’Farrell N. Genital ulcers, stigma, HIV, and STI control in sub-Saharan Africa. Sex Transm Infect. 2002;78(2):143–146. doi:10.1136/sti.78.2.143
50. Marx MA, Murugavel KG, Tarwater PM, et al. Association of hepatitis C virus infection with sexual exposure in Southern India. Clin Infect Dis. 2003;37(4):514–520. doi:10.1086/376639
51. Shirin T, Ahmed T, Iqbal A, Islam M, Islam MN. Prevalence and risk factors of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infections among drug addicts in Bangladesh. J Health Popul Nutr. 2000;18(3):145–150.
52. Strathdee SA, Abramovitz D, Lozada R, et al. Reductions in HIV/STI incidence and sharing of injection equipment among female sex workers who inject drugs: results from a randomized controlled trial. PLoS One. 2013;8(6):e65812. doi:10.1371/journal.pone.0065812
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.