Back to Journals » International Journal of Women's Health » Volume 12
Seroprevalence of Hepatitis B Virus and Associated Factors Among Pregnant Women Attending Antenatal Care in Public Health Facilities in Jigjiga Town, Eastern Ethiopia
Authors Roble AK , Roba KT , Mengistie B , Abdurke Kure M
Received 15 August 2020
Accepted for publication 25 November 2020
Published 5 January 2021 Volume 2020:12 Pages 1299—1310
DOI https://doi.org/10.2147/IJWH.S276526
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Abdurahman Kedir Roble,1 Kedir Teji Roba,2 Bizatu Mengistie,3 Mohammed Abdurke Kure2
1Department of Midwifery, College of Medicine and Health Sciences, Jigjiga University, Jigjiga, Ethiopia; 2College of Health and Medical Sciences, School of Nursing and Midwifery, Haramaya University, Harar, Ethiopia; 3College of Health and Medical Sciences, Department of Environmental Health, Haramaya University, Harar, Ethiopia
Correspondence: Mohammed Abdurke Kure
College of Health and Medical Sciences, School of Nursing and Midwifery, Haramaya University, P.O. Box: 235, Harar, Ethiopia
Tel +251919231736
Fax +251256668081
Email [email protected]
Background: Hepatitis B virus (HBV) remains a major global public health problem affecting millions of people across the world. The risk of developing a chronic hepatitis B virus infection is affected by the age at the time of acquiring infection. For instance, around 95% of these infections are acquired during the perinatal period. Although evidences indicate the wider effects of hepatitis B virus and its negative consequences, there are limited studies and a scarcity of data in Eastern Ethiopia. Therefore, this study was aimed at determining seroprevalence of HBV and associated factors among pregnant women attending antenatal care in the public health facilities of Jigjiga town, Eastern Ethiopia, from March 4 to April 4, 2019.
Methods: A facility-based cross-sectional study was employed among pregnant women in the public health facilities of Jigjiga town, Eastern Ethiopia. A total of 589 pregnant women were enrolled in the study using a systematic sampling technique. Data were collected using a pretested interviewer administered questionnaire. Five milliliters of venous blood samples was collected and tested for HBV using ELISA diagnostic test. The collected data were entered in to Epidata version 3.1 and exported to SPSS version 20 for statistical analysis. Descriptive statistics was carried out using frequency tables and summary measures. Multivariable analysis was done to identify the true effects of the selected predictor variables on the outcome variable after controlling for possible confounders. Statistical significance was declared at p-value < 0.05.
Results: Overall, 8.5% (95% CI: 6.5– 10.7) of the study participants were seropositive for HBsAg. Having any surgical history [AOR = 3.41, 95% CI (1.26– 9.24)], family history of HBV [AOR = 4.96, 95% CI (2.11– 10.60)], history of sharing sharps [AOR = 2.78, 95% CI (1.13– 6.83)] and having multiple sexual partners [AOR = 6.12, 95% CI (2.12– 17.64)] were significant predictors of HBV infection.
Conclusion: The seroprevalence of HBV was relatively high in this study area. Having a history of surgery, family history of hepatitis, history of sharing sharps and multiple sexual partners were significantly associated with HBV infection. Therefore, health information dissemination and awareness creation on mode of transmission of HBV are very crucial.
Keywords: HBV, associated factors, ANC, pregnancy, Eastern Ethiopia
Introduction
Viral hepatitis is the principal cause of chronic liver disease, liver cirrhosis and hepatocellular carcinoma in the world.1 There are five common causes of viral hepatitis out of which three can cause persistent infection and chronic hepatitis such as hepatitis B virus (HBV), hepatitis C virus (HCV) and hepatitis D virus (HDV). The other two viruses such as hepatitis A and hepatitis E can cause acute, but self-limited disease.2 Hepatitis B and C viruses are the top two most serious and fatal forms of chronic hepatitis which cause around 96% of hepatitis related deaths.3 Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). It is a major global health problem that causes chronic infection and puts people at high risk of death from cirrhosis and liver cancer.4 HBV has three different antigens (HBsAg, HBeAg and HBcAg). Both HBsAg and HBeAg can be detected in the serum, while HBcAg can only be detected in the liver tissue of persons with acute or chronic HBV infection.5 Hepatitis B surface antigen is used to diagnose and monitor the progress of HBV infection while HBeAg is an indication of high HBV infectivity.1
According to the estimates of the World Health Organization (WHO), viral hepatitis was considered as one of the public health challenges. In 2015 alone, approximately 1.34 million deaths and mortality from viral hepatitis are comparable to annual deaths caused by tuberculosis and higher than those caused by HIV and malaria.4 Globally, the prevalence of HBV infection in the general population is 3.5% and the geographical distribution of HBV is the highest in WHO western Pacific and Africa regions with a prevalence of 6.2% and 6.1% respectively.6
Worldwide, approximately 65 million women of reproductive age are infected with HBV and 9 in every 10 mothers with hepatitis B infection transmit the disease to their babies at or around the time of birth.3,4,7 Similarly, other study findings revealed that 90% of the neonates born to HBV infected mothers can develop chronic hepatitis B if they do not receive hepatitis B immune globulin and hepatitis B vaccine at the time of birth.1 Researchers have also reported that the possibility of developing chronic hepatitis B infection among individuals who acquire the infection during the perinatal period was around 90%, but only 5% was reported in adulthood.8 Moreover, researchers have indicated HBV infected pregnant women are a potential source of infection for their sexual partners as well as for health care providers during labor and delivery.9 The infection of hepatitis B virus during pregnancy can cause serious obstetrics complications such as miscarriage, preterm labor, gestational diabetics, and low birth weight.10−12
Further, researchers have found that the burden of hepatitis HBV can be reduced if certain interventions are accessibly used. For instance; screening of HBV for all pregnant women, universal HBV birth dose within one day of delivery and hepatitis B vaccination are effective methods and can decrease mother to child transmission of the disease by 90%.13 However, HBV birth dose and vaccine coverage are still low in most developing countries.14,15 For instance; in Africa, only 11 countries have incorporated the HBV birth dose vaccine as part of a routine expanded program of immunization (EPI). In contrast, currently all East African countries are not implementing this EPI program.16 Although WHO recommended routine antenatal screening for HBV, vaccination of HBV birth dose and universal hepatitis B vaccination are still lacking in Ethiopian public health facilities.8,17,18 However, some private owned health facilities are currently providing HBV vaccination which is expensive for the community at large.19,20
In Ethiopia, national representing data on seroprevalence of HBV in the general population including pregnant women is limited. However, press conferences released by the ministry of health indicated that the prevalence of HBV is about 8% in the general population.21 Previous studies conducted in different parts of Ethiopia indicated the seroprevalence of HBV as 8.4% in Dire Dawa, 6.9% in Oromia, 6.3% in Harar, 5.5% in Tigray and 3.5% in Southern Nation Nationalities and Peoples. Moreover, researchers have also reported the seroprevalence of HBV in pregnant women is associated with a history of blood transfusion, any surgical procedural, dental extraction, abortion, genital mutilation, noise and ear piercing.22–26 In contrast, one similar study conducted in Southern Ethiopia showed that no factor was significantly associated with the seropositivity of HBV in pregnant women.27
In the Somali region, particularly in Jigjiga town, although some health facilities are currently screening HBV for pregnant women as a routine test, data related to seroprevalence of HBV are limited. Therefore, this study aimed to fill these gaps by collecting the primary data from pregnant women and to investigate the seroprevalence of HBV and its associated factors among pregnant women who attended ANC in public health facilities of Jigjiga town, Eastern Ethiopia.
Methods
Study Setting, Period and Design
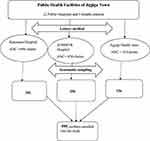
A facility based cross-sectional study design was employed among pregnant women attending antenatal care at the public health facilities of Jigjiga town from March 4 to April 4, 2019. Somali Regional state is one of the nine regions of the Federal Democratic Republic of Ethiopia. Geographically it is situated in the eastern Ethiopian lowlands and has boundaries with Djibouti, Somalia, and Kenya, locally bordering with Oromia and Afar regions.28 Regarding health facilities, the town has one public referral hospital and one general hospital, 3 health centers, and 20 health posts. There is one private general hospital, 27 higher clinics, and 10 medium clinics. The study was conducted at Jigjiga University Sheik Hassen Bare Referral Hospital (JUSYRH), Karamara hospital (KH), and Jigjiga health center from March 4 to April 4, 2019.
Study Population, Inclusion and Exclusion Criteria
Eligible pregnant women who attended ANC services at randomly selected public health facilities of Jigjiga town during the data collection period, whose pregnancy was confirmed by clinical history and physical examination or an obstetric ultrasound scan were enrolled in to study. Those pregnant women who were unable to provide appropriate information because of severe disability and critical illness, and those who came for another ANC visit for the second time during the data collection period were excluded from the study.
Sample Size Determination and Sampling Techniques
The minimum required sample size of this study was determined by using Statistical Software Epi-info version 7.0 by considering the following assumptions. The proportion of hepatitis B virus (p = 6.3%) was taken from a previous study report in Harar town, Eastern Ethiopia,24 precision or degree of error 2%, 95% confidence level and 5% contingency for non-response rate, the final sample size was 595. A systematic sampling technique was used to select eligible study participants. First, Jigjiga town was selected purposely. In Jigjiga town, currently there are five public health facilities that are providing ANC services. From the total of five health facilities, three public health facilities (Two public hospitals and one health center) were randomly selected (using a lottery method). According to the previous information obtained from 6 months ANC followers’ registry, an estimated 1451 pregnant women visit these public health facilities. Accordingly, the total sample size was proportionally allocated for each health facility based on ANC client load, obtained from ANC registry of each health facility. Eligible pregnant women attending ANC clinic at the time of data collection were carefully chosen using a systematic sampling method (Figure 1). To decide the interval (k) estimated total number of ANC attendants was divided to yje allocated sample size of the study, ie, K = N/n, where N = total ANC attendants, n = required sample size, K = sampling interval. Thus, K = 1451/595 = 2.4 ≈ 2, so that every 2nd eligible pregnant woman was enrolled and interviewed until the required sample size was achieved.
Data Collection Methods and Procedures
The data were collected using a pretested interviewer administered questionnaire that was adapted from the Ethiopian Demographic and Health Survey (EDHS) data collection tools.29 The questionnaires were initially prepared in English and then translated into Somali language. Then, the information on variables such as socio-demographic characteristics; risky socio-cultural behavior and practices, health care related factors were collected.
Data Collectors, Blood Sample Collection and Procedure
Three midwives and three senior laboratory technologists (one midwife and one laboratory technologist for each health facility) were recruited in the respective ANC clinics and laboratory departments. Similarly, one Bachelor of Science (BSc) in midwifery and one senior laboratory technologist were also assigned to respective health facilities in order to monitor and supervise the data collection processes. In addition, one senior laboratory technologist was employed to investigate HBsAg serum using a HEUMAN ELISA machine. The 5 mL of venous blood was drawn under aseptic conditions in disposable vacutainer tubes by experienced laboratory personnel. These tubes were labelled and processed at the time of sample collection. The blood samples taken from the participants were centrifuged at 3000 revolutions per minute (RPM) for at least 15 minutes at room temperature. All serum samples had been tested for HBsAg using the wondfo rapid test method according to the manufacturer’s instructions. Based on the result screened women received the result as usual. The leftover serum collected in to other plain tubes using micropipette, was then stored at −20 °C at respective study health facilities (JUSRH, KH, and JHC) until transported to regional blood banks to perform the ELISA test.30
Blood Sample Processing and Testing
Five mL of venous blood were collected in plane tubes under aseptic conditions from a peripheral vein by experienced laboratory personnel from all eligible pregnant women consecutively. Routine standard operating procedures for sample collection were strictly followed. The blood samples were taken from the individuals and centrifuged at 3000 revolutions per minute (RPM) for at least 15 minutes at room temperature.30,31 The rapid HBsAg was performed to deliver the women’s results immediately. Then, left over serum blood was separated using a micropipette in to another sterilized plain tube, and kept frozen at −20 °C until it was transported from each health facility. Lastly, the collected serum was sent to the blood bank of the regional laboratory using an ice box to test the serum for the presence or absence of HBsAg using enzyme-linked immunosorbent assay (ELISA Human type, Germany product) based on the manufacturer’s manual instructions.
Variables and Measurements
Dependent Variable
In this study, the outcome variable of interest was serostatus of hepatitis B virus. Independent variables: the explanatory variables were socio-demographic characteristics such as: maternal age, marital status, educational level, family income, ethnicity, religion, occupation, gravidity and parity were taken as socio-demographic variables. Health care related characteristics like: surgical procedures, working in hospital, blood transfusion and dental extraction were taken as health related variables. Risky behavior and practice related characteristics such as: body tattooing, nose piercing, ear piercing, genital mutilation, abortion and sharing sharps materials, contact with infected person, polygamous marriage and multiple sexual partners were taken as risky behaviors and practice variables.
Rapid HBsAg Screening Test
Rapid HBsAg screening test has a sensitivity of 99.8% and specificity of greater than 99.7%.30 It is a qualitative, solid phase, two-site sandwich immunoassay for the detection of HBsAg in serum. The membrane was precoated with anti-HBsAg antibodies on the test band region and anti-mouse antibodies on the control band region. During testing, the serum sample reacts with the dye conjugate (mouse anti HBsAg antibody colloidal gold conjugate) that was coated on the test strip. Then, the mixture by capillary action reacts with anti-HBsAg antibodies on the membrane and generates a red band. Presence of this red band indicates a positive result while its absence indicates a negative result.
ELISA Method Test
WANTAI HBV diagnostic kit which has a sensitivity of 100% and specificity of 99.92%.31 It uses an antibody sandwich ELISA method in which polystyrene micro-well strips are precoated with monoclonal antibodies specific to HBsAg. A patient’s serum sample was added to the micro wells together with a second antibody conjugated with horseradish peroxidase (HRP) and formed in case of the presence of HBsAg in the sample, which is captured on the solid phase. The amount of color was measured as it was proportional to the amount of antigen in the sample. Wells containing samples which were negative for HBsAg remain colorless. Every procedure was followed according to the manufacturer's instructions. The standard procedures of HBsAg and AiDTM-HBsAg ELISA tests were performed. First reactive results were repeated using AiDTM HBsAg ELISA to declare the results as a positive.31
Operational Definitions
Hepatitis B surface antigen (HBsAg):- A marker presents in persons who are currently infected with HBV (ie, persons with both acute and chronic infection).32 Prevalence:- Is the number of cases of a disease in a specific place at a specific time.33 Seronegative:- Is the status of an individual who gives a negative reaction to a serological test.33 Seropositive:- Is the status of an individual who gives a positive reaction to a serological test.34 Risky behavioral and practice factors: - Refers to some of the behavioral malpractice and some of the social and cultural activities of the subjects that exposed them to the risk of acquiring HBV infection from different sources like abortion in the past, ear or nose piercing, contact with family members during visiting and caring of the sick in the past.35,36
Data Quality Control
First, the data collection tools were prepared in English versions. Then, they were translated to a local language (Somali language) that can be understandable by study participants. Finally, the questionnaires were translated back to English versions to ensure their consistence with the previous initial version. Data collectors, along with supervisors, were trained on the data collection procedure. The questionnaire pretest was conducted on 5% (30 samples) before the actual data collection process in another health center in the town. The result was used to correct the questionnaires. The collected data were checked by supervisors and the principal investigator on a daily basis for completeness and consistency. Codes were given for the completed questionnaires. Double data entry verification was done. Serological data quality was assured following all health facilities' Standard of Procedure (SOP). Standardized procedure was strictly followed during the blood sample collection, storage, and analytical process. Positive and negative controls were run according to the test manual and quality assurance were followed.
Data Processing and Analysis
The collected data were checked, coded and entered into Epi-data version 3.1 and exported to SPSS version 20 for analysis. Descriptive statistics was done using frequencies tables. Bivariable logistic regression analysis were carried out using cross tabulation to see the association between HBsAg serostatus and independent variables. Variables having a p-value less than 0.25 were included in the multiple logistic regression analysis. The multivariable logistic regression was done to control for possible confounders and identify the true effect of the selected predictor variables. The model adequacy was checked using Hosmer and Leme show goodness of fitness tests. Finally, the strength of associations between outcome and predictor variables was assessed using adjusted odds ratio (AOR) with 95% confidence and the significance of the association was declared at a p-value of less than 0.05.
Ethical Consideration
An ethical clearance was obtained from the Institutional Health Research Ethics Review Committee (IHRERC) of Haramaya University, College of Health and Medical Sciences. A copy of the ethical letter was submitted to Jigjiga University Institutional Review Board of College of Medicine and Health Science and Somali regional health bureau. Official letters were written to Jigjiga University Sheik Hassan Yabare Referral Hospital and the Somali regional health bureau. Somali regional health bureau wrote supporting letters to Karamara Hospital and Jigjiga health center. The informed, voluntary, written and signed consent was obtained from each study participant. In addition, informed, voluntary, written and signed assent was also obtained from the guardians and parents if a mother’s age was less than 18 years. Participation in the study was on a voluntary basis and participants were informed about their right to withdraw/refuse at any stage of the study if they did not want to participate. Moreover, confidentiality of the information was assured. The data collectors interviewed the participants in separated areas and the testing was done free of charge.
Results
Socio-Demographic Characteristics of Study Participants
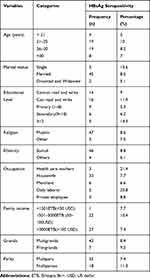
A total of 589 pregnant women attending ANC were enrolled in to the study with a response rate of 98.8%. The participant's ages ranged between 17 and 42 years with a mean and standard deviation of 26.9 and ±4.85 respectively. About 239 (40.6%) women were in the age group of 25–29 years. About 169 (28.7%) of the women attended primary school and only 35 (5.9%) of the women attended above grade 12. The majority, 426 (72.3%), of the women were housewives and only 14 (2.5%) women were health care workers (Table 1).
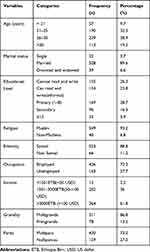 |
Table 1 Socio-Demographic Characteristics of Pregnant Women Who Attended ANC Follow Up in Public Health Facilities of Jigjiga Town, Eastern Ethiopia (N=589), April 2019 |
Health Related and Risk Behaviors of Study Participants
From the 589 pregnant women who participated in the study, only 14 (2.7%) participants had a history of ever working in health institutions. Around 458 (77.8%) had a history of ear piercing and the majority, 477 (81%) of the women had a history of circumcision or FGM. Out of the total 589 study participants, 46 (7.8%) women had a history of contact with an infected person in the family and 44 (7.5%) of the women shared sharp materials (Table 2).
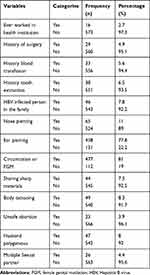 |
Table 2 Health Related and Risk Behavior of the Pregnant Women Who Attended ANC Follow Up in Public Health Facilities of Jigjiga Town, Eastern Ethiopia (N=589), April 2019 |
Seroprevalence of HBV Among Pregnant Women Attending ANC Services
Of 589 pregnant women tested for HBsAg, 50 (8.5%) were seropositive for HBV (95% CI, 6.5–10.7). The highest seroprevalence of HBsAg was observed among the 20–24 years age group (11.2%) and lowest (3.1%) in the 15–19 years age category. The prevalence of HBsAg was highest in those who were health care workers (21.4%) followed by those who working as daily laborers (20%). Regarding education status, seroprevalence of HBV was highest among those who attended above 12 grades (14.3%) and lowest in those who attended primary school (5.3%). The seroprevalence of HBV was 9% and 8.4% in primigravida and multigravida respectively (Table 3).
Factors Associated with Seroprevalence of HBV
Bivariable and Multivariable Analysis
In bivariable analysis, history of surgery, HBV infected person in the family and sharing sharp materials, body tattooing and ever had multiple sexual partners were significantly associated with HBsAg seropositivity. However, other predictors were selected as candidate variables for multivariable analysis when a p-value <0.25. However, in the final model of multivariable analysis: Having a surgical history, a HBV infected person in the family, sharing sharp materials and history of multiple sexual partners were significantly associated with seropositivity of HBsAg. The odds of having HBV infection was about 3 times higher among pregnant women who had a history of surgical procedures [AOR = 3.01, 95% CI (1.2–8.00)] compared to those who had no history of surgery. Having a family history of HBV infection meant 5 times the chance to have HBsAg seropositivity [AOR = 4.96, 95% CI (2.11–10.60)] when compared to their counterparts. Mothers who shared sharp materials were 3 times more likely to be infected with HBV than those who did not share sharp materials [AOR = 2.78, 95% CI (1.13–6.83)]. The odds of seropositivity for HBsAg was about 6 times higher among pregnant women who had multiple sexual partners [AOR = 6.12, 95% CI (2.12–17.64)] when compared to those who had not had multiple sexual partners (Table 4).
Discussion
In this study, the overall seroprevalence of HBV infection among pregnant women was 8.5%. This study also revealed that having a history of surgery, family history of hepatitis B virus, history of shared sharp materials and having a history of previous multiple sexual partners were significantly associated with seropositivity of HBsAg. In this study, the seroprevalence HBV is relatively in line with previous studies such as: 8.4% in Dire Dawa, Eastern Ethiopia,22 7.8% in Hawassa, Southern Ethiopia,27 6.9% in Deder hospital, Eastern Ethiopia,23 8.1% in Mekelle, Northern Ethiopia,37 7.2% in Yirgalem, Southern Ethiopia38 and 8.5% from Thailand.39 In contrast, higher seroprevalence of HBV was observed in similar study populations in different parts of the world. For instance; the current study finding is considerably higher than other previous study reports such as: 2.26% from Bulgaria,40 2.78% from Pakistan,41 2.04% from India,42 4.6% from Nigeria43 and 3.5% from Dawuro, Southern Ethiopia.26 These differences in the seroprevalence might be due to differences in cultural and behavioral characteristics of the pregnant women. In contrast, the finding was lower than the reports from Juba Teaching Hospital, South Sudan 11%,44 16.6% in Nigeria45 and 9.5% in Ghana.46 This difference in estimates might be attributed to the time gap between study periods, geographical setting of study population and difference in sample size of the study.
In this study, the final model of multivariable analysis showed that the odds of being positive for HBV infection was 3 times higher among pregnant women who had a history of surgical procedure compared to those who had no history of surgery. This result is in agreement with the study report from Harare,24 Addis Ababa,47 Bahir Dar city48 and Dader Hospital.23 This may be due to poor infection prevention practice during the surgical procedures. However, contradictory results were reported from South Sudan,44 Rwanda,10 Hawassa, Southern Ethiopia27 and Dire Dawa, Eastern Ethiopia.22 The possible reasons for this discrepancy may be due to variation in the sample size and demographic features of the study population.
Final multivariate analysis of this study also revealed that pregnant women who had a family history of HBV infection were 5 times more likely to have HBsAg seropositivity compared to their counterparts. The finding was incongruent with previous study reports from Turkey (Antioch, University Hospital),49 Felege Referral Hospital,50 Tigray Public Hospital51 and a survey reported from Ethiopian hospitals.47 The possible explanation might be due to the fact that having contact with someone who is chronically ill or a carrier of hepatitis B virus may increase the probability of exposure to an infected person’s body fluid which is believed to be a source of infection.
In addition, this study demonstrated that HBsAg seropositivity was statistically significant among pregnant women who had a history of shared sharp materials. The odds of having HBV infection was about 3 times higher among pregnant women who shared sharp material when compared with those who had not shared. This is supported by a similar study report from Uganda, Mulago National Referral Hospital.52 The conceivable justification for this may be due to poor practice on infection prevention and control. In this regard, the sharp materials may have been contaminated with blood which might be a potential source of HBV infection.
Furthermore, this study revealed that the probability of acquiring HBV infection was higher among pregnant women with multiple sexual contacts. The odds of having HBsAg was 6 times higher among pregnant women who had a history of multiple sexual partners compared to their counterparts. This finding is consistent with previous study reports from Legos, Nigeria,53 Dawuro, Southern Ethiopia26 and Yirgalem Hospital.38 The possible justification could be due to the fact that HBV might be found in blood, semen and other body fluids that are exchanged during sexual contact. For instance; women who had multiple sexual partners are more likely to have sexual contact that increases the risks of HBV and other sexually transmitted infections.
Strengths and Limitations
Strengths of the study: The blood samples were collected using ELISA diagnostic method which has high sensitivity and specificity instead of using only rapid test which have low sensitivity and specificity. The study also used a standardized questionnaire to collect information from the study participants. Limitations of the study: Since the study was conducted at a point in time, no causal association could be made. The study was conducted only in public health institutions; pregnant women who attended ANC services at private health facilities were not enrolled in to the study. Moreover, since the study is institutionally based, it may not be generalizable to the entire population.
Conclusion
The overall seroprevalence of HBV infection among pregnant women was relatively high. This result indicates that HBV infection may be a serious public health problem in Jigjiga town. Factors such as surgical history, family history of hepatitis, sharing sharp materials, and a history of multiple sexual partners were significantly and positively associated with HBV infection. Screening of pregnant women for HBV irrespective of the basis of risk factors and intensified prevention targeting this group may reduce mother to child transmission of HBV infection. Health education programs on the mode of HBV transmission, high-risk behaviors, and methods of prevention should be instituted at antenatal care clinics, aseptic technique should be followed during surgical procedures at health facilities. Awareness should be created among families to avoid contact with HBV infected people, sharing sharp materials and sexual contact with multiple people. Other studies to determine the causal association with HBV should be done. Further, community-based studies should be conducted to determine the exact seroprevalence of HBV among the general population.
Abbreviations
ANC, antenatal care; CDC, Center for Disease Control; CSA, Central Statistical Agency; ELISA, enzyme linked immunosorbent assay; HBcAg, hepatitis B core antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IHRERC, Institutional Health Research Ethics Review Committee; JUSYRH, Jigjiga University Sheik Hassan Yabare Referral Hospital; KH, Karamara Hospital; WHO, World Health Organization.
Data Sharing Statement
The datasets used for analysis are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
Ethical clearance was obtained from the Institutional Health Research Ethics Review Committee (IHRERC) of the College of Health and Medical Sciences, Haramaya University. Support letters were written to all public health facilities where the study was actually conducted. The informed, voluntary, written and signed consent was obtained from each study participant. In addition, informed, voluntary, written and signed assent was also obtained from the guardians and parents of mother’s aged less than 18 years. Study participants were clearly informed about their right to withdraw/refuse at any stage of the study if they did not want to participate and confidentiality of the information was assured. The data collectors interviewed the participants in separated areas and the testing was done free of charge. Confidentiality of information and privacy of participants were respected. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
The authors thank Institutional Health Research Ethics Review Committee coordinating office of Haramaya University College of Health and Medical Sciences; School of Nursing and Midwifery, Somali Regional Health Bureau, and Jigjiga University Referral Hospital, Karamara Hospital, Jigjiga blood bank and Jigjiga health center for their cooperation and unreserved contribution for this research paper, without them this work would not have been realized.
Author Contributions
All authors have made a significant contribution to the conception, study design, acquisition, data analysis and interpretation of the result. They also took part in drafting the manuscript, critically reviewed and agreed on the journal to which the article has to be submitted. All authors read and approved the final version of the manuscript and agreed to be accountable for the all contents of the manuscript.
Funding
This study was funded by Haramaya University and Ethiopian Ministry of Science and Higher Education. The funding organizations had no role in the study design, data collection, data analysis and writing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. Guideline for prevention,care,and treatment of person with chronic HB infection WHO. Available from:http://www.who.int/hiv/topics/hepatitis/en/978.
2. WHO. World Health Organizaton Hepatitis B Manual for care WHO. 2018. Available from: http://www.who.int/hepatitis/Cdr/lyo.
3. WHO. Pevention, Care and Treatmant of Viral hepatitis in the Africa region: frameworkfor Action,2016-2020. Available from: http://apps.who.int/iris.
4. WHO. Global Hepatitis Report.world Health Organization. world Health Organizazation; 2018.
5. Kang FB, Wang L, Sun DX. Hepatitis B virus infection in an HBsAb-positive lymphoma patient who received chemotherapy: a case report. Medicine (Baltimore). 2017;96(44):e8518. doi:10.1097/MD.0000000000008518
6. WHO. Hepatitis B facts sheets World Health Organization. 2018. Available from: www.who.int/news-room/fact-sheets/detail/hepatitis-b.
7. WHO. Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis.: World Health Organization. 2018. Available from: http://www.who.int/hepatitis/.
8. Chen DS, Locarnini S, Wait S, et al. Report from a viral hepatitis policy forum on implementing the WHO Framework for Global Action on viral hepatitis in North Asia. J Hepatol. 2013;59(5):1073–1080. doi:10.1016/j.jhep.2013.06.029
9. Hearld KR, Budhwani H. Maternal health care use by pregnant women living with hiv: factors associated with prenatal, delivery, and postnatal care in Haiti. J Health Care Poor Underserved. 2017;28(4):1452–1461. doi:10.1353/hpu.2017.0126
10. Nyamusi M, M’Imunya J, Habtu CM. Factors associated with Hepatitis B surface antigen seropositivity among pregnant women in Kigali, Rwanda: a cross sectional study. J Commun Public Health Nurs. 2017;3(3). doi:10.4172/2471-9846.1000182
11. Kristina L, Helen C, Mark W, Stephen E, Renee H. Maternal Hepatitis B Infection and Pregnancy Outcomes in the United States: A Population-Based Cohort Study. Oxford University Press on behalf of Infectious Diseases Society of America; 2018; doi:10.1093/ofid/ofy134
12. Sirinart S, Kuntharee T, Pannee S, Theera T. Pregnancy outcomes among chronic carriers of hepatitis B virus. Int J Gynecol Obstetrics. 2014. doi:10.1016/j.ijgo.2014.02.019
13. CDC. Working Together to Eliminate the Threat of Hepatitis CDC. 2018. Available from: www.cdc.gov/getce.
14. UNDP. Sustainable development goals booklet. 2015. http://wwwundporg/content/undp.
15. Bongomin B, Oliver M. Delayed introduction of the birth dose of Hepatitis B vaccine in EPI programs in East Africa: a missed opportunity for combating vertical transmission of Hepatitis B. Pan Afr Med J. 2017;27(3):19. doi:10.11604/pamj.supp.2017.27.3.11544
16. UNICEF, WHO. WHO/UNICEF coverage estimates 2014 revision. 2015. WHO/UNICEF. noveber 144, 2018. Available from: http://apps.who.int/immunization/globalsumarry
17. Shiferaw F, Letebo M, Bane A. Chronic viral hepatitis: policy, regulation, and strategies for its control and elimination in Ethiopia. BMC Public Health. 2016;16(1):769. doi:10.1186/s12889-016-3459-1
18. Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B virus infection in The United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018;67(1):1–31. doi:10.15585/mmwr.rr6701a1
19. Biset M, Adugna B. Hepatitis B vaccination status among health care workers in a Tertiary Hospital in Ethiopia. Hepat Res Treat. 2017;2017:647–658. doi:10.1155/2017/6470658
20. Shiferaw F, Letebo M, Bane A. Erratum to: chronic viral hepatitis: policy, regulation, and strategies for its control and elimination in Ethiopia. BMC Public Health. 2016;16(1):1065. doi:10.1186/s12889-016-3745-y
21. Sintayehu T Ethiopia sustaing HBV Iintervation success stories All African News 17. Available from: http://african.com/stories/201710662.html.
22. Robel M, Dawit A, Meseret B. Sero-prevalence of Hepatitis B virus and associated factors among pregnant mothers attending antenatal care in public health facilities, Dire Dawa. J Med Microbiol Diagn. 2018;7(3):8. doi:10.4172/2161-0703.1000281
23. Abdi U, Berhan S, Tesfaye G, Tamirat H. Hepatitis B virus infections and associated factors among pregnant women attending antenatal care clinic at Deder Hospital, Eastern Ethiopia. PLoS One. 2016;11:1–11. doi:10.1371/journal.pone.0166936
24. Getahun T, Kasiye S, Firehiwot T. Seroprevalence of Hepatitis B virus infection and associated factors among pregnant women attended antenatal care services in Harar City, Eastern Ethiopia. J Women’s Health Care. 2018;7(3). doi:10.4172/2167-0420.1000436
25. Araya Mezgebo T, Niguse S, Gebrekidan Kahsay A, Hailekiros H, Berhe N, Asmelash Dejene T. Hepatitis B virus infection and associated risk factors among pregnant women attending antenatal care in health facilities of Tigray, Northern Ethiopia. J Med Virol. 2018;90(3):503–509. doi:10.1002/jmv.24987
26. Asrat C, Aman Y, Amsalu A. Seroprevalence of Hepatitis B virus surface antigen and factors associated among pregnant women in Dawuro zone, SNNPR, Southwest Ethiopia: a cross sectional study. BMC Res Notes. 2017;10(1):418. doi:10.1186/s13104-017-2702-x
27. Yeshi M, Walelign D, Ibrahim A, Anteneh A. Seroprevalence and associated risk factors of hepatitis B virus among pregnant women in southern Ethiopia: a hospital-based cross-sectional study. Epidemiol Health. 2016;38:e2016027. doi:10.4178/epih.e2016027
28. Summary and statistical report of the 2007 population and housing census: Population size by age and sex. 2008.
29. Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey 2016: Key Indicators Report. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2016.
30. one step HBsAg serum/plasm Test 2008.
31. WANTAI. AiDTM HBsAg ELISA. In: Beijing Wantai Biological Pharmacy Enterprise Co. L, editor. Wantai Hepatitis B Virus Diagnostic.
32. Jaroszewicz J, Serrano BC, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52(4):514–522. doi:10.1016/j.jhep.2010.01.014
33. Hughes E, Bassi S, Gilbody S, Bland M, Martin F. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. The Lancet Psychiatry. 2016;3(1):40–48. doi:10.1016/S2215-0366(15)00357-0
34. Erena AN, Tefera TB. Prevalence of hepatitis B surface antigen (HBsAg) and its risk factors among individuals visiting Goba General Hospital, South East Ethiopia, 2012. BMC Res Notes. 2014;7:833. doi:10.1186/1756-0500-7-833
35. Hussain R, Mansoor H, Tehreem I, Rakhshanda A, Ulfat S, Hameed U. Prevalence of Hepatitis b (HBV) Infection in Pregnant Women in District Kohat, Khyber Pakhtunkhwa. Pak J Entomol Zool Stud. 2018;6(1):254–257.
36. Kebede K, Abateneh D, Belay A. Hepatitis B virus infection among pregnant women in Ethiopia: a systematic review and meta-analysis of prevalence studies. BMC Infect Dis. 2018;18(1):322. doi:10.1186/s12879-018-3234-2
37. Semaw A, Awet H, Yohannes M. Sero-prevalence of hepatitis B surface antigen and associated factors among pregnant mothers attending antenatal care service, Mekelle, Ethiopia: evidence from institutional based quantitative cross-sectional study. Int J Med Health Sci. 2015;2:9.
38. Anteneh A, Getachew F, Setegn E, Agete T, Demissie A. Prevalence, infectivity, and associated risk factors of hepatitis b virus among pregnant women in Yirgalem Hospital, Ethiopia: implication of screening to control mother-to-child transmission. J Pregnancy. 2018;2018:84–87. doi:10.1155/2018/8435910
39. Banks T, Kang J, Watts I, et al. High hepatitis B seroprevalence and risk factors for infection in pregnant women on the Thailand-Myanmar Border. J Infect Dev Ctries. 2016;10(4):384–388. doi:10.3855/jidc.7422
40. Tsankova G, Kostadinova T, Todorova T. Seroprevalence of Hepatitis B among pregnant women in Varna Region, Bulgaria. J Med Virol. 2016;4(1):25–28. doi:10.1002/jmv
41. Jamil A, Hamid S, Aziz Q. Prevalence of Hepatitis B amongst pregnant women. JIMDC. 2018;7(2):195.
42. Ruchika G, Asha N, Saroj S, Richa S, Shikha S, Rekha R. Seroprevalence of Hepatitis B surface antigen among pregnant women in a Tertiary Care Health Center of North India. J South Asian Fed Obstetrics Gynecol. 2017;9(2):164–168. doi:10.5005/jp-journals-10006-1484
43. Kotingo L, Allagoa B, Omietimi E, Aigere E, Oweisi W, John T. Seroprevalence and clinico-epidemiological correlates of Hepatitis B Infection in pregnancy at a booking antenatal clinic, Federal Medical Centre. Eur Sci J. 2018;14(6):16. doi:10.19044/esj.2018.v14n6p279
44. Kirbak ALS, Ng’ang’a Z, Omolo J, Idris H, Usman A, Mbabazi W. B. Sero-prevalence for Hepatitis B virus among pregnant women attending antenatal clinic in Juba Teaching Hospital, Republic of South Sudan. Pan Afr Med J. 2017;26. doi:10.11604/pamj.2017.26.72.11410
45. Kolawole. Seroprevalence of hepatitis B surface antigenemia and its effects on hematological parameters in pregnant women in Osogbo, Nigeria.. Virol J. 2012;9:317. doi:10.1186/1743-422X-9-317
46. Richard E, Isaac D, Samuel A, Joyce A, Hope A. Seroprevalence and risk factors of Hepatitis B and Hepatitis C infections among pregnant women in the Asante Akim North Municipality of the Ashanti region, Ghana; a cross sectional study. Afr Health Sci. 2015;15:3.
47. Zelalem D, Adane M, Habtamu BB, et al. Survey of Hepatitis B virus infection and risk factors among pregnant women at public hospital in Ethiopia. Int J Biomed Res. 2016;7(07):450–456. doi:10.7439/ijb
48. Yohannes Z, Wondemagegn M, Mulat Y, Bayeh A. Sero-prevalence and risk factors of hepatitis B virus and human immunodeficiency virus infection among pregnant women in Bahir Dar city, Northwest Ethiopia: a cross sectional study. BMC Infect Dis. 2014;14:118. doi:10.1186/1471-2334-14-118
49. Cetin S, Cetin M, Turhan E, Dolapcıoglu K. Seroprevalence of hepatitis B surface antigen and associated risk factors among pregnant women. J Infect Dev Ctries. 2018;12(10):904–909. doi:10.3855/jidc.10018
50. Sefinew M, Endalkachew N. Seroprevalence of hepatitis B surface antigen and anti HCV antibody and its associated risk factors among pregnant women attending maternity ward of Felege Hiwot Referral Hospital, northwest Ethiopia: a cross-sectional study. Virol J. 2015;12:204. doi:10.1186/s12985-015-0437-7
51. Araya Mezgebo T, Niguse S, Gebrekidan Kahsay A, Hailekiros H, Berhe N, Asmelash Dejene T. Hepatitis B virus infection and associated risk factors among pregnant women attending antenatal care in health facilities of Tigray, Northern Ethiopia. J Med Virol. 2018;90(3):503–509. doi:10.1002/jmv.24987
52. Allen N, Bashir M, Mugisha T. Prevalence and associated factors of Hepatitis B virus infection among pregnant women attending antenatal care clinic at Mulago National Referral Hospital, Uganda. Int Blood Res Rev. 2017;7(4). doi:10.9734/IBRR/2017/36972
53. Adegbesan M, Okunade K, Gbadegesin A, Olowoselu O, Oluwole A, Omilabu S. Seroprevalence of hepatitis B virus infection among pregnant women at the antenatal booking clinic of a Tertiary Hospital in Lagos Nigeria. Niger J Clin Pract. 2015;18(6):819–823. doi:10.4103/1119-3077.163283
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.