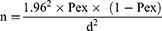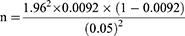Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Seroprevalence of Bovine Brucellosis in Selected Sites of Central Highland of Ethiopia
Authors Getahun T , Urge B, Mamo G
Received 11 October 2022
Accepted for publication 26 January 2023
Published 15 February 2023 Volume 2023:14 Pages 11—22
DOI https://doi.org/10.2147/VMRR.S388970
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Temesgen Getahun,1 Beksisa Urge,1 Gezahegne Mamo2
1Animal Health Research National Program, Ethiopian Institute of Agricultural Research, Holeta Agricultural Research Center, Oromia, Ethiopia; 2Department of Veterinary Microbiology and Public Health, College of Veterinary Medicine and Agriculture, Addis Ababa University, Oromia, Ethiopia
Correspondence: Temesgen Getahun, Tel +251911199867, Email [email protected]; [email protected]
Background: Brucellosis is a contagious, economically significant bacterial disease that affects animals worldwide and is one of the most neglected zoonotic diseases in the world. The disease poses a barrier to the trade of animals and animal products, represents a public health hazard, and is an impediment to free animal movement.
Methods: A cross-sectional study was carried out from December 2019 to May 2020 in order to determine seroprevalence and identify potential risk factors for brucellosis in dairy cows in the Central Highlands of Ethiopia with recent cases of abortion. Purposive sampling was carried out on the farms and kebeles in question to screen for recent cases of abortion in dairy cows. For the purpose of performing serological testing, 352 blood samples from dairy cattle were obtained. The Rose Bengal Plate test was used to initially screen the serum samples, and the Complement Fixation test was utilized as a confirmatory test.
Results: Using combined RBPT and CFT tests, the overall seroprevalence of bovine brucellosis was 0.6% (95% CI: 0.16– 2.09). Retained fetal membrane (OR = 32.74, p = 0.006), market-based stock replacement (OR = 16.55, p = 0.002), breeding method (OR = 7.58, p = 0.027), and late stage of abortion (OR = 14.74, p = 0.0002) are all significantly associated risk factors.
Conclusion: The present seroprevalence study revealed that brucellosis is prevalent at a lower rate among dairy cattle in the study areas. However, there is a possible risk of brucellosis transmission in dairy cattle and the exposed human population in research locations because no control measures were put in place there. Implementing a test and slaughter method with compensation for farmers is advised due to the low prevalence of bovine brucellosis in government-owned and small-holder farms.
Keywords: abortion, bovine brucellosis, risk factors, seroprevalence
Introduction
Brucellosis is a contagious, economically significant bacterial disease that affects animals worldwide and is one of the most neglected zoonotic diseases in the world. Although it is still widespread in parts of Southern Europe, the Middle East, Central and Southeast Asia, and Central and South America, the bulk of developed countries have completely eradicated it.1,2 Brucellosis is still underreported and underdiagnosed while being common in underdeveloped countries.3 This serious disease affects both people and livestock in sub-Saharan Africa.4
Bovine brucellosis is an infectious and contagious disease that mostly affects sexually mature animals. It is typically brought on by B. abortus, however B. melitensis and B. suis have also been known to cause it. In the majority of the world’s nations, it has a significant economic impact. In the world, it affects about 5% of the livestock population, and its range is expanding. The disease is a barrier to free animal movement, a threat to public health, and a barrier to the commerce of animals and animal products.5 Economic losses from culling, delayed heat, lost calves, decreased milk output, and trade restrictions in the tropics and subtropics.6
Contact between animals following an abortion and a retained placenta is typically how the disease is transmitted in cattle.3 Shepherd and guard dogs are a main reservoir and source of transmission for brucellosis in the rural environment, through the consumption of foetal membranes and abortions, and through their promiscuity with humans.7 The organisms are most typically consumed after having been exposed to grassland or animal barns that are polluted. Other options include inhalation and conjunctival inoculation. Infections can also spread when pooled colostrum is given to newborn calves. The epidemiology of bovine brucellosis is typically not greatly affected by sexual transmission. However, only animals whose disease-free status has been established should have their semen collected because artificial insemination can spread the disease.6 In mixed livestock farming, there exists the mixed feeding in inter-species between cattle and sheep. Therefore, not only does there exist infection in internal species but also exist mixed cross infection between two species, B. abortus and B. melitensis are the most important Brucella species in cattle and sheep, respectively.8
Abortions that occurring late in pregnancy are the main feature of bovine brucellosis. Endometritis and fetal membrane retention typically follow, and the latter may make future pregnancies infertile. In herds that are completely vulnerable, the abortion rate may range from 30 to 80%.9,10 Since the first case of brucellosis in Ethiopia was reported in the 1970s,11 the illness has been emphasized as one of the main livestock illnesses in the nation.
In Ethiopia, there is no documented information on how and when bovine brucellosis was introduced and established. However, in the last two decades, several serological surveys have shown that it is endemic and widespread.12,13 The disease is prevalent in cattle in highland and lowland areas.14–16 Though there is limited information on the seroprevalence of bovine brucellosis in some farms in Holeta Town, there is no previous seroprevalence report of brucellosis in medium and small-holder dairy cattle in Holeta Town, Wolmera District, and Ethiopian Institute of Agricultural Research (EIAR) Holeta Agricultural Research Center (HARC) Adda Berga dairy farm, which are located in the milk-shed areas for Addis Ababa and its surroundings. Therefore, this study was carried to determine the current seroprevalence status of brucellosis in bovine with recent history of abortion in the study area and to assess the associated risk factors of bovine brucellosis in the study area.
Materials and Methods
Description of Study Areas
The study was conducted in Holeta Town, Wolmera District and Adea Berga EIAR dairy farm, Oromia regional state, Ethiopia, which are known for their well-developed dairy production and constitute the major milk sheds of Addis Ababa. Holeta Town hosts the Ethiopian Institute of Agricultural Research dairy farms.
A town in the Wolmera District called Holeta is situated in the Oromia Special Zone, which surrounds Addis Ababa, the capital of Ethiopia. The town, which is a part of Ethiopia’s central highlands, is situated 29 kilometers west of Addis Ababa at 9°30′ N and 38°30′ E. Its elevation ranges from 2300 to 3800 meters above sea level. The average annual minimum and maximum temperatures were 6 °C and 22 °C, respectively. Rainfall varies between 900 and 1100 mm every year. The town’s population was 23,296 (men: 11,512, women: 11,784) as per the 2007 population and housing census.17 The main livestock production methods in the region are mixed crop-livestock farming, market-driven peri-urban dairy production, and urban dairy production systems.18 The estimated total number of cattle in the area is 175,741, of which 172,769 (98.3%) are local breeds and 2972 (1.7%) are crossbred cattle handled under extensive and semi-intensive management systems. There are eleven medium-sized dairy farms in Holeta town.19
Adda Berga is a woreda in Ethiopia’s Oromia Region, located at 9° 15′ N and 38° 25′ E. It has a dairy farm substation for the Ethiopian Institute of Agricultural Research. The Adea Berga dairy farm was built in 1986 at the Adea Berga wetland using 400 pure Jersey pregnant heifers and two sires (foundation stock) imported from Denmark for commercial milk production under government state farming.20 The farm had been producing and raising Jersey cattle of the pure breed from the foundation stock to give milk to dairy development businesses as well as serving as a bull dam station for the National Artificial Insemination Center (NAIC). Then, the farm was transferred to Holeta Agricultural Research Center for a genetic improvement research program in 2007. Currently, this research dairy farm has 350 pure Jersey, Boran, and Holstein Friesian and Jersey crossbreeds kept under a semi-intensive rearing system.
Study Population
The target study populations were dairy cattle with recent cases of abortion. The occurrence of abortion cases in one month, referred to as “recent abortion”, was assessed at the respective site during the entire period of this study. The dairy cows under study comprised pure Holstein Friesian and Jersey breeds, indigenous breeds, and Boran Holstein Friesian and Boran Jersey crossbreeds, which have no history of vaccination. Study animal-related traits such as species, age, body condition score, lactation, reproductive status, parity number, period of abortion, and history of abortion were collected and recorded at the time of sampling. Dairy cows were classified into three age groups: <4 years, 4–8 years, and >8 years as young, adult, and old, respectively, based on Ibrahim et al.13 Body condition score (BCS) was estimated subjectively using 17 guidelines.21
Study Design
A cross-sectional study was conducted from November 2019 to May 2020 to study brucellosis in dairy cows with a recent history of abortion.
Sampling Technique and Sample Size Determination
A purposive sampling technique was applied to select medium-, large-, and small-scale farms. Accordingly, all eight kebeles, all eleven medium-scale farms, and one large-scale farm of the Holeta Agricultural Research Center from Holeta Town were included. On the other hand, fourteen kebeles out of twenty-three kebeles of Wolmera District were selected purposively based on accessibility and the number of dairy cows. One large scale farm of the Ethiopian Institute of Agricultural Research located in Adda Berga District was also purposefully included in the study.
The sample size for the serological study of brucellosis was estimated based on the previous study results by.21 In Holeta Town, which were 0.92% seroprevalence. The sample size for the study was calculated using the formula described by22 with a defined precision of 5% and a 95% level of confidence interval.
where n=required sample size, Pex=expected prevalence, and d=desired absolute precision.
Hence, based on the above formula and taking into account 0.92% prevalence, the minimum sample size is:
n = 14.
However, in order to increase precision and reduce standard error, all recently aborted cows in the study area during the study duration were included. Therefore, a total of 352 recently aborted cows were sampled in the study duration.
Sample Collection
Blood Sample Collection
To prevent unanticipated harm to people and to reduce any unneeded stress that might be placed on the animals, the dairy cows with a history of recent abortions were adequately segregated and restrained. After cleaning the jugular vein site on each cow, we took blood samples of 7–10 mL in sterile, plain vacutainer tubes. In accordance with the Thrusfield23 manual, the blood samples were maintained in a slanting position overnight at room temperature to separate the serum. Each serum was then carefully transferred into sterile screw-capped Eppendorf tubes (1.8 mL), labeled, and kept at −20 °C in the Animal Health Microbiology laboratory of the Ethiopian Institute of Agricultural Research (EIAR) Holeta Agricultural Research Center (HARC) until it was tested for antibodies against natural Brucella exposure analysis using RPBT and CFT for confirmation of the RBPT positive samples. In the serology lab of the NVI (National Veterinary Institute), Bishoftu, RBPT and CFT tests were performed on all serum samples taken from animals.
Laboratory Diagnosis
Rose Bengal Plate Test (RBPT)
All serum samples collected from bovines were screened using RBPT according to the procedures described by OIE24, the World Organization for Animal Health23 and the manufacturer’s instructions. The antigen used was Rose Bengal antigen, which constitutes a suspension of Brucella. For the method, 30μL of serum and 30μL of antigen were mixed on a test plate and rocked for 4 minutes. After four minutes of rocking, visible agglutination was considered positive. Agglutinations were recorded as 0, +, ++, and +++, according to the degree of agglutination. A score of 0 indicates the absence of agglutination; + indicates barely visible agglutination; ++ indicates fine agglutination; and +++ indicates coarse clumping. The presence of agglutination was considered a positive reaction, while the absence of agglutination was considered negative. Brucella positive and negative control sera were also tested along with the test sera to guide in the reading of the results.25 The results were recorded and stored in Microsoft Excel.
Complement Fixation Test (CFT)
Serum that tested positive for RBPT was then subjected to a CFT test to confirm the results using the common Brucella antigen. Titration was used to test the reagent preparation, and it was done in accordance with the World Organization for Animal Health’s suggested protocols.23 Sera with a significant reactivity, more than 75% fixation of complement (3+) at a dilution of 1:5, or at least 50% fixation of complement (2+) at a dilution of 1:10 or higher, were classified as positive, while complete hemolysis or lack of fixation were classified as negative.
Data Management and Analysis
Data was coded and saved in Microsoft Office Excel spreadsheets before being transferred to R software version 4.0 for statistical analysis. Data were obtained from the field and from serological tests. On the basis of RBPT and CFT positive, the seroprevalence for the animal level was computed by dividing the number of Brucella reactors by the total number of examined animals. Descriptive questioner findings were analyzed using Chi-square, and Firth’s bias-reduced logistic regression analysis was used to determine the relationship between seropositivity and possible risk factors.22
Ethical Considerations
An ethical clearance certificate was obtained from the animal research ethical review committee of the College of Veterinary Medicine and Agriculture (Date: 15/10/2019GC, Ref. No. VM/ER/10/01/12/2020) based on the assessment of the research proposal. The standard ethical principles and conduct were implemented for animal study participants. Written and oral informed consents were obtained from human study participants and livestock owners.
Result
Seroprevalence of Brucellosis in Dairy Cattle
Out of 352 dairy cows having a history of recent abortion testing (222 cross and 130 local breeds), the current study found that 4 (1.14%) (95% CI: 0.47–2.97) had RBPT results that were positive. CFT was used to confirm the serum samples that tested positive for RBPT. In the research area, only 2 samples were bovine brucellosis confirmed seropositive. According to CFT tests conducted in the research area, the overall seroprevalence of bovine brucellosis was 0.6% (95% CI: 0.16–2.09) (Figure 1).
 |
Figure 1 The overall seroprevalence of brucellosis in dairy cow with a history of recent abortion by RBPT and CFT diagnostic techniques. |
Association of Risk Factors with Bovine Brucellosis Seropositivity
A Univariable Firth’s Bias-Reduced Logistic Regression analysis was computed to evaluate the association between brucellosis seropositivity and different risk factors. Out of 352 serologically screened dairy cows, 1 (0.3%) from the Ethiopian Institute of Agricultural Research Holeta Agricultural Research Center (HARC) large-scale dairy farm, 1(0.3%) from Fayiru medium-scale farm, and 2(0.6%) from Burka Harbu kebele were positive by RBPT, and 2(0.6%) from Burka Harbu kebele were further confirmed by CFT. The analysis indicates that there was no statistically significant association between animal origins and bovine brucellosis (P > 0.05) (Table 1).
 |
Table 1 Univariable Firth’s Bias-Reduced Logistic Regression Analysis of Risk Factors Associated with Bovine Brucellosis Seropositivity with Combined RBPT and CFT |
The seroprevalence of bovine brucellosis in the late stage of abortion (OR = 14.76, p = 0.0002), retained fetal membrane (OR = 32.74, p = 0.0064), market source of stock replacement (OR = 16.548, p = 0.0022), and parturition pen (OR = 11.533, p = 0.027) were statistically significant, while other factors were not statistically significant (P > 0.05) (Table 1).
The results of multivariable Firth’s Bias-Reduced Logistic Regression analysis showed the association of predictor variables with bovine brucellosis seropositivity. There was no multicollinearity between variables. Accordingly, the stepwise multivariable Firth’s Bias-Reduced Logistic Regression analysis results showed important risk factors for bovine brucellosis seropositivity. Therefore, stage of abortion, retained fetal membrane, source of animal, and presence of parturition pen were included in the final model. However, abortion stages, RFM, and animal source for replacement were all significantly associated with brucellosis seropositivity (Table 2).
 |
Table 2 Multivariable Firth’s Bias-Reduced Logistic Regression Analysis of Risk Factors Associated with Dairy Cow Brucellosis Seropositivity |
Thus, the reduced model revealed that cows with late stage abortion, retained fetal membrane, and market purchase herd replacement were 1.283, 1.046, and 1.0638 times more likely to be seropositive to Brucella infection, respectively, than those with early stage abortion, without retained fetal membrane, and own or government source of herd replacement.
Farm Characteristics of Different Scale Farms
Two large-scale farms have semi-intensive management systems, eight medium-scale farms have intensive and four of them have semi-intensive management systems, while most small-holder (164) farmers have extensive management systems. It was also found that 57 (34.8%) of sampled cows from large scales were using the AI breed system, while 107 (97.3%) of small-holder farmers were dependent on natural mating and also 76 (97.4%) of small-holder farmers were using both AI and natural mating (Table 3).
 |
Table 3 Farm Characteristics of Different Scale Farms |
Risks Among Farm Workers and Dairy Cattle Owners
In the study area, about 95 (37.4%) of small-holder farmers had common housing with dairy animals. The result showed that only 8 (42.1%), 1 (9.1%) and 15 (5.9%) of respondents from large-scale, medium-scale and small-scale farms were aware of brucellosis, respectively. Up to 11 (57.9%), 10 (90.9%) and 239 (94.1%) of the respondents from large-scale and medium-scale farms and small-holder animal owners reported that they had poor knowledge about brucellosis, while most of them drank raw milk (Table 4).
 |
Table 4 Occupational Risks and Awareness Among Farm Workers and Owners About Brucellosis |
Discussion
The present study revealed that the overall seroprevalence of bovine Brucellosis in the study areas was 0.6%. The seroprevalence in this study was slightly higher than the finding of Nielsen26 who reported an overall prevalence of 0.14% in the North Gondar Zone,27 0.2% in Ambo and Debre Berhan, and16 0.06% in the Addis Ababa area. While in other ways, Bashitu et al28 and Alemu et al29 failed to find any seroreactive cattle in the Eastern and Western Showa zones of central Ethiopia and in intensive dairy farms in the Addis Ababa area, respectively. This variation in seroprevalence might be seen due to the difference in the study animal management system. Most of the reactive animals in our study were from small-holder farmers kept under an extensive management system. The dependency of most of the farmers on outside sources for stock replacement could be one possible way of introducing the disease into unaffected herds. This could also be due to differences between the study areas regarding conditions that could facilitate the rate of transmission of the disease.30
The finding of my study was in close agreement with the findings of Gay et al31 (0.69%); Tesfaye32 (0.77%); Tolosa et al33 (0.9%); Gumi et al34 (1.0%) from Ethiopia and Adugna35 (1.0%) from Kenya. On the other hand, there were reports with a relatively higher seroprevalence of bovine brucellosis in other parts of the country; Zemmouri et al7 (3.19%);15 Yilma et al10 (11.0%); Kang’Ethe et al36 (2.9%); Jergefa et al37 (4.9%); Li et al8 (3.1%); Haileselassie et al38 (1.38%); Jergefa et al37 (6.1%); Angara et al44 (3.5%); Megersa et al39 (1.9%); Asmare et al41 (4.3%); Acha et al6 (1.4%). Similarly, relatively higher seroprevalence was reported in other African countries by other authors: (8.5%) Tibesso et al42 from Eritrea, (24.5%) Omer et al43 from Sudan; (24.0%) Angara et al44 from Zimbabwe; (5.5%) Matope45 from Nigeria were some of the reports.
The observed variation in prevalence might be explained by variations in production systems and animal care. The majority of previous research indicating higher prevalence, which varied authors reported, was carried out in intensively managed herds where cattle from numerous owners gathered at grazing or watering places. The findings of this study showed that all of the confirmed instances came from small-holder dairy farmers who kept their dairy animals under extensive management. Therefore, the reasons for the low prevalence of bovine brucellosis in these study areas could potentially be explained by improved hygiene practices, separation of cows during parturition, cleaning and disinfection activities, culling of infected animals, relying on their own herd to replace stock in two large-scale farms and eleven medium-scale farms, and the prevailing management differences between intensive, semi-intensive, and extensive production systems. This is also reflected the relatively good hygienic status of the farms and practices in disposing aborted materials to ward off contact with animals.
In addition to the estimation of seroprevalence, this study was also carried out to assess the risk factors associated with disease occurrence. The previous history of abortion stage has a statistically significant association with the seropositivity of bovine brucellosis. This was in agreement with previous reports by.6 This could be explained by the presence of higher seropositivity in cows in the last trimester, which may be due to the preferential localization of Brucella in the uterus, in which allantoic fluid factor and erythritol stimulate the growth of Brucella in the uterus and increase in the placenta and fetal fluid from about the 5th month of gestation.46,47
In the current study, there was also a highly significant association between seropositivity for brucellosis and cows having a history of retained fetal membrane. Following a brucellosis-related abortion, the placenta is frequently retained and the uterine wall becomes inflamed (metritis). According to Constable et al48 report, Brucella infected cows were expected to abort 3 to 4 times more than unexposed cows. This could also be explained by the fact that retained fetal membrane is a typical outcome of brucellosis. Other studies have also shown a significant association between seropositivity and retained fetal membrane.7,8,12,13,23,27,29,33–35,40
There were statistically significant differences in seroprevalence of brucellosis seropositivity and breeding methods. In the present study area, most farms used artificial insemination (38.9%) more often than bulls (22.7%) for breeding purposes. There was a higher seroprevalence rate in the AI service, whereas there was no seropositive record in the bull mating method. The sources of replacement stock were shown to significantly affect the prevalence of bovine brucellosis in study areas. Those animals purchased from other areas were relatively more susceptible to brucellosis than cattle grown and replaced the stock.
According to the results of study, Brucella infection did not show significant variation between breeds. The present finding agrees with the previous reports of Acha et al, Yilma et al, Adugna et al and Angara et al6,10,35,44 who reported that seropositivity of Brucella infection was independent of the breeds. The variation in the number of animals sampled per breed group might be responsible for the absence of significant variation in Brucella infection between the breeds. Crossbreds, on the other hand, were becoming more common in my study area, and farmers treated crossbred cows better than local breeds.
According to the present study, there was no statistically significant difference among age groups for Brucella seropositivity. All positive cows (0.6%) were found in the adult age group, whereas no Brucella seropositivity was observed in the young and old age groups of dairy cattle in the study sites. Similar findings were also reported by Acha et al, Gay et al and Angara et al6,11,31,37,44,50 where age was not significantly associated with Brucella seropositivity.
The higher seroprevalence of brucellosis among adult cows may be related to the higher number of adult cows included in the study. In addition, it may be related to their advanced age as the organism may remain latent or chronic for an unspecified period before manifesting as a clinical disease. The other justification is also possible as age is one of the intrinsic factors which influence the susceptibility to Brucella infection. Brucellosis appears to be more associated with sexual maturity.25,31 It is essentially a disease of sexually mature animals and susceptibility increases with sexual maturity and pregnancy due to the influence of sex hormones and placenta erythritol on the pathogenesis of brucellosis. On the other hand, younger animals tend to be more resistant to infection and frequently clear infections, although latent infections can occur.45,51
Conclusion and Recommendations
In Holeta town, Wolmera District, and HARC Adea Berga dairy farm in West Shoa, Oromia Region Ethiopia, the overall seroprevalence of bovine brucellosis with recent abortion history was 0.6%. The finding of positive serological reactors did not only suggest the presence of the disease in the cattle population in the areas but also indicated the presence of foci of infection that could serve as sources of infection for the spread of the disease into unaffected animals and humans. In this finding, stage of abortion, retained fetal membrane, source of stock replacement, and breeding methods were statistically significant risk factors associated with dairy animal brucellosis seropositivity.
Based on the above conclusions, the following recommendations are forwarded to curb further spread of the disease in both cattle and human populations:
- Isolation of aborted animals and proper disposal of aborted fetuses and fetal membranes, preferably, by incineration.
- Replacement stock should be purchased from herd known to be free of brucellosis.
- Strict movement control of animal from one area to another in order to prevent the spread and transmission of the disease from infected cattle to the non-infected ones.
- The implementation of test and slaughter policy with compensation payment to the farmers as the prevalence of the disease is low in the study area.
- Adoption of replacement stock vaccination with the aim of eradicating the diseases and prevention of its impact on the public and economic sector.
Data Sharing Statement
All the required raw data is readily available.
Consent for Publication
I fully agree that this paper can be published in our journal.
Acknowledgments
- The data sent with the manuscript are raw data.
- This paper is based on the thesis of Temesgen Getahun. It has been published on the institutional website (http://etd.aau.edu.et/handle/123456789/25436).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Donev D, Karadzovski Z, Kasapinov B, Lazarevik V. Epidemiological and public health aspects of brucellosis in the Republic of Macedonia. Prilozi. 2010;31(1):33–54.
2. Awah-Ndukum J, Mouiche M, Bayang H, et al. Seroprevalence and associated risk factors of brucellosis among indigenous cattle in the Adamawa and north regions of Cameroon. Vet Med Int. 2018;2018:1–10. doi:10.1155/2018/3468596
3. Holt HR, Bedi JS, Kaur P, et al. Epidemiology of brucellosis in cattle and dairy farmers of rural Ludhiana, Punjab. PLoS Negl Trop Dis. 2021;15(3):e0009102. doi:10.1371/journal.pntd.0009102
4. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775–786. doi:10.1016/S1473-3099(07)70286-4
5. Deka RP, Magnusson U, Grace D, Lindahl J. Bovine brucellosis: prevalence, risk factors, economic cost and control options with particular reference to India-a review. Infect Ecol Epidemiol. 2018;8(1):1556548. doi:10.1080/20008686.2018.1556548
6. Acha PN, Szyfres B. Zoonoses and Communicable Diseases Common to Man and Animals: Volume 3: Parasitoses. Vol. 580. Pan American Health Org; 2001.
7. Zemmouri L, Besbaci M, Mammeri A, Lafri M. Sero-epidemiological investigation of the major abortive bacterial agents in Ewes of M’Sila Governorate, Algeria. Bull Univ Agric Sci Vet Med ClujNapoca Vet Med. 2020;77(2):24–34. doi:10.15835/buasvmcn-vm:2020.0004
8. Li M-T, Sun G-Q, Wu Y-F, Zhang J, Jin Z. Transmission dynamics of a multi-group brucellosis model with mixed cross infection in public farm. Appl Math Comput. 2014;237:582–594. doi:10.1016/j.amc.2014.03.094
9. Boris S. Zoonoses and Communicable Diseases Common to Man and Animals. Scientific publication/Pan American Health Organization (USA); 1987.
10. Yilma M, Mamo G, Mammo B. Review on brucellosis sero-prevalence and ecology in livestock and human population of Ethiopia. Achiev Life Sci. 2016;10(1):80–86. doi:10.1016/j.als.2016.05.008
11. Minda AG, Gobena A, Tesfu K, et al. Seropositivity and risk factors for Brucella in dairy cows in Asella and Bishoftu towns, Oromia Regional State, Ethiopia. Afr J Microbiol Res. 2016;10(7):203–213. doi:10.5897/AJMR2015.7707
12. Berhe G, Belihu K, Asfaw Y. Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray region of Ethiopia. Int J Appl Res Vet Med. 2007;5(2):65.
13. Ibrahim N, Belihu K, Lobago F, Bekana M. Sero-prevalence of bovine brucellosis and its risk factors in Jimma zone of Oromia Region, South-western Ethiopia. Trop Anim Health Prod. 2010;42(1):35–40. doi:10.1007/s11250-009-9382-z
14. Ahmed EY, Ali A, Mesfin A, Deressa A, Girmaye T. Brucellosis as a zoonosis in chifra district, Afar Regional State, Ethiopia. Bull Anim Health Prod Afr. 2008;56:357–361.
15. Kebede T, Ejeta G, Ameni G. Seroprevalence of bovine brucellosis in smallholder farms in central Ethiopia (Wuchale-Jida district). Rev Med Vet. 2008;159(1):3.
16. Edao BM, Hailegebreal G, Berg S, et al. Brucellosis in the Addis Ababa dairy cattle: the myths and the realities. BMC Vet Res. 2018;14(1):1–9. doi:10.1186/s12917-018-1709-4
17. CSA. Ethiopian Livestock sector current status and future prospects Introduction: livestock production; 2007:15–24.
18. CSA. Population and housing census of administrative. 2016:320–330.
19. WoWAHA. WolmeraWereda animal health agency. statisticalabstract. Wolmera, FinfineLiyuZuria Zone, Oromia, Ethiopia. J Vet Med Anim Health. 2015;10(6):153–158.
20. Siyoum T, Yohannes A, Shiferaw Y, Asefa Z, Eshete M. Major reproductive disorders on Jersey breed dairy cattle at Adea Berga dairy farm, West Shewa Zone, Oromia Region, Ethiopia. Ethiop Vet J. 2016;20(1):91–103. doi:10.4314/evj.v20i1.7
21. Wildman E, Jones G, Wagner P, Boman R, Troutt JH, Lesch T. A dairy cow body condition scoring system and its relationship to selected production characteristics. J Dairy Sci. 1982;65(3):495–501. doi:10.3168/jds.S0022-0302(82)82223-6
22. Shanko K. Sero- Epidemiological Study of Bovine Brucellosis in Selected Dairy Farms of Bishoftu and Holeta Towns, Oromia Regional State, Central Ethiopia [Thesis] Addis Ababa Univ; 2017:50.
23. Thrusfield M. Sample size determination. Vet Epidemiol. 2007;3:185–189.
24. OIE. Bovine Brucellosis, World Organisation for Animal Health Manual of Diagnostic Tests and Vaccines (Mammals, Birds and Bees). Cambridge University Press; 2009.
25. Alton GG, Jones LM, Angus R, Verger J. Techniques for the Brucellosis Laboratory. Institut National de la recherche Agronomique (INRA); 1988.
26. Nielsen K. Animal Brucellosis. CRC press; 2018.
27. Negash Y, Gebre B, Benti D, Bejiga M. A community based study on knowledge, attitude and practice (KAP) on HIV/AIDS in Gambella town, Western Ethiopia. Ethiop J Health Dev. 2003;17(3):205–213.
28. Bashitu L, Afera B, Tuli G, Aklilu F. Sero-prevalence study of bovine brucellosis and its associated risk factors in Debrebirhan and Ambo towns. J Adv Dairy Res. 2015;3(131):2. doi:10.4172/2329-888X.1000131
29. Alemu F, Admasu P, Feyera T, Niguse A. Seroprevalence of bovine brucellosis in eastern Showa, Ethiopia. Acad J Anim Dis. 2014;3(3):27–32.
30. Belihu K. Analysis of Dairy Cattle Breeding Program in Selected Areas of Ethiopia [PhD]. Land wirtsch Aftlich-Gartene rische Fakultat der Humbodt Universistat ZuBerlin; 2002.
31. Gay CC, Hinchcliff DC, Kenneth W, Radostits OM. Veterinary Medicine; a Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. Elsevier Health Sciences; 2000.
32. Tesfaye A. Brucellosis in cattle and small ruminants in selected sites of Tigray Region, North Ethiopia [Unpublished DVM Thesis]. Addis Ababa University; 2003.
33. Tolosa T, Regassa F, Belihu K. Seroprevalence study of bovine brucellosis in extensive management system in selected sites of Jimma Zone, Western Ethiopia. Bull Anim Health Prod Afr. 2008;56(1). doi:10.4314/bahpa.v56i1.32823
34. Gumi B, Firdessa R, Yamuah L, et al. Seroprevalence of brucellosis and Q-fever in southeast Ethiopian pastoral livestock. J Vet Sci Med Diagn. 2013;2(1). doi:10.4172/2325-9590.1000109
35. Adugna K, Agga G, Zewde G. Seroepidemiological survey of bovine brucellosis in cattle under a traditional production system in western Ethiopia. Rev Sci Tech. 2013;32(3):765–773. doi:10.20506/rst.32.2.2218
36. Kang’Ethe E, Ekuttan C, Kimani V, Kiragu M. Investigations into the prevalence of bovine brucellosis and the risk factors that predispose humans to infection among urban dairy and non-dairy farming households in Dagoretti Division, Nairobi, Kenya. East Afr Med J. 2007;84(11 Suppl):S96–S100. doi:10.4314/eamj.v84i11.9583
37. Jergefa T, Kelay B, Bekana M, Teshale S, Gustafson H, Kindahl H. Epidemiological study of bovine brucellosis in three agro-ecological areas of central Oromiya, Ethiopia. Rev Sci Tech. 2009;28(3):933. doi:10.20506/rst.28.3.1939
38. Haileselassie M, Kalayou S, Kyule M, Asfaha M, Belihu K. Effect of Brucella infection on reproduction conditions of female breeding cattle and its public health significance in Western Tigray, Northern Ethiopia. Vet Med Int. 2011;2011:1–7. doi:10.4061/2011/354943
39. Megersa B, Biffa D, Abunna F, Regassa A, Godfroid J, Skjerve E. Seroprevalence of brucellosis and its contribution to abortion in cattle, camel, and goat kept under pastoral management in Borana, Ethiopia. Trop Anim Health Prod. 2011;43(3):651–656. doi:10.1007/s11250-010-9748-2
40. Degefu H, Mohamud M, Hailemelekot M, Yohannes M. Seroprevalence of bovine brucellosis in agro pastoral areas of Jijjiga zone of Somali National Regional State, Eastern Ethiopia. Ethiop Vet J. 2011;15(1). doi:10.4314/evj.v15i1.67683
41. Asmare K, Sibhat B, Molla W, et al. The status of bovine brucellosis in Ethiopia with special emphasis on exotic and cross bred cattle in dairy and breeding farms. Acta Trop. 2013;126(3):186–192. doi:10.1016/j.actatropica.2013.02.015
42. Tibesso G, Ibrahim N, Tolosa T. Sero prevalence of bovine and human brucellosis in Adami Tulu, Central Ethiopia. World Appl Sci J. 2014;31(5):776–780.
43. Omer M, Skjerve E, Woldehiwet Z, Holstad G. Risk factors for Brucella spp. infection in dairy cattle farms in Asmara, State of Eritrea. Prev Vet Med. 2000;46(4):257–265. doi:10.1016/S0167-5877(00)00152-5
44. Angara T, Ismail A, Agab H, Saeed N. Sero-prevalence of bovine brucellosis in Kuku Dairy Scheme, Khartoum North, Sudan. Sud J Vet Sci Anim Husb. 2004;48(1):2.
45. Matope G, Bhebhe E, Muma J, Lund A, Skjerve E. Risk factors for Brucella spp. infection in smallholder household herds. Epidemiol Infect. 2011;139(1):157–164. doi:10.1017/S0950268810000968
46. Mai HM, Irons PC, Kabir J, Thompson PN. A large seroprevalence survey of brucellosis in cattle herds under diverse production systems in northern Nigeria. BMC Vet Res. 2012;8(1):1–14. doi:10.1186/1746-6148-8-144
47. Coetzer J, Thomson G, Tustin R. Infectious diseases of livestock with special reference to Southern Africa; 1994.
48. Constable PD, Hinchcliff KW, Done SH, Grünberg W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. Elsevier Health Sciences; 2016.
49. Díaz A. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev Sci Tech. 2013;32(1):1.
50. Amenu K, Thys E, Regassa A, Marcotty T Brucellosis and tuberculosis in Arsi-Negele District, Ethiopia: prevalence in ruminants and people’s behavior towards zoonoses. 2010.
51. Svendsen E. Parasites abroad. In: The Professional Hand Book of the Donkey.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.