Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Seroprevalence of Bluetongue Virus Antibodies in Ovine in Maji District of West Omo Zone, Southwest Ethiopia
Authors Haile T , Abera M, Teklemariam T, Sibhatu D , Asres F
Received 4 July 2022
Accepted for publication 5 September 2022
Published 19 September 2022 Volume 2022:13 Pages 257—264
DOI https://doi.org/10.2147/VMRR.S375482
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Tamirat Haile,1 Mulugeta Abera,1 Tsegaye Teklemariam,1 Demeke Sibhatu,2 Fasil Asres2
1Mizan Regional Veterinary Laboratory Center, Mizan-aman, Ethiopia; 2National Animal Health Diagnostic and Investigation Center, Sebeta, Ethiopia
Correspondence: Tamirat Haile, Tel +251920110182, Email [email protected]
Background: Bluetongue (BT) disease is an arthropod-transmitted viral disease of domestic and wild ruminant species caused by Bluetongue virus (BTV). It is of most importance in sheep and endemic primarily in the tropical and subtropical regions where vectors (Culicoides species) are present.
Materials and Methods: A cross-sectional study was conducted in July–November 2019 to examine the seroprevalence of BTV infection in ovine in Maji district of West Omo zone. Serum samples were examined for the presence of specific antibodies of BTV using competitive enzyme-linked immunosorbent assay (c-ELISA) test. The collected data was coded and analyzed using STATA version 13 software. Associations between sero-prevalence and its risk factors were tested in a Chi-square analysis and with a P< 0.05 were considered as statistically significant.
Results: The individual animal prevalence was revealed as 39.23% (153/390). Herd size prevalence was: small size herd (37.42%; 61/163), medium size herd (32.35%; 55/170), and large size herd (64.91%; 37/57). Species-based prevalence showed ovine (38.00%; 141/371) and caprine (63.15%; 12/19). Age-based prevalence revealed adult (39.26%; 150/382) and young (37.5%; 3/8). The cumulative sex prevalence for both ovine and caprine was male (37.95%; 52/137) and female (39.92%; 101/253).
Conclusion: The current prevalence of BTV antibodies in the area was found to be high. Lack of application of bluetongue disease control mechanisms like vaccination for the animals is a key factors for the high prevalence of the disease in the areas besides the existence of the vectors.
Keywords: bluetongue, caprine, c-ELISA, Maji district, ovine, sero-prevalence
Introduction
Bluetongue virus is non-contagious and categorized under the family Reoviridae and genus Orbivirus.1 Bluetongue (BT) disease is an arthropod-transmitted virus disease of domestic and wild ruminant species caused by bluetongue virus (BTV). BT occurs most commonly in sheep (sore-muzzle, catarrhal fever) and certain species of wildlife, particularly white-tailed deer.2 Bluetongue disease is of most importance in sheep, with economic losses resulting from death and loss of body condition in sheep that survive. The disease has emerged as a major non-tariff trade barrier, ie, suspicion of its presence is used at the convenience of importing countries to limit live sheep, lamb, and mutton imports.
In sheep, bluetongue is characterized by fever that may last several days before hyperemia, excess salivation, and frothing. There is nasal discharge, initially serous but becoming mucopurulent and speckled with blood.3 It affects animals by causing fetal death, abortion, and congenital defects.4 It is reported that BTV can cause 25% abortion and a 50% decrease in fertility in sheep.4 The severity of the disease varies markedly depending on the virus strain, breed of sheep, and environmental stresses.1 The tongue may become cyanosed, hence the name “bluetongue”. There is loss of body condition and the sheep may die, often through aspiration pneumonia.3 Isolation through embryonated chicken egg, identification with polymerase chain reaction (PCR), and serological tests like indirect ELISA or agar gel immune diffusion test (AGID) are the most common laboratory identification tests for BTV.5 It has been reported that specific sero-diagnostic techniques, such as competitive ELISA or BTV neutralization tests, should be used for bluetongue surveillance in Ibaraki virus (IBV)-endemic areas because IBV-positive serum samples may result in false-positive bluetongue agar gel immunodiffusion test reactions.6
Bluetongue disease activity can be found on all continents except Antarctica; comprising different serotypes and strains causing variable disease.5 Bluetongue disease has been known in South Africa for over a hundred years.7 Currently, 21 serotypes from 27 known BTV serotypes have been isolated from sheep in Africa.8 Midges are the only significant natural vectors of BTV; thus prevalence of the disease is influenced by favorable ecological factors like rainfall, temperature, humidity, and soil characteristics.5
Bluetongue infection is seasonal because Culicoides may breed in damp, wet, irrigation channels, muddy, and fecal runoff areas around farms.9 Vectors obtain the virus from blood on an infected animal and then transmit to other animals after an extrinsic incubation period of 4–20 days. During this period, the virus infects and disseminates from the midgut of the insect to its salivary glands.2 The infected midges can remain infective for life.10 Mechanical transmission may also occur by the Melophagus ovinus sheep ked, Amblyomma variegatum, and Ornithodorus coviaceus ticks and blood sucking flies such as Stomoxys species and Tabanus species.10 Additional mechanisms of transmission through dam to fetus (vertical transmission) and the sire to dam via infected semen (horizontal transmission) may occur.11 Sheep and young age groups are more susceptible than goats and adult age groups, respectively.10 Recovered animals may harbor the virus for some months. Domestic animals like cattle and wild animals may be considered as reservoirs of the virus.10,12 Morbidity and mortality in sheep may reach 100% and between 30–70% in more susceptible breeds, respectively; while mortality can reach 90% in wild deer and antelopes.5 Direct losses associated with loss of body weight, death, and condition, drop in milk production, and poor subsequent reproductive performance, as well as indirect loss as a result of export restrictions of live animals and their products are considered a greater economic effect.11
The livelihood of most peoples’ in Ethiopia is closely related with farming cereals crops and rearing animals. However, there is limited research in other area of Ethiopia and no published research study was found in Maji district on the sero-epidemiology of BTV. Hence, the current study contributes a lot in improving animal health, being a baseline data for animal health workers when intervention activities are needed. Furthermore, noting the disease prevalence in the area to the scientific community by publishing in journals is important. The objective of the study was to assess the seroprevalence rate of Bluetongue virus infection, to examine associated risk factors for the occurrence of the disease, to indicate all possible prevention and control methods to minimize the disease.
Materials and Methods
Study Area Description
A study was conducted in Maji district of West Omo zone located in Southwest Ethiopia Peoples’ Regional State (SWEPRS). Maji district is bordered on the South by the Kibish River which separates it from South Sudan, on the west by Surma, on the northwest by Bero, on the north by Meinit Shasha, and on the east by the Omo River which separates it from the Debub Omo Zone. Towns in Maji district include Tum and Maji. Climatic conditions of the district were 12% highland, 42.8% midland, 40.6% lowland, and 4.6% desert: temperature ranges from 15–35°C. The altitude ranges from 900–2,000 mm above sea level and the annual rainfall ranges from 900–2,000 mm. The total livestock population of the district was cattle 244,500, sheep 11,832, goats 13,980, equine 246, and poultry 7,351.13 Agro-ecologically, Maji 01 and Gebarku PAs are highland, while Tum and Balt are midland. Four peasant associations (PAs), namely Balt, Gabarku, Maji 01, and Tum were selected randomly (Figure 1).
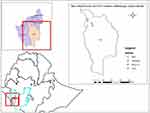 |
Figure 1 Map of the study area. |
Study Animals and Study Design
The study animals were local breed ovine and caprine species found in selected different peasant associations. Animals of different ages, sex, and flock population for both species (ovine and caprine) were included for sampling. Animals health status and vaccination history against the BTV were recorded. A cross-sectional study was conducted to estimate the seroprevalence of bluetongue virus (BTV) antibodies from July 2019 to November 2019 in Maji district to examine the seroprevalence and associated risk factors of BTV through short structured questionnaires. A study in California stated that the disease occurrence is highly seasonal (late July–November) months, thus the inter-seasonal period (November–July) when BTV is apparently “inactive” lasts approximately 8 months.14 Therefore, in this study July–November was considered to estimate the sero-prevalence of the BTV in the area. Tum and Balt have a midland type of agro-ecology, while Gebarku and Maji PAs have highland types of agro-ecology, therefore, there is the variation in altitude and temperature between each PA.
Sample Size Determination
The total sample size was calculated by a formula stated in Thrusfield,15 having a 95% confidence level and 5% absolute precision. There was no published previous research report in the study area. Therefore, the expected prevalence rate was considered as 50%.
where
N = required sample size,
Z-value = 1.96,
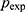 = expected prevalence (50% = 0.50), and
= expected prevalence (50% = 0.50), and
d = desired absolute precision (5% = 0.05).
Therefore, a total sample size of 384 animals was obtained from formulas. However, a total of 390 ovine and caprine were observed by adding six animals as a contingency sample.
Sampling Techniques
A stratified simple random sampling method was used to select animals from the flock during sample collection. Animals were selected randomly from the flock and then a number of animals were sampled until the sample total for that herd was reached. In total, 390 animals (371 sheep and 19 caprine) were selected. In the study area the availability of ovine population is higher than caprine, which is why the population of sampled ovine was greater than caprine during sampling. Species, age, flock size, and sex were recorded. Sheep and goats <1 year were considered as the young group while those ≥1 year were considered as the adult group. Animal flock sizes were categorized into three groups, with small flock size (holding 1–5 animals), medium flock size (holding 6–10 animals), and large flock size (holding above 10 animals).
Sample Collection
About 5 mL of blood was collected aseptically from the jugular vein of each sheep and goat using plain serum vacutainer tubes and needles. The blood was allowed to clot for 1–2 hours at room temperature, stored horizontally overnight at 4°C in a refrigerator, and then the serum was separated. All the sera were stored at −20°C and transported on ice and submitted to the National Animal Health Investigation and Diagnostic Center (NAHDIC) to investigate for the presence of BTV group-specific antibodies.
Laboratory Examination
Serum samples from animals were tested and examined for the presence of BTV group specific antibodies using a competitive ELISA (ID vet, 310, rue Louis Pasteur-Grabels-France) test kit, which is based on recombinant VP7 protein. The overall test procedures were performed in accordance with the manufacturer’s protocol indicated.
Data Collection, Management, and Analysis
The raw data were collected through a structured questionnaire comprising the detailed information regarding environment and animal. And, the collected data has been recorded in a computer Microsoft Excel spreadsheet and coded for ease of analysis with data analysis software. After coding, the data analysis was conducted using STATA version 13 software. Associations between seroprevalence and its possible potential risk factors were analyzed by using a Chi-square (χ2) analysis. The risk factors with a P<0.05 were considered as statistically significant while P>0.05.
Results
The individual seroprevalence was shown as 39.23% (153/390). Herd-based seroprevalence revealed: small (37.42%, 61/163), medium (32.35%, 55/170), and large (64.91%, 37/57) (Table 1). Ovine and caprine species were the only species included in the study with a prevalence of 38.00% (141/371) and 63.15% (12/19), respectively (Table 2). Age was categorized into adult and young groups with the prevalence of 39.26% (150/382) and 37.5% (3/8), respectively (Table 3). From both species, males had a prevalence of 37.95% (52/137) and females 39.92% (101/253) (Table 4). The prevalence in four peasant associations (PAs) was Tum (80.80%, 80/99), Maji 01 (24.52%; 52/212), Gebarku (18.75%; 9/48), and Balt (38.70%; 12/31) (Table 5).
 |
Table 1 Herd-Based Seroprevalence of BTV |
 |
Table 2 Species-Based Seroprevalence of BTV |
 |
Table 3 Age-Based Seroprevalence of BTV |
 |
Table 4 Sex-Based Seroprevalence of BTV |
 |
Table 5 PAs-Based Seroprevalence of BTV |
Discussion
The total seroprevalence of ovine and caprine bluetongue in the area was 39.23% (153/390). Since there are few published article available regarding Bluetongue in Ethiopia the current result was compared with results from other countries. The result was quite different from the 0% (negative report) seroprevalence of bluetongue antibodies in goats in small ruminant farms in Serdang, Malaysia.16 It is greater than seroprevalence rates of 5.3% to BTV antibodies in Kerala, India;17 34.1% seropositivity to BTV in indigenous sheep of Northwestern Ethiopia;18 and 129 (30.6%) seroprevalence of small ruminants in South Western Ethiopia.19 The current study result was less than the 89.2% (922/1034) seropositivity of bluetongue in sheep and goats in North East Iran;20 65.21% overall seroprevalence of bluetongue in selected areas of Ethiopia;21 and 46.67% seroprevalence rate of bluetongue in sheep in central Ethiopia.22 Depending on the above result it is possible to conclude that the seropositivity of the animals was related to the prevalence of carrier vectors in the areas. Ecological factors (high rainfall, temperature, soil type and humidity) in the areas was suitable to maintain the growth of the vector Culicoides.22 There was no information obtained regarding vaccination for the animals against BTV infection in the area, so the antibodies detected in the sera were from past natural infection of animals with BTV. Due to the seasonality of the vector’s appearance, the occurrence and transmission of the virus was higher during warmer months of the year. Recently evidence from California has indicated the possibility of the virus surviving during the winter season in long-living female midges, Culicoides sonorensis.23 However, it is impractical to control the vector of insects (mosquitoes) by spraying with insecticides and destroying their breeding habitats, which can minimize the occurrence and transmission of bluetongue virus disease in the areas.
Herd-based prevalence showed that small (37.42%, 61/163), medium (32.35%, 55/170), and large (64.91%, 37/57) with a statistical significance with P<0.00. Bluetongue is naturally non-contagious. However, limited literature was found to compare for relative herd size affecting the probability of being BTV seropositive, Pascual-Linaza et al,24 indicated that herd size was significantly associated with a BTV epidemic in Spain. However, higher prevalence showed in larger herd population in this study; the existence of vectors in small herd population remains the source of infection for the rest of the herd. The survival vector Culicoides through host–vector relationship living style and the existence of seropositive animals increases the endemic character of the BTV.
Ovine and few caprine species were the only species included in the study, with prevalences of 38.00% (141/371) and 63.15% (12/19), respectively. It is clear that comparing the prevalence between ovine and caprine is not important because the number of ovine population sampled was higher than the caprine population. However, this data may indicate the BTV disease has an ability to infect caprine species. The present study was in agreement in the seroprevalence of small ruminants in south western Ethiopia, with ovine being 57 (23.2%)19 and caprine 72 (40.9%), respectively. The variation of rate might be that sheep are higher susceptible to BTV and manifest clearer clinical signs than goats. Goats show less clinical signs of manifestation while maintaining a high titer of BTV. Goats may act as the source of infection to other susceptible animals.25 Species-based seroprevalence was statistically significant (P<0.05).
Age-based prevalence was revealed that adult and young with 39.26% (150/382) and 37.5% (3/8), respectively (P>0.05). Age-based seroprevalence in this study revealed with a minimum difference prevalence rate in each age group with a higher prevalence in young aged animals than old aged animals. Most authors stated that aged animals are more susceptible for bluetongue than young ones;26 an increase in prevalence rate with an increase of age in sheep and goats.27 However, the result was in agreement with the increasing of age in sheep and the seroprevalence infection rates were decreased.28 This could be due to the reality that equal probability of exposure of flock for vector which transmit Bluetongue virus from one to another. Therefore, the variation with seroprevalence rate might be with variation in sampling between adult and young animals. Most animals observed in this study were 382 adults animals (382) and the other eight animals were young. This is due to the greater availability of adult animals than young during sampling in the area and the random sampling methods used made adult animals more likely to be randomized than young animals.
From both species, males had prevalence of 37.95% (52/137) and females 39.92% (101/253). This is not significant. The present study findings showed both sexes are nearly equally infected without significant variation. The present sex-based study was also statistically not significant (P>0.05).
The prevalence in four Peasant associations (PAs) were quite different, with the highest rate in Tum *80.80%, 80/99), followed by Balt (38.70%, 12/31), Maji 01 (24.52%, 52/212), and the lowest rate was in Gebarku (18.75%, 9/48) with statistical significance (P<0.05). This report clearly showed the midland type of egro-ecology (Maji 01 and Gebarku PAS) favored the growth of the vectors compared to highland areas (Tum and Balt PAs). Since Culicoides requires warmth and moisture for both breeding and feeding period, the distribution of infection in the areas is determined by the climatic conditions (temperature, rainfall and humidity), geography, and altitude, which affect the occurrence and activity of the Culicoides vectors and availability of susceptible hosts.28,29 Different prevalence range was directly related with suitability of ecology and environment for survival of the vectors. The transmission period is limited to the times when adult vectors are active. Vector activity is in association with the ambient temperature for spreading of the disease, which is between 28°C and 30°C.28 Vectors control by spraying with insecticide chemicals and destroying their breeding environments can minimize the occurrence and transmission of the bluetongue virus disease in the areas.
Conclusion
The current study showed a high sero-prevalence rate of BTV in the area. The area had high rainfall which favors the existence of biting midges. There is no effective treatment choice for the bluetongue virus disease but controlling of entry of animals from endemic into non-endemic areas could minimize the spreading of the disease. Control of the disease is achieved mainly by vaccination, since the elimination of vector insects is impossible/impractical. Control of vector insects (mosquitoes) by spraying with insecticides and destroying their breeding habitats can minimize the occurrence and transmission of bluetongue virus disease in the areas. Finally, a further study in relation with the reproductive problem (abortion and decrease in fertility) in ovine and caprine should be conducted.
Data Sharing Statement
The data used and analyzed in the current research study are available and provided from the corresponding author in accordance with reasonable request.
Ethical Approval and Consent to Participation
This study has obtained ethical clearance from Mizan Regional Veterinary Laboratory Center animal ethics Review Board. Written informed consent was obtained for sample collection to keep the confidentiality of the owners at the time of sample collection. Animal owners consented, and the importance and outcomes of the research study briefed for the owners. Animals were handled with great care and all methods were carried out depending on procedures indicated in the guidelines and regulations for ethics of animal research.
Acknowledgments
I hereby acknowledge that the abstract of this paper was presented at the Academic journal conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Journal of Veterinary Medicine and Animal Health . Currently, the abstract is not available in the journal listed above since it was withdrawn. We would like to thank Mizan Regional Veterinary Laboratory Center for provision of logistics and budget for the research work. We extend great thanks to the National Animal Health Diagnostic and Investigation Center for their collaboration in conducting laboratory tests of the collected samples.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research work was funded by Mizan Regional Veterinary Laboratory Center and National Animal Health Diagnostic and Investigation Center.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. James NM, Edward JD. Fenner’s Veterinary Virology.
2. Scott DM, Kennedy M, Chengappa MM. Veterinary Microbiology.
3. Murphy AF, Gibbs JP, Horzinek CM, Studdert JM. Veterinary Virology.
4. Toussaint JF, Sailleau C, Mast J, et al. Bluetongue in Belgium. Emerg Infect Dis. 2007;13:614–616. doi:10.3201/eid1304.061136
5. OIE (Organization International des Epizooties). Manual of diagnostic tests and vaccines for terrestrial animals contagious caprine pleuropneumonia; 2014.
6. Shimizu S, Toyota I, Arishima T, Goto Y. Frequency of serological cross-reactions between Ibaraki and bluetongue viruses using the agar gel immunodiffusion test. Vet Ital. 2004;40:583–586.
7. Maclachlan NJ. Bluetongue: history, global epidemiology and pathogenesis. Prev Vet Med. 2011;102:107–111. doi:10.1016/j.prevetmed.2011.04.005
8. Mogajane ME. Trade implications of bluetongue in Africa. Vet Ital. 2004;40(4):691–692.
9. Sperlova A, Zendulkova D. A review article of bluetongue. Vet Med. 2011;56(9):430–452. doi:10.17221/3206-VETMED
10. Lughano K, Dominic K. Disease of Small Ruminants: Hand Book.
11. Aradaib IE, Mohamed ME, Abdalla TM, et al. Serogrouping of United States and some African serotypes of bluetongue virus using RT-PCR. Vet Microbiol. 2005;111:145–150. doi:10.1016/j.vetmic.2005.09.014
12. Dwight CH, Yuan CZ. Veterinary Microbiology.
13. MDLFO (Maji Agricultural and Rural Development office) (2012). Socio-economic data of the Maji district (unpublished document).
14. Mayo CE, Mullens BA, Gerry AC, et al. The combination of abundance and infection rates of Culicoides sonorensis estimates risk of subsequent bluetongue virus infection of sentinel cattle on California dairy farms. Vet Parasitol. 2012;187:295–301. doi:10.1016/j.vetpar.2012.01.004
15. Thrusfield M. Veterinary Epidemiology.
16. Peter I, Abba Y, Jesse F, Malek A, Bitrus A, Hambali I. Seroprevalence of bluetongue antibodies among goats in selected small ruminant farms in serdang, Malaysia. Int J Livest Res. 2018;8(3):39–440. doi:10.5455/ijlr.20180109033854
17. Ravishankar C, Krishnan Nair G, Mini M, Jayapraksan V. Seroprevalence of bluetongue virus antibodies in sheep and goats in Kerala State, India. Rev Sci Tech. 2005;24(3):953–958.
18. Gulima D. Seroepidemiological study of bluetongue in indigenous sheep in selected districts of Amhara National Regional State, North Western Ethiopia. Ethiop Vet J. 2009;13(1):1–15.
19. Abera T, Bitew M, Gebre D, Mamo Y, Deneke Y, Nandi S. Bluetongue disease in small ruminants in south western Ethiopia: cross‑sectional sero‑epidemiological study. Bio Med Cen Res Notes. 2018;11:112. doi:10.1186/s13104-018-3222-z
20. Vahid N, Mahin R. Seroepidemiology of bluetongue disease in small ruminants of Northeast of Iran. Asian Pac J Trop Biomed. 2013;3(6):492–495. doi:10.1016/S2221-1691(13)60102-1
21. Daniel G, Demeke S, Brehan A, Mesfin S. Sero-prevalence study of bluetongue infection in sheep and goats in selected areas of Ethiopia. Ethiop Vet J. 2016;20(1):105–114. doi:10.4314/evj.v20i1.8
22. Woldemeskel M, Tilahun G, Tibbo M, Potgieter LN. Prevalence of bluetongue virus antibodies in sheep in central Ethiopia. Dtsch Tierarztl Wochenschr. 2000;107(10):408–410.
23. Mayo CE, Mullens BA, Reisen WK, et al. Seasonal and interseasonal dynamics of bluetongue infection of dairy cattle and Culicoides sonorensis midges in northern California - Implications for virus overwintering in temperate zones. PLoS One. 2014;9(9):e106975. doi:10.1371/journal.pone.0106975.eCollection.2014
24. Pascual-Linaza AV, Martı´nez-Lo´pez B, Pfeiffer D, Moreno JC, Sanz C, Sa´nchez-Vizcaı´no JM. Evaluation of the spatial and temporal distribution of and risk factors for Bluetongue serotype 1 epidemics in sheep Extremadura (Spain), 2007–2011. Prev Vet Med. 2014;3:279–295. doi:10.1016/j.prevetmed.2014.05.009
25. Yilma M, Mekonnen M. Competitive enzyme linked immuno-sorbent assay (c-ELISA) based sero-prevalence of bluetongue virus (BTV) on small ruminants in selected areas of Wolaita, Southern Ethiopia. J Virol Mycol. 2015;4:148. doi:10.4172/2161-0517.1000148
26. Miller MM, Brown J, Cornish T, et al. Investigation of bluetongue disease epizootic caused by bluetongue virus serotype 17 in sheep in Wyoming. J Am Vet Med Assoc. 2010;237(8):955–959.
27. Mozaffari AA, Khalili M, Sabahi S. High seroprevalence of bluetongue virus antibodies in goats in Southeast Iran. Asian Pac J Trop Biomed. 2014;4(1):275–278. doi:10.12980/APJTB.4.2014B599
28. Wilson AJ, Mellor PS. Bluetongue in Europe: past, present and future. Philos Trans R Soc B. 2009;364:2669–2681. doi:10.1098/rstb.2009.0091
29. Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PPC, Baylis M. Climate change and the recent emergence of bluetongue in Europe. Nat Rev Microbiol. 2005;3:171–181. doi:10.1038/nrmicro1090
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

