Back to Journals » Hepatic Medicine: Evidence and Research » Volume 11
Seroprevalence and associated factors of hepatitis B virus infection among HIV-positive adults attending an antiretroviral treatment clinic at Wolaita Sodo University Referral Hospital
Authors Goa A, Dana T, Bitew S , Arba A
Received 26 February 2019
Accepted for publication 19 June 2019
Published 6 September 2019 Volume 2019:11 Pages 137—147
DOI https://doi.org/10.2147/HMER.S206870
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Video abstract presented by Aseb Arba.
Views: 887
Abraham Goa, Tadele Dana, Shimelash Bitew, Aseb Arba
Wolaita Sodo University, College of Medicine and Health Sciences, Wolaita Sodo, Ethiopia
Correspondence: Aseb Arba
Wolaita Sodo University, College of Medicine and Health Sciences, PO. 138, Areka, Ethiopia
Tel +25 191 603 8833
Email [email protected]
Background: Hepatitis B virus infection (HBV) constitutes major public health problems in sub-Saharan Africa from different infections occuring in HIV positive patients. Ethiopia is a part of sub-Saharan Africa with 1.5% adult HIV prevalence, and also belongs to the intermediate to high HBV prevalence category. Hence, this study aimed to measure the seroprevalence and associated factors of HBV infection among HIV-positive adults attending an antiretroviral treatment (ART) clinic at Wolaita Sodo University Referral Hospital.
Methods: An institution-based cross-sectional study was conducted from October 15 to December 10, 2017 using a systematic random sampling technique. After getting informed written consent, data were collected by a structured and interviewer-administered questionnaire. Venous blood was collected and centrifuged to separate serum. Hepatitis B surface antigen (HBsAg) was detected from serum using an advanced quality one-step rapid test kit. Data were entered into EpiData version 3.01 and exported to SPSS version 20. Summary statistics, bivariate analysis, and multivariate analyses were performed. The variables having significant association of P<0.05 in the multivariate logistic regression were taken as independent factors. OR and 95% CI were used to measure the strength of the association.
Results: A total of 442 study participants, 187 males and 255 females, were included in this study. Overall prevalence of HBsAg was 37 (8.4%). Family history of HBV (adjusted OR=8.83, 95% CI=2.56–30.49), multiple sexual partners (adjusted OR=7.08, 95% CI=2.29–21.9), and CD4 count <200 cells/μL (adjusted OR=15.34, 95% CI=4.77–49.3) were found to be significantly associated with HBsAg positivity.
Conclusion: The prevalence of HBsAg in this study was high. Family history of HBV, multiple sexual partners, and CD4 count <200 cells/μL were independently associated with HBsAg positivity. Therefore, screening for HBV is recommended before initiation of ART in HIV patients and providing appropriate treatment for co-infection. Furthermore, accurate information on risk factors for HBV transmission should be provided.
Keywords: hepatitis B surface antigen, HIV, co-infection, HIV-positive adults, highly active antiretroviral treatment regimens, hepatitis B virus infection
Introduction
Background
Viral hepatitis is an inflammation of the liver affecting millions of people every year. Of the five different types of hepatitis viruses (A, B, C, D, and E), hepatitis B virus (HBV) infects the liver more than other viruses. HBV is a DNA virus that replicates in the liver cells and causes acute and chronic infections of the liver. It is a major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Most people are unaware of their infection with viral hepatitis and unknowingly transmit the infection to other people, so it is a silent epidemic due to its highly asymptomatic nature.1–3
About 80% of countries, including all countries in the African region, have recognized viral hepatitis to be an urgent public health issue. Most persons who become chronic carriers of HBV live in Asia and Africa. Ethiopia, part of Africa, has significant hepatitis B transmission in both children and adults, displaying around 6–12% hepatitis B surface antigen (HBsAg) prevalence. It also displays 12% of hospital admissions and 31% of mortality on medical wards due to acute viral hepatitis, chronic viral hepatitis, cirrhosis of the liver, and hepatocellular carcinoma.4–6
HBV is reported to be 50–100 times more infectious than HIV; it can survive for several weeks even in dried blood.7,8 Blood is the most important vehicle for its transmission. Semen, tears, and saliva are also possible vehicles for its transmission. Percutaneous contact with infected blood, unprotected sexual contact, infected mother-to-infant, unsafe blood transfusion, sharing razors, toothbrushes, needles, and syringes, tattooing, and body piercing are some modes of HBV transmission.1,2,9
The seromarkers and biomarkers associated with HBV infection are HBsAg, hepatitis B surface antibody, hepatitis E antigen, and hepatitis B core antigen. From these seromarkers, the presence of a confirmed HBsAg result is indicative of ongoing HBV infection. So, all HBsAg-positive persons should be considered infectious and it is the only serologic marker detected during the first 3–5 weeks after infection in newly infected persons.10
Because of common routes of transmission, frequent co-infection of viral hepatitis and HIV is common. An estimated 5–25% of people living with HIV are also infected with HBV (2–4 million). The mean HIV/HBV co-infection in 20 sub-Saharan African countries was reported as 15%.11 Some hospital-based studies documented 3.9–14% HBsAg prevalence among HIV patients, which is intermediate to high prevalence, in Ethiopia.8,12
HIV-infected individuals are more likely to develop chronic hepatitis B than are HIV-negative individuals after infection with hepatitis B in both groups. HIV accelerates the progression of HBV-related liver disease. Cirrhosis is more common in co-infected cases than in HBV mono-infected cases. HIV/HBV co-infected men are also more likely to die of liver-related causes compared to those mono-infected with HBV.13
HBV infection is a serious public health problem that occurs all over the world. According to the WHO estimation, more than 2 billion people are HBV infected and about 378 million people are chronic carriers of HBV in the world. There are approximately 620,000 HBV-related deaths each year. Approximately 4.5 million new HBV infections are occurring worldwide each year and a quarter progress to liver disease from these new infections. It was also estimated that more than 50% of liver cancers were caused by HBV.2
The introduction of HBV vaccines to prevent HBV infection in the 1980s is to be considered the major achievement.2 The introduction of highly active antiretroviral treatment (ART) for treatment of HIV infection has decreased morbidity and mortality from HIV infection. However, the management of HIV/HBV co-infection has become increasingly important since the management of the co-infected patient is complex, as the presence of one infection can affect the management of the other in a number of ways.12,14,15
Since several antiviral agents have activity against HIV or HBV alone or HIV and HBV co-infection, the issues of HIV and HBV drug resistance must be considered when selecting therapeutic regimens. When stopping ART regimens in patients with HIV/HBV due to treatment failure, toxicity, or other reasons, agents with anti-HBV activity may be stopped together although the patient is positive for HBV. This causes a hepatitis flare reaction which is an elevation of aminotransferases to more than 10 times the upper limit of normal and more than twice the baseline value during the natural course of a chronic HBV infection. So, clinicians need to be mindful for this problem in HIV patients before stopping ART regimens containing agents effective against HBV.13,14
Approximately, 10% of the 40 million HIV-positive individuals in the world have chronic hepatitis B infection.15 Their co-infection is reported as high as 10–20% in countries where HBV infection is either endemic or intermediate to high.15 HBV constitutes a major public health challenge in sub-Saharan Africa from different infections in HIV patients.16 Ethiopia is part of sub-Saharan Africa with 1.5% HIV prevalence in adult,17 and also belongs in the intermediate to high HBV prevalence category8 according to the global clustering of the prevalence of chronic HBV infection.
However, data on the prevalence of hepatitis B infection and associated risk factors among HIV/AIDS patients in Ethiopia are scarce. The majority of the previous studies on hepatitis B infection were conducted in pregnant women and healthy blood donors in Ethiopia.18 A few studies on HBV infection were conducted among HIV patients9,18,19 but these studies have not well addressed the distribution and associated factors for HBV transmission in HIV-positive adults, particularly in the study setting. Thus, assessing the prevalence of HBV infection and associated factors among HIV-positive adults becomes most important, because they are productive age groups who can move from place to place and can be exposed to many risk factors for HBV infection including unsafe sexual intercourse. Once exposed, they can serve as a reservoir of HBV transmission due to the silent nature of the virus.
Moreover, since therapeutic management is dependent on the correct diagnosis and staging, the state of HBV infection in the HIV-infected individual should be accurately diagnosed and assessed.15 However, treating of HIV/AIDS-infected individuals with ART without prior screening for HBV infection is common in many Ethiopian health facilities. This results in high ART drug resistance, and severe liver toxicity and cirrhosis.18 So, early screening using serological markers and preventing transmission is very essential because HBV is preventable with a safe and effective vaccine available.
Significance of the study
The quality of treatment and life in HIV patients co-infected with HBV can be improved by appropriate management and monitoring. Knowing the magnitude of HBV infection and associated risk factors for its transmission is fundamental data for health planners and health professionals working in the ART clinic for appropriate medical management and monitoring. Moreover, this can reduce the occurrence of multiple complications due to viral HBV co-infection in HIV patients and can also help to undertake effective prevention measures once the status of HBV infection is recognized.
Therefore, this study aimed to measure the seroprevalence and associated factors of HBV infection among HIV-positive adults attending an ART clinic at Wolaita Sodo University Referral Hospital (WSURH). Data from this study can also be the baseline for further large-scale study.
Objectives of the study
This study aimed to determine the magnitude of HBV infection among HIV-positive adults attending an ART clinic at WSURH.
The study also aimed to identify associated factors of HBV infection among HIV-positive adults attending an ART clinic at WSURH.
Methods and materials
Study setting
The study was conducted at WSURH which is located in Sodo town, southern Ethiopia. The hospital has more than 200 beds for inpatient services which are in medical, emergency, and critical care units, pediatrics, surgical, gynecology, eye unit, and obstetric wards. Currently, there are 1445 HIV patients under the ART service, of which 112 are children and 1333 are adults.
Study design and period
An institution-based cross-sectional study was conducted among HIV-positive adults attending an ART clinic at WSURH from October 15 to December 10, 2017.
Population
Source population
All HIV-positive adults attending an ART clinic at WSURH were considered the source population.
Study population
The study population consisted of all HIV-positive adults aged 15 years and older attending an ART clinic at WSURH during the study period.
Eligibility criteria
Inclusion criteria
HIV-positive adults aged 15 years and older who signed informed consent and were able to answer the questionnaire were included.
Exclusion criteria
HIV-positive adults who were critically sick or were unable to speak and hear were excluded.
Study variables
Dependent variable
Infection by HBV was the dependent variable.
Independent variables
Sociodemographic variables (age, sex, residence, marital status, occupation, education, income), route-associated factors (history of sharing sharp instruments, history of blood transfusion, multiple sexual partners, family history of HBV, history of hospital admission, surgical history, tattooing, tooth extraction, ear piercing, circumcision, unsafe therapeutic injection), and clinical characteristics (history of opportunistic infection, years lived with HIV virus, history of sexually transmitted infection [STI], CD4 count, and highly active ART regimens) were independent variables.
Sample size determination
The sample size was calculated for both objectives
Sample size for first specific objective
The sample size was calculated using a single population proportion formula by assuming the prevalence of HBV to be 6.9%,19 with 95% CI and 2.5% marginal error, and considering 10% contingency for possible nonresponse. Since the source population of the study population was 1333, which was less than 10,000, a finite population correction formula was used to adjust the necessary sample size. After considering a contingency of 10% for the nonresponse rate, the final sample size became 303+303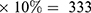 .
.
Sample size for second specific objective
The sample size was calculated using OpenEpi version 3.03, and using factors statistically associated with HBsAg positivity among HIV patients taken from Mekelle Hospital18 and Hawassa University Referral Hospital.19 The study in Mekelle Hospital found a history of multiple sexual partners and the study in Hawassa University Referral Hospital found a history of surgical procedure and opportunistic infection were statistically associated with HBsAg prevalence.
The 442 participants calculated as the maximum sample size would have better representativeness taken as the final sample size.
Sampling technique
A systematic random sampling technique was used to select the study participants among HIV patients attending an ART clinic, with a sampling interval of three. Of the first three subjects, one study participant was selected by lottery method, and then every third study participant was selected to participate in the study. Duplication was controlled by marking on the patient's card.
Data collection method
Enrollment and data collection procedure
Prior to data collection, 1 day of training was given to two nurses holding a BSc degree in nursing for data collection, one MPH student for supervision, and one laboratory technologist for laboratory analysis, respectively. Then, a pretested structured questionnaire containing sociodemographic and other explanatory variables was delivered to eligible HIV patients after informed consent was taken to collect data through face-to-face interview by two trained BSc nurses working at the ART clinic. Then, the patients were ready to give blood samples.
Specimen collection and processing
Five milliliters of venous blood was collected following standard operational procedures by a trained laboratory technologist from each study subject. 3 mL was added into a red top vacutainer tube for HBsAg test and 2 mL was added into a K3EDTA test tube for CD4+ count. The blood specimen for the HBsAg test was allowed to clot at room temperature and serum was separated by centrifugation at 5000 rpm for 15 min. The collected serum specimen was tested for HBsAg using an advanced quality one-step rapid test kit according to the manufacturer's guidelines.20
For the CD4+ count, the whole blood was incubated in a CD4+/CD8+ reagent vial and a fixative was added to measure the CD4+ count using a FACS count analyzer (BD FACS count; BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instruction.
Laboratory testing method
Sample testing for HBsAg was done using an advanced one-step HBsAg test which is 98.89% sensitive and 98.87% specific.20 This is a colloidal gold enhanced immunoassay for the determination of HBsAg in human whole blood, serum, or plasma. Goat anti-HBsAg antibody is immobilized in the test region on a nitrocellulose membrane. During the assay, the specimen was allowed to react with the colored conjugate (antibody–colloidal gold conjugate); the mixture then migrated chromatographically on the membrane by capillary action. An HBsAg-positive specimen produced a distinct color band in the test region, formed by the specific antibody–HBsAg-colored conjugate complex. Absence of this colored band in the test region suggested a negative result. A colored band in the control region served as procedural control regardless of the test result.
Data quality control
The questionnaire was translated into Amharic language to make it appropriate and understandable; a pretest was made on 5% of the sample size at Sodo Health Center 1 week before actual data collection. Necessary modifications were made to the questionnaire after conducting the pretest. Close supervision was undertaken during data collection to avoid bias. Standardized procedures were strictly followed during blood sample collection, storage, and the analytical process.
Positive and negative control samples within the test kit were run to assess the performance of the test kit as an internal quality control. Known positive and negative serum samples for HBsAg confirmed by ELISA were obtained from the Ethiopian Red Cross Society Blood Bank, Arba Minch branch. These known serum samples were analyzed before the actual investigation as an external quality control of the test kit.
Data management
The completeness and consistency of questionnaires were checked by the principal investigator and supervisor on each day of data collection. After checking the completeness, accuracy, and clarity, the data were entered into EpiData version 3.01.
Data analysis procedure
The data entered into EpiData version 3.01 were exported to Statistical Package for Social Science (SPSS) software version 20 for analysis. Summary statistics such as frequencies and percentages were computed. Bivariate analysis was conducted to check the association of each variable with the dependent variable. Variables that had association with the dependent variable at a P-value of 0.25 were entered into multivariate logistic regression to control for possible effects of confounding. The final model was then tested for its goodness of fit by the Hosmer and Lemeshow test, and P>0.05 was taken as best fit. The variables which had significant association with P<0.05 in the multivariate logistic regression were considered independent factors. The OR and 95% CI were used to measure the strength of the association.
Ethical consideration
This study was conducted in accordance with the Declaration of Helsinki that provides guidance for researchers to protect research subjects. The study was approved by the institutional research review committee of Wolaita Sodo University. Then, the participants were informed that participation is on a voluntary basis, and about the benefits and harms of participation. Sample collection was done based on their agreement. Samples were handled properly and participants and physicians were informed of results based on permission of the participants for their benefit. Each respondent was informed about the objective of the study and informed written consent was obtained. Confidentiality and voluntariness were kept at each step of data collection and processing. The participant was interviewed alone to keep privacy and all participants did not pay for the test. Finally, positive HBsAg test results of the study participants were communicated to the clinicians who are working at the ART clinic of the hospital for further diagnosis and better management of the patients.
Results
Sociodemographic and economic characteristics
A total of 442 HIV-positive adults comprising 187 (42.3%) males and 255 (57.7%) females were included in this study. The mean (±SD) age of study participants was 36.79 (±9.97) years with the highest category of age being 35–44 years. 361 (81.7%) were living in an urban setting, 236 (53.4%) were married, and 116 (26.2%) were housewives. 117 (26.5%) study participants had no formal education. Of 442 study participants, 182 (41.2%) had ≤500 ETB average monthly income, as shown in Table 1.
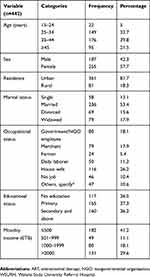 |
Table 1 Sociodemographic and economic characteristics of HIV-positive adults attending an ART clinic at WSURH from October 15, 2017 to December 10, 2017 |
Route-associated risk factors
From a total of 442 study participants, 210 (47.5%), 20 (4.5%), 50 (11.3%), and 86 (19.5%) had a history of hospital admission, blood transfusion, surgical procedure, and sharing sharp materials, respectively. 35 (7.9%) had family members with HBV infection. Those who had a history of tattooing, ear piercing, and tooth extraction were 123 (27.7%), 233 (52.7%), and 221 (50%), respectively. 235 (53.2%) had more than one sexual partner, as shown in Table 2.
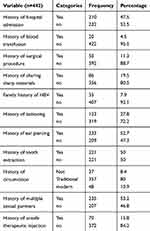 |
Table 2 Route-associated risk factors of HBV infection among HIV-positive adults attending an ART clinic at WSURH from October 15, 2017 to December 10, 2017 |
Clinical characteristics
318 (71.9%) had a history of opportunistic infections (OIs) since their HIV status had been known. 137 (31%) had a history of STIs other than HIV and HBV infections. The mean (±SD) years that study participants lived with HIV was 8.4 (±3.7) with a range of 1–20 years. Of the total study participants, 87 (19.7%) had CD4 count<200 cells/µL. For the treatment protocol, 35.3% were taking TDF, 3TC, and EFV, 30.8% were taking AZT, 3TC, and NVP, 11.5% were taking AZT, 3TC, and EFV, and 12.7% were taking second-line ART drugs, as shown in Table 3.
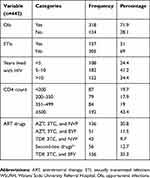 |
Table 3 Clinical characteristics of HIV-positive adults attending an ART clinic at WSURH from October 15, 2017 to December 10, 2017 |
Prevalence of HBsAg
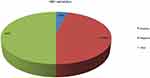
From 442 HIV-positive study participants included in this study, the overall prevalence of HBsAg was 37 (8.4%). The prevalence of HBV infection was 16 (8.6%) among males and 21 (8.2%) among females, respectively. The highest prevalence was detected among participants with CD4 count<200 cells/µL (25.3%) followed by family with HBV history (22.9%) followed by second-line drug users (19.6%), respectively. It was also high among those who had a history of sharing sharp materials (16.3%) and those who had a history of multiple sexual partners (13.2%), respectively, as shown in Figure 1.
Multivariate analysis of variables associated with HBV infection
In the bivariate analysis, occupational status, hospital admission, sharing sharp materials, family history of HBV, tooth extraction, multiple sexual partners, OIs, STIs, CD4 count<200 cells/μL, and second-line ART drugs have shown statistically significant association (p<0.25) with HBsAg seropositivity. When these significantly associated variables were entered into the multivariate model, family history of HBV (adjusted OR [AOR]=8.83, 95% CI=2.56–30.49, p=0.001), multiple sexual partners (AOR=7.08, 95% CI=2.29–21.9, P=0.001), and CD4 count<200 cells/μL (AOR=15.34, 95% CI=4.77–49.3, P=0.000) were found significantly associated with HBsAg positivity.
Although there was no statistically significant association, those taking AZT, 3TC, and EFV (AOR=2.41, 95% CI=0.56–10.43) and those taking second-line ART drugs (AOR=2.47, 95% CI=0.72–8.42) showed an increased HBsAg positivity compared with those who were taking TDF, 3TC, and EFV based combined ART therapy, as displayed in Table 4.
 |
Table 4 Multivariate analysis of variables associated with HBV infection among HIV-positive adults attending an ART clinic at WSURH from October 15, 2017 to December 10, 2017 |
Discussion
The overall prevalence of HBsAg among 442 HIV-positive adult study participants in this study was 37 (8.4%). It was 8.6% among males and 8.2% among females. The finding of the present study is similar to previous studies conducted in Uganda (8.3%),22 in Vietnam (8.4%),23 in Myanmar (8.7%),24 and in Taiwan (7.8%)25 among HIV-infected patients.
On the other hand, this finding was relatively higher as compared with studies conducted in Tigray (5.9%),18 in Hawassa (6.9%),19 in Gondar (6.1%),9 and in Addis Ababa (3%)11 among HIV-infected patients in Ethiopia. It was also relatively higher than studies done in Brazil (2.5%),26 in Canada (5.5%),27 and in Botswana (5.3%)28 among HIV patients. This might be due to the low awareness about transmission and prevention of HBV in our study population. The other possible reason might be lack of hepatitis B vaccine which is a cornerstone for HBV prevention in our study setting.
The finding of this study was also higher compared to some studies done among different population groups in Ethiopia like 3.7% in Jimma,29 4.3% in Arba Minch,30 and 6% in Jigjiga among pregnant women attending an antenatal clinic, 4.2% in Bahir Dar31 among military personnel, and 2.4% in Addis Ababa32 among health professionals. This is an indication that the prevalence of HBsAg seropositivity among HIV-infected individuals is higher than among the general population.
However, the prevalence of HBsAg was found lower compared to 14% in Shashemene8 and 19% in Bahir Dar31 in Ethiopia among HIV-infected patients. It was also similarly lower compared to studies conducted in different areas in Nigeria among HIV patients; 26.5% in Gombe,33 20% in Nsukka,34 12.1% in Uyo,7 and 12.3% in northwestern Nigeria.16 It was also lower than studies done in Sudan (11.7%),35 in Iran (35.5%),36 and in India (19.5%).37 The high prevalence of HBV in these studies compared to our study might be due to the more advanced diagnostic tools they used like the HBV DNA real-time PCR and HBsAg ELISA technique, but in our study we used only a one-step rapid HBsAg serological kit. The lower rate of HBsAg prevalence in this study might also be due to the effect of Tenofovir, which is active against both HBV and HIV. In our study, 199 (45%) were using Tenofovir, a nucleotide reverse-transcriptase inhibitor, with potent in vitro activity against lamivudine-resistant HBV. The other possible reasons could be due to the lower proportion of high-risk groups such as intravenous drug users and homosexual men in the study setting.
The present study found a statistically significant association between HBsAg and family history of HBV (AOR=8.83, 95% CI=2.56–30.49, P=0.001). This finding is supported by a study conducted in central Brazil.26This might be due to close contact of family members with the HBV-positive patient in the family (family environment).
In this study, 53.2% of HIV patients had a history of multiple sexual partners. Among patients with this exposure, the prevalence of HBsAg was 13.2% which is significantly higher than those without such exposure (13.2% versus 2.9%, AOR=7.08, 95% CI=2.29–21.9, P=0.001). This finding is in line with similar studies conducted in South Gondar, Ethiopia,9 in Brazil,38 and in Mexico.39 This finding supports the fact that people who have multiple sexual partners will be prone to HBV infection and other sexually transmitted disease.40 It also supports the fact that adolescents having sex with multiple partners are at higher risk of acquiring SDTs including HIV and unplanned pregnancy.41
A higher prevalence of HBsAg was seen in HIV patients with CD4 count<200 cells/μL than in those with CD4 count≥500 cells/μL; this study showed statistically significant association between HBV infection and low CD4 count (AOR=15.34, 95% CI=4.77–49.3, P=0.000). This finding is similar to studies done in Tigray, northern Ethiopia,18 in Nepal,42 and in Thailand.43 This significant association might be due to the fact that at a lowered CD4 count, re-emergence of HBV replication occurs due to spontaneous reverse seroconversion marked by the disappearance of anti-hepatitis B surface antibodies and reappearance of HBsAg.
Furthermore, the seroconversion rate of HBsAg between study participants who were taking AZT, 3TC, and EFV and second-line drugs was compared with those who were taking TDF, 3TC, and EFV combined ART therapy. Although there was no statistically significant association observed between those taking AZT, 3TC, and EFV (AOR=2.41, 95% CI=0.56–10.43) and those taking second-line ART drugs (AOR=2.47, 95% CI=0.72–8.42), there was an increased HBsAg positivity compared with those who were taking TDF, 3TC, and EFV-based combined ART therapy. This was similar to studies done in Tigray18 and in Kenya.44
The reason for this can be justified by the efficacy difference between these three treatment regimens. The HIV antiretroviral drugs tenofovir (TDF), lamivudine (3TC), and emtricitabine (EFV) are the backbone of nucleoside/nucleotide analogues effective against both HIV and HBV. Tenofovir plus emtricitabine or tenofovir plus lamivudine and efaviranz is the preferred first-line therapy for HIV/HBV co-infected patients.14,45 Starting from 2010, the WHO also recommended that tenofovir should be contained in the first-line regimen for HIV patients.46
Conclusion
The prevalence of HBsAg among HIV-positive adults was high compared with the intermediate endemicity among the general population in previous studies in Ethiopia. Family history of HBV, history of multiple sexual partners, and CD4 count<200 cells/μL were significantly associated with HBsAg positivity among HIV-positive adults.
HBsAg seropositivity was also higher among the AZT, 3TC, and EFV users and second-line ART drug users than the TDF, 3TC, and EFV combination ART drug users due to the high efficiency of TDF. So, early screening of HBV and providing appropriate treatment for HIV/HBV co-infection are essential. Moreover, health education about the transmission and prevention of HBV is needed.
Recommendations
- The ART clinic should screen HIV-positive patients for HBV before ART initiation.
- The ART clinic should provide two active HBV agents, TDF plus 3TC or TDF plus EFV, for co-infected individuals when ART is initiated.
- Health institutions giving HIV-related services should provide accurate awareness on risk factors such as family with HBV, having multiple sexual partners, and low CD4 count.
- Federal Ministry of Health should incorporate routine HBV screening into the ART treatment guideline.
- WSU should provide a hepatitis B vaccination service for HIV patients not infected with HBV.
- Further study should be conducted using advanced modern machines.
Abbreviations list
ABC, Abacavir; AIDS, Acquired immune deficiency syndrome; ALT, Alanine aminotransferace; AOR, Adjusted odds ratio; ART, Antiretroviral therapy; ARV, Antiretroviral; ATV, Atazanavir; AZT, Zidovudine; CI, Confidence Interval; DNA, Deoxyribonucleic acid; EDTA, Ethylene diamine tetra acetic acid; EFV, Efavirenz; ELISA, Enzyme linked immunosorbant assay; HAART, Highly active antiretroviral therapy; HBcAg, Hepatitis B core antigen; HBeAg, Hepatitis B e antigen; HBs, Hepatitis B surface antibody; HBsAg, Hepatitis B surface antigen; HBV, Hepatitis B virus; HCC, Hepatocellular carcinoma; HCV, Hepatitis C virus; HIV, Human immunodeficiency virus; OR, Odds ratio; PCR, Polymerase Chain Reaction; RPM, Revolution per minute; SPSS, Statistical Package for Social Science; STI, Sexually transmitted infection; WHO, World Health Organization; WSURH, Wolaita Sodo University Referral Hospital.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki that provides guidance for researchers to protect research subjects. The study was approved by the institutional research review committee of Wolaita Sodo University. Then, the participants were informed, as participation is on a voluntary basis, about the benefits and harms of participation. Sample collection was done based on their agreement. Samples were handled properly and participants and physicians were informed of results based on permission of the participants for their benefit. Informed written consent is obtained from participants aged ≥18 years, and for those younger than 18 years old, informed written consent was obtained from parents or guardians.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization Prevention and Control of Viral Hepatitis: Frame Work for Global Action; 2012. Available from: https://www.who.int/hiv/pub/hepatitis/Framework/en/#targetText=WHO's%20Prevention%20and%20Control%20of,regional%20and%20country%2Dspecific%20strategies.
2. Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):74–80. doi:10.4254/wjh.v4.i3.74
3. WHO. Regional action plan for viral hepatitis in the Western Pacific 2016–2020: a priority action plan for awareness, surveillance, prevention and treatment of viral hepatitis in the Western Pacific Region. 2014. ISBN 978 92 9061761 7
4. WHO. Global policy report on the prevention and control of viral hepatitis in WHO Member States: a Strategy for Global Action. Geneva: World Health Organization; 2013. ISBN 978 92 4 156463 2.
5. Azage GAAM. Hepatitis B vaccine knowledge and vaccination status among health care workers of Bahir Dar City administration Northwest Ethiopia. BMC Infect Dis. 2015;15(p):30. doi:10.1186/s12879-015-0756-8
6. FMOH. Plan for the Introduction of Hepatitis B and Haemophilus Influenza Type B Vaccine into Routine Immunization in Ethiopia; 2005.
7. Ekanem U.S., Eyoh JE Esubok NU. Prevalence of hepatitis- B virus infection among HIV patients seen in university of UYO teaching hospital (UUTH), UYO. Int J Res Biosci. 2013;2(1):92–98.
8. Negero A, Sisay Z, Medhin G. Prevalence of Hepatitis B surface antigen (HBsAg) among visitors of Shashemene General Hospital voluntary counseling and testing center. BMC Res Notes. 2011;4:35. doi:10.1186/1756-0500-4-35
9. Balew M, Moges F, Yismaw G, Unakal C. Assessment of hepatitis B virus and hepatitis C virus infections and associated risk factors in HIV infected patients at Debretabor hospital, South Gondar, Northwest Ethiopia. Asian Pac J Trop Dis. 2014;4(1):1–7. doi:10.1016/S2222-1808(14)60304-2
10. Hoofnagle JH, Di Bisceglie AM. Serologic diagnosis of acute and chronic viral hepatitis. Semin Liver Dis. 1991;11:73–83. doi:10.1055/s-2008-1040426
11. Manyazewal T, Sisay Z, Biadgilign S, Abegaz WE. Hepatitis B and hepatitis C virus infections among antiretroviral-naive and -experienced HIV co-infected adults. J Med Microbiol. 2014;63:742–747. doi:10.1099/jmm.0.063321-0
12. Bezabeh YH, Muluken DM, Solomon GS, Helmut K. Higher prevalence of Hepatitis B virus Infection among ARV- exposed than naive HIV-infected individuals in North Shewa Zone, Ethiopia. J AIDS HIV Res. 2015;7(1):10–17. doi:10.5897/JAHR
13. Pittman C, Plitt S, Birse T, et al. Prevalence and correlates of HIV and hepatitis B virus coinfection in Northern Alberta. Can J Infect Dis Med Microbiol. 2014;25:1:e8-e13
14. Brito M, Alhyraba M. Hepatitis B in Patients Coinfected with HIV. Hospital Physician. 2008;30:17–23.
15. Thio CL. Hepatitis B and Human Immunodeficiency Virus Coinfection. Hepatology. 2009;49(5). doi:10.1002/hep.22883
16. Hamza M, Samaila A, Yakasai A, Babashani M, Borodo M, Habib A. Prevalence of hepatitis B and C virus infections among HIV-infected patients in a tertiary hospital in North-Western Nigeria. Niger J Basic Clin Sci. 2013;10:2. doi:10.4103/0331-8540.122765
17. Central Statistical Agency, Ethiopa; ICF International. Ethiopia Demographic and Health Survey; 2011. Available from: https://dhsprogram.com/pubs/pdf/PR10/PR10.pdf.
18. Weldemhret L, Asmelash T, Belodu R, Gebreegziabiher D. Sero‑prevalence of HBV and associated risk factors among HIV positive individuals attending ART clinic at Mekelle hospital, Tigray, Northern Ethiopia. AIDS Res Ther. 2016;13:6. doi:10.1186/s12981-016-0090-2
19. Belayneh F. Prevalence of hepatitis B virus infection and associated factors among HIV Positive adults attending ART Clinic at hawassa referral hospital, SNNPR, Ethiopia. Open Access Lib J. 2015;2:e1490. doi.org/10.4236/oalib.1101490.
20. In Tec Products, I.x., China. Advanced Quality in Medical Diagnostics, One Step HBsAg Test Card; 2016.
21. World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children; 2007. Available from: https://apps.who.int/iris/handle/10665/43699.
22. Bwogi J, Makumbi I, Mishra V, Bakamutumaho B, Nanyunja M. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 2009;9:98–108.
23. Huy BV, Vernavong K, Nguy N. HBV and HCV coinfection among HIV/AIDS Patients in the National Hospital of Tropical Diseases, Vietnam. December. 2014;8:5.
24. Zaw SKK, Tun STT, Thida A, et al. Prevalence of hepatitis C and B virus among patients infected with HIV: a cross-sectional analysis of a large HIV care programme in Myanmar. Trop Doct. 2013;43(3):113–115. doi:10.1177/0049475513493416
25. Sun C-YC, Lee N-Y, Yang C-J, et al. Seroprevalence of hepatitis B virus among adults at high risk for HIV transmission two decades after implementation of nationwide hepatitis B virus vaccination program in Taiwan. PLoS One. 2014;9(2):e90194.
26. Freitas, S.Z., Soares CC, Tanaka TS et al. Prevalence, risk factors and genotypes of hepatitis B infection among HIV-infected patients in the State of MS, Central Brazil. Brazj Infect Dis. 2014;18(5):473–480.
27. Negussie A, Beyene E. Seroprevalence of Hepatitis B Surface Antigenemia among Pregnant Women Attending Antenatal Clinic in Jigjiga, East Ethiopia: A Cross-Sectional Study. Int J Res Rev. 2016;3(2):1-7.
28. Patel P, Anabwani G, Davis S, Mabikwa V, Patel P. Prevalence of hepatitis B and hepatitis C coinfections in an adult hiv centre population in Gaborone, Botswana. Am J Trop Med Hyg. 2011;85(2):390–394. doi:10.4269/ajtmh.2011.10-0510
29. Awole M, Gebre-Selassie S. Seroprevalence of HBsAg and its risk factors among pregnant women in Jimma, Southwest Ethiopia. Ethiop J Health Dev. 2005;19(1):45–50. doi:10.4314/ejhd.v19i1.9970
30. Yohanes T, Zerdo Z, Chufamo N. Seroprevalence and predictors of hepatitis b virus infection among pregnant women attending routine antenatal care in arba minch hospital, South Ethiopia. Hindawi Publ Corp Hepatitis Res Treat. 2015;2016:7.
31. Birku T, Gelaw B, Moges F, Assefa A. Prevalence of hepatitis B and C viruses infection among military personnel at Bahir Dar Armed Forces General Hospital, Ethiopia. BMC Res Notes. 2015;8:737. doi:10.1186/s13104-015-1719-2
32. Desalegn Z, Selassie SG. Prevalence of hepatitis B surface antigen (HBsAg) among health professionals in public hospitals in Addis Ababa, Ethiopia. Ethiop J Health Dev. 2013;27:1.
33. Jibrin SKM, Jibrin YB. The prevalence of hepatitis B surface antigenaemia in patients with HIV infection in Gombe, Nigeria. Ann Afr Med. 2004;3(1):10–12.
34. Uju MED, do GEO, Obukwelu C. Co-infection of Hepatitis B virus (HBV) and Hepatitis C virus among Human Immunodeficiency Virus (HIV) infected people: case study of Nsukka. Int J Curr Microbiol App Sci. 2013;2(120):89–103.
35. Mudawi H, Hussein W, Mukhtar M, et al. Overt and occult hepatitis B virus infection in adult Sudanese HIV patients. Int J Infect Dis. 2014;29:65–70. doi:10.1016/j.ijid.2014.07.004
36. Davarpanah MA, Motazedian N, Fallahzadeh E, Rasti M, Rahmati H, Motazedian N. Hepatitis B virus infection serology and the associated risk factors among patients with HIV in Shiraz, Iran. Shiraz E-Med J. 2015;16(4). doi:10.5812/semj.
37. Antala SK, Joshi TK. Seroprevalence of Hepatitis B, Hepatitis C and Syphilis in HIV positive cases at ICTC, Rajkot. Gujarat Med J. 2010;65:1.
38. Lewis-Ximenez LL, Do Ó KM, Ginuino CF, et al. Risk factors for HBV infection in Rio de Janeiro, Brazil. BMC Public Health. 2002;2:26. doi:10.1186/1471-2458-2-26
39. Méndez-Sánchez N, Motola-Kuba D, Zamora-Valdés D, et al. Risk factors and prevalence of hepatitis virus B and C serum markers among nurse at a tertiary care hospital in Mexico City, Mexico: a descriptive study. Ann Hepatol. 2006;5:276–280.
40. Cruz HM, Scalioni LP, Paula VS, et al. Poor sensitivity of rapid tests for the detection of antibodies to the hepatitis B virus: implications for field studies. Mem Inst Oswaldo Cruz. 2017;112(3):209–213. doi:10.1590/0074-02760160394
41. WHO. WHO Case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV related disease in adults and children. 2006. ISBN 978 92 4 159562 9.
42. Bhattarai, M, Baniya JB, Aryal N et al. Epidemiological profile and risk factors for acquiring HBV and/or HCV in HIV-infected population groups in Nepal. Hindawi BioMed Res Int. 2018;2:7.
43. Phuangchoei P, Chotiyaputta W, Chayakulkeeree M. Clinical characteristics of hepatitis B and C virus infections in HIV-infected patients. J Med Assoc Thai. 2015;98(3):226–231.
44. Harania RS, Karuru J, Nelson M, Stebbing J. HIV, hepatitis B and hepatitis C coinfection in Kenya. AIDS. 2008;22(10). doi:10.1097/QAD.0b013e32830162a8
45. Sarkar J, Saha D, Bandyopadhyay B, et al. Baseline characteristics of HIV & hepatitis B virus (HIV/HBV) co-infected patients from Kolkata, India. Indian J Med Res. 2016;143:636–642. doi:10.4103/0971-5916.187113
46. World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection; 2015. Available from: https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.