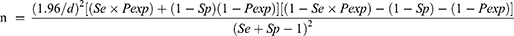Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Seroepidemiology of Neospora caninum in Cattle of Pastoral Production System in Teltelle District of Borana Zone, Southern Ethiopia
Authors Jilo Tache K, Getachew Y, Negussie H
Received 20 June 2022
Accepted for publication 5 September 2022
Published 14 September 2022 Volume 2022:13 Pages 247—256
DOI https://doi.org/10.2147/VMRR.S377408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Kula Jilo Tache,1 Yitbarek Getachew,2 Haileleul Negussie2
1School of Veterinary Medicine, Borena University, Borena, Yabelo, Ethiopia; 2Department of Clinical Studies, College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu, Ethiopia
Correspondence: Haileleul Negussie, Department of Clinical Studies, College of Veterinary Medicine and Agriculture, Addis Ababa University, P. O. Box; 34, Bishoftu, Ethiopia, Email [email protected]
Background: Neosporosis is a major cause of abortion in smallholder dairy farms in Ethiopia. However, its status and impact in pastoral cattle production settings were uncovered. This study was performed with the aims of estimating the seroprevalence and associated potential risk factors for Neospora caninum in Boran cattle in Teltelle district of Borana zone, Ethiopia.
Methods: 180 blood samples were collected from 48 randomly selected pastoral herds using a multistage sampling technique and subjected to an indirect enzyme-linked immunosorbent assay (ELISA) test to detect antibodies specific to N. caninum. A questionnaire survey was also used to identify the potential risk factors of N. caninum in the study area. Evaluation of the associated risk factors was conducted using a multivariable logistic regression model.
Results: Antibodies against N. caninum exposure were detected in 5% of cattle (95% CI: 1.816– 8.184) from 180 animals tested. Similarly, the seroprevalence of N. caninum in herds with at least one positive animal was 14.6% (95% CI: 4.598– 24.567) from 48 herds examined. A multivariable logistic regression model identified the following as significant risk factors: a history of abortion (AOR = 23; 95% CI: 2.354– 188.702; P = 0.006), dystocia (AOR = 11; 95% CI = 22.275– 55.860; P = 0.003), wells water sources (AOR = 9; 95% CI: 1.599– 47.568; P = 0.012), and dogs fed with raw animal products (AOR = 6; 95% CI: 11.213– 27.222; P = 0.028).
Conclusion: This study revealed the first serological evidence of N. caninum exposure in cattle reared under pastoral production system. Our findings suggest N. caninum is likely to be an important cause of abortion and dystocia in cattle in Ethiopia. Management practices, such as provision of hygienic water and restriction of dogs fed with raw animal products, are likely to reduce the risk of infection. Thus, maximizing community awareness about these disease management practices is suggested.
Keywords: abortion, cattle, ELISA, Neospora caninum, pastoral, seroepidemiology
Introduction
Neospora caninum is a protozoan intracellular parasite that significantly causes reproductive disorders in dairy industries.1,2 N. caninum causes abortion and stillbirth in cattle2 and neonatal neuromuscular diseases in dogs.3,4 Dogs are the definitive hosts of N. caninum that shed oocysts in their feces.4 Cattle and other domestic animals are intermediate hosts and acquire the infections via ingestion of feed or water contaminated with oocysts of the parasite.2 The major routes of transmission are horizontal and vertical routes, and lactogenic route is also suspected.4,5
Neospora caninum is the most serious cause of economic losses in dairy industries worldwide.6 It is highly abortifacient in cattle, where the risk of abortion is three to seven times higher in infected cows and as high as 7.4 folds in congenitally infected heifers.4 Similarly, N. caninum was found attributable for 12.5–16% proportions of all the annual bovine abortions in several countries.7,8 Even though there was no firm evidence about N. caninum infection in humans, detections of genetic materials and antibodies in humans and primates could suggest a zoonotic potential of neosporosis.9–11
N. caninum is distributed worldwide; however, it is more prevalent in warm climates and humid areas than in cold and dry regions.10 Primarily, the epidemiology of neosporosis is associated with the presence of definitive hosts.2 A large number of serological surveys revealed that neosporosis is highly prevalent in dairy cattle worldwide.1 In Ethiopia, N. caninum antibody was detected in dairy cattle in urban, peri-urban, and commercial production systems.18,26,30 Asmare et al reported a seroprevalence ranging from 13.3% to 23.8% in dairy cattle of selected milk shed areas in Ethiopia,12–14 and it was identified as the leading cause of abortion followed by bovine viral diarrhea virus and Brucella species in dairy cattle of urban and peri-urban smallholder production systems in Ethiopia.13 However, data on the epidemiology and potential risk factors of N. caninum in pastoral cattle production settings were uncovered. Reproductive inefficiency significantly affects the social security and livelihoods of the pastoralists. In Borana pastoral community, cattle play a pivotal role in the livelihoods as a source of milk and immediate cash income. Cats and dogs are also an integral part of livestock for protection against predators and rodents.15 Particularly, dogs are used as the second herdsman and kept with cattle at grazing land and watering points. Knowledge of the epidemiology of N. caninum in a pastoral setting is important for a better understanding of the impact of the disease and the implementation of the management practices that decrease the risk of exposure of cattle. Therefore, the present study aimed to provide the first insight into the seroprevalence and associated risk factors of N. caninum exposure among the pastoral cattle in the Teltelle district of Borana zone.
Materials and Methods
Descriptions of the Study Area
Teltelle district is one of the 13 districts of Borana zone located at the southernmost tip of Ethiopia as shown in Figure 1. Teltelle has 23 administrative peasant associations (PA), of which it has 12 PA inhabited by pure pastoralists, and the remaining 11 PA are dominated by agro-pastoralists. “Kolla” agro-climatic is dominant with latitude ranging from 500 to 1420 meters above sea level. Distinct bimodal rainfall varying from 400 to 650mm is received from September to November (short rainy season) and March–May (long rainy season) annually, whereas the annual temperature ranges from 17 to 34°C. Teltelle district is sparsely populated with 72,476 human populations, and their livelihoods rely almost on livestock husbandry and to some extent crop production. Due to the scarcity of water and pasture during recurrent droughts in the district and the surroundings, the tension of animal mobility across the Kenyan border is very high. The livestock components in the area are cattle, goats, sheep, camel and equines. The numbers of livestock of the district are 215,918 cattle, 170,055 goats, 76,785 sheep, 2646 camels, and 9201 donkeys.16
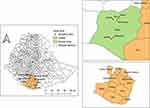 |
Figure 1 Map of Ethiopia showing the location of the study areas. This map was developed from Ethiopian’s administrative boundaries shapefile 2021 using QGIS version 3.1.1.2. |
Study Population, Study Design, and Procedures
The study population was Boran cattle aged greater than 3 years and managed under a pastoral production system. A cross-sectional study was conducted from October 2020 to May 2021. A multistage sampling method was applied to select 6 peasant associations from the district, and then, the random selection was used to select 8 herds having a minimum of 4 female animals aged ≥3 years.
The optimal sample size to establish the seroepidemiology of N. caninum was computed using the formula designated for diagnostic kits with predetermined sensitivity and specificity.17
Where n = the required sample size, Pexp = expected prevalence, Se = test sensitivity, Sp = test specificity, d = desired absolute precision. Accordingly, 188 sample size was determined optimum to detect the minimum expected seroprevalence of 13.3% reported by Asmare et al14 with 5% precision by considering the predetermined sensitivity (98.7%) and specificity (99.5%) described for ID Screen® Neospora caninum Indirect ELISA kit.18
Blood Sample Collection
About 10mL of blood samples were collected from the jugular vein of cattle using plain vacutainer tubes. Samples were kept at room temperature until the serum was decanted. Each sample was then decanted into labeled cryovials and transported in an icebox containing icepack to the National Veterinary Institute laboratory (NVI), Bishoftu. The samples were kept at −20°C until serological examination.
Serology Procedure
The serological test was undertaken for the presence of anti-N. caninum antibodies using indirect ELISA (ID.vet Innovative diagnostics, ID Screen® Neospora caninum Competition, and Montpellier, France) according to the manufacturer’s instructions. Positive and negative controls were included in each test, and an animal was considered infected if the serum was presented with an optical density (OD) of greater than 50%. The validity of serological tests was checked by the mean value of positive control OD (>0.350) and the ratio of mean values of positive and negative controls OD (>3).
Questionnaire Survey
A questionnaire survey was conducted to gather information about the potential risk factors of N. caninum using a semi-structured questionnaire. Data on physiological risk factors (age, body condition score, gestation status, and the number of parity) and history of reproductive disorders (abortion, dystocia, retained placenta, stillbirth, infertility, repeated breeding, and neonatal mortality) were considered the hypothesized risk factors for N. caninum infection. Similarly, information on the risk factors on the management and community practices (herd size, type of dog feed, type of dog housing, disposal way of fetal membrane, and the status of barn hygiene) as well as environmental factors (ecology, source of water, presence of dog and wild felid contacts with the animals) were collected.
Statistical Analysis
Data generated from laboratory investigation and questionnaire survey were recorded in a Microsoft Excel spreadsheet (Microsoft Corporation), coded, and analyzed using R software version 3.6.2. The association of the risk factors with seropositivity of N. caninum at the animal level was analyzed using Firth’s bias reduced logistic regression analysis.19 An ordinary logistic regression model was used to measure the association of the risk factors with seropositivity of N. caninum at the herd level. The association of risk factors was screened out by univariate logistic regression analysis and variables with P-value <0.25 (maximum likelihood ratio test) were offered to the final multivariable model. The risk factors having a P-value <0.05 were considered statistically significant. Multicollinearity and goodness of fit of the models were checked using variance inflation factor (VIF) and Hosmer and Lemeshow tests.
Results
Seroprevalence of N. caninum
In this study, from 180 animals tested, antibodies against N. caninum were detected in 5% (95% CI: 1.816–8.184) of animals. Similarly, the seroprevalence of N. caninum in herds with at least one positive animal was 14.6% (95% CI: 4.598–24.567) from the 48 herds examined.
Physiological Risk Factors
In this study, higher seroprevalence was recorded in animals aged ≥8 years (6.67%; 95% CI = 0.657–9293.418; P = 0.244) compared to other age groups; however, this difference was not statistically significant as shown in Table 1. Although not statistically significant (P = 0.260), higher seroprevalence was found in pregnant cattle (19.35%) compared to non-pregnant (2.01%). Similarly, higher seropositivity was recorded in animals with the number of parity ≥5 (6.67%) followed by parity 3–4 (5.66%) and parity 0–2 (4.46%), but the difference was not statistically significant (P = 0.456) as shown in Table 1.
 |
Table 1 Univariable Logistic Regression Analysis for Physiological Factors and Clinical Disorders Associated with N. caninum Infection |
Environmental Risk Factors
Higher seroprevalence was found in animals managed in lowland (6.79%) as compared to midland (3.89%); however, no statistically significant difference (P = 0.265) as shown in Table 2. Similarly, significantly higher seroprevalence (P = 0.012) was recorded in animals in which water sources were from wells (10.34%) compared to pipe water (2.45%). The odds of acquiring N. caninum exposure were 9 times higher in animals that drank well water than those that drank piped water (AOR = 9; 95% CI = 1.599–47.568; P = 0.012). Although it was not significant (P = 0.181), the seroprevalence of N. caninum was recorded higher in animals where wild felids are present (5.80%) (Table 3).
 |
Table 2 Univariable Analysis of Environmental Factors and Community Practices Associated with N. caninum Infection at Animal Level |
 |
Table 3 Multivariate Logistic Regression Analysis of N. caninum Seropositivity at Animal Level |
Clinical Reproductive Disorders
In the current study, significantly higher seroprevalence was recorded in animals with a history of abortion (15.55%) as compared to those without history of abortion (1.48%). Similarly, higher seroprevalence was recorded in animals with a history of dystocia (9.09%) compared to those without history of dystocia (4.08%). The odds of exposure to N. caninum infection were 23 (AOR = 23; 95% CI: 2.354–188.702; P = 0.006) and 11 (AOR = 11; 95% CI = 22.275–55.860; P = 0.003) times higher in cattle with the history of abortion and dystocia, respectively, as shown in Table 3.
Management and Community Practices
In this study, although not statistically significant (P = 0.952), higher seroprevalence was recorded in medium herd sizes (5.26%) as compared to other herd sizes. Lower seroprevalence was observed in cattle owners who disposed the fetal membrane properly (4.31%) compared to those who gave it to their pets (6.25%); however, this difference was not statistically significant (P = 0.08) (Table 2). Cattle owners who fed their dogs raw animal products had a 6-times higher risk of exposure to N. caninum compared to those owners who fed their dogs a human leftover (AOR = 6; 95% CI = 1.213–27.222; P = 0.028). Higher seroprevalence was recorded in animals that had dog contacts (6.38%) than those without dog contacts (3.48%); however, the difference was not statistically significant (P = 0.580). Although not statistically significant (P = 0.449), the seroprevalence of 5.35% (n = 6) and 4.41% (n = 3) was recorded in animals managed in poor and good hygiene barns, respectively, as shown in Table 2.
Herd Level Seroprevalence and Associated Risk Factors
In this study, the history of clinical reproductive disorders had a significant association with the seropositivity of cattle herds. The highest seroreactor was recorded in herds with a history of retained placenta (31.25%), abortion (25%), dystocia (25%), and presence of wild felid (24%) as shown in Table 4. Multivariable analysis showed that dystocia and abortion had significantly associated (P < 0.05) with seropositivity of N. caninum in the cattle herds. Herds with a history of abortion had a 16-times higher risk of N. caninum exposure compared to the herd without a history of abortion (AOR = 16; 95% CI = 1.446–175.939; P = 0.024). Similarly, the odds of acquiring N. caninum infection was 7 times higher in herds with a history of dystocia compared to those without a history of dystocia (AOR = 7; 95% CI = 1.008–45.071; P = 0.049).
 |
Table 4 Multivariate Logistic Regression Analysis of N. caninum seropositivity at Herd Level |
Discussion
In the present study, the overall seroprevalence was 5% in cattle managed in pastoral production systems. This finding was comparable with findings from Korea (4.1%),20 Tanzania (4.5%),21 Nepal (4.84%),22 Japan (5.7%),23 and the Czech Republic (5.83%).24 However, the prevalence was considerably lower than the previous findings in Ethiopia which range from 13.3% to 23.8% in cattle managed under intensive and semi-intensive animal production systems.18,30 The seroprevalence N. caninum in cattle varies depending on the country, region, age, gender, breed of the animals, and type of serologic test used.25,26 In previous studies, it was reported with a prevalence ranging between 7.6% and 97.2% in America,27–29 3.9% and 24.1% in Africa,13,21,30,31 0.5% and 60% in Asia,1,22,32 0.7% and 76% in Europe,33–35 and 3.2% and 46.7% in Australia.36,37 So far, it has been stated that animals with free access to pasture might have a greater opportunity of horizontal N. caninum exposure compared to those raised in intensive and semi-intensive production systems.38 However, in our current findings, the lower seroreaction in the free-range rearing system was quite contradicting with several previous findings.1,22,38 The difference in prevalence observed in the current study could arise from the differences in sample size, where the number of samples used for this study was minimal. In addition, the lower prevalence might be associated with the duration of the study, where this study was conducted during harsh climate conditions and severe drought that may hinder the maintenance and sporulation of N. caninum oocysts. Biologically, warm temperature and humid climate conditions are favorable for the maintenance of N. caninum oocysts and subsequent sporulation.39 On the other hand, the oocysts of N. caninum readily desiccate in warm and dry climate conditions.32 Due to the unavailability of pasture during severe droughts, cattle shift to browse on drought-tolerant forage trees and shrubs, which may reduce the chance of pasture-related infections. Moreover, during severe droughts in Borana pastoral areas, the herd of cattle temporarily separated from the household and all other domestic animals and settled nearby water sources until the commencement of the rainy season. This segregation reduces the dog contacts and subsequently may reduce the chance of N. caninum exposure in cattle during drought periods.
In the present study, abortion and dystocia were the clinical reproductive disorders that were found to be significant predictors of N. caninum exposure in cattle both at the animal and herd level. N. caninum is a main cause of reproductive disorders in bovines that particularly induces abortion storms in cattle.2,13 In this study, a cow with a history of abortion was 23 times more likely to be seropositive for N. caninum exposure compared to those without a history of abortion. Similarly, at the herd level, the odds of N. caninum seropositivity was 16 times higher in the herd with a history of abortion than those without an abortion record. Comparably, the strong association of abortion with seropositivity of N. caninum supported many scholars.12,13,40,41 In line with our findings, it has been reported that a cow infected with N. caninum was 3 to 7 times more likely to abort than an uninfected cow.4 Another study also revealed that the risk of abortion was 7.4-fold higher in congenitally infected heifers than in seronegative.6 Additionally, it has been confirmed that 12–42% of aborted fetuses from dairy cattle are infected with N. caninum.2 This strong association might be due to the pathogenic nature of the agent and/or placental and fetal tissue infections.
Out of 33 dams with a history of dystocia, 3 (9.09%) of them were found to be seroreactors for N. caninum exposure. With the presence of dystocia, the odds of N. caninum seropositivity were 11 times higher at the animal level and 7 folds higher at the herd level. It has been stated that N. caninum can cause gross lesions and deformities in calves that may pave the way for the occurrence of dystocia during parturition.25 A more recent study revealed that N. caninum is capable of destroying a variety of nerve cells including those of cranial and spinal nerves.38 Thus, destructions of cranial and spinal nerves may cause paralysis of abdominal musculature that might diminish the expulsion power of the dam during parturition.
In the current study, a source of water and a type of dog feed were found to be statistically significant predictors of N. caninum exposure in cattle. Cows that drank open water (wells) were 7 times higher at risk of N. caninum exposure than those drank pipe water. This finding was in consistent with other findings.27,38,42 The management system is a crucial factor in determining disease occurrence in animal husbandry worldwide.43 In dairy farm industries, the likelihood of disease occurrence is mainly determined by housing type, the hygienic status of watering and feeding troughs, humidity and air circulation, and condition of biosecurity standards.44,45 However, in pastoral settings, disease occurrence largely arises from environmental contamination (water and pasture contamination), the presence of high contact rates with different species of animals, and management practices. During this study, surface water sources such as lakes and rivers were dried up due to severe drought, so public shallow wells and rarely available motorized piped water were assessed.
In the current study, the feeding habit of dogs was assessed and found to be a determinant factor of N. caninum exposure in cattle. Cattle owned by individuals who fed their dogs raw animal products had a 6-times higher risk of acquiring N. caninum infection than that managed by the individuals who fed their dogs a household leftover. According to Borana pastoralists, pets are reared as an integral part of livestock for the protection against rodents and predators.15 In line with our findings, it has been reported that the risk of infection increases by 3 folds in farms that have dog access to the placentas and fetuses in Canada.46 A dog is a definitive host of N. caninum and acquires the parasite by ingestion of contaminated materials, aborted fetuses, or placentas.2,25 So far, N. caninum has been found in naturally infected placentas, and dogs that were fed placentas of naturally infected cows shed N. caninum oocysts.47,48 Moreover, N. caninum oocysts have been identified by bioassay and PCR in feces of naturally infected dogs.49
Conclusion
This study revealed the serological evidence of N. caninum exposure in cattle reared under a pastoral production system with an overall seroprevalence of 5% and 14.6% at individual and herd levels, respectively, in Teltelle district of Ethiopia. Our results suggest that N. caninum is a significant factor in the occurrence of abortion and dystocia in cattle reared in pastoral settings. To our knowledge, the current study was the first attempt at Neosporosis investigation in cattle managed under a pastoral production system in Ethiopia. Management practices, such as the provision of hygienic water and restriction of dogs fed with raw animal products, are likely to reduce the risk of infection, and maximizing community awareness about these disease management practices is suggested.
Data Sharing Statement
The data collected and used to support this article can be offered by the first or corresponding author upon request.
Ethical Approval
Ethical approval for this study was granted by the animal research ethical review committee of the College of Veterinary Medicine and Agriculture, Addis Ababa University (Certificate Ref. number: VM/ERC/19/5/13/2021). Every investigation was done according to the international ethics of animal handling, and animals were handled according to the best veterinary care guidelines. Before conducting the research, animal owners were informed with the objectives and the benefits of the study, and they gave consent for their animal’s inclusion in the study. The consent obtained from cattle owners was verbal because they are unable to write and read. These consents were taken in the presence of a third independent party.
Acknowledgments
The authors would like to acknowledge the National Veterinary Institute, Ethiopia, for the provision of laboratory facilities and Addis Ababa University Thematic Research Project (Number: VPRTT/LT-497/2019) for the financial support provided. We also acknowledge the Teltelle District Livestock Resource Development Office and pastoralists for their support to the success of this research work.
Funding
This study was supported by the Addis Ababa University thematic research fund (VPRTT/PY-120/2019), Ethiopia. The funder had no role in the conception, design of the study, data collection, analysis, and interpretation of the data reported in this manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Dubey JP, Schares G. Veterinary parasitology neosporosis in animals—the last five years. Vet Parasitol. 2011;180:90–108. doi:10.1016/j.vetpar.2011.05.031
2. Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. 2007;20(2):323–367. doi:10.1128/CMR.00031-06
3. Webster JP. Review of “ toxoplasmosis of animals and humans (Second Edition)” by J. P. Dubey. Parasit Vectors. 2010;3:112. doi:10.1186/1756-3305-3-112
4. Innes EA, Bartley PM, Maley SW, Wright SE, Buxton D. Comparative host–parasite relationships in ovine toxoplasmosis and bovine neosporosis and strategies for vaccination. Vaccine. 2007;25(30):5495–5503. doi:10.1016/j.vaccine.2007.02.044
5. Al-qassab SE, Reichel MP, Ellis JT. On the biological and genetic diversity in Neospora caninum. Diversity. 2010;2:411–438. doi:10.3390/d2030411
6. Wouda W. Diagnosis and epidemiology of bovine neosporosis: a review. Vet Q. 2000;22(2):71–74. doi:10.1080/01652176.2000.9695028
7. Reichel MP, Alejandra Ayanegui-Alcérreca M, Gondim LFP, Ellis JT. What is the global economic impact of Neospora caninum in cattle – the billion dollar question. Int J Parasitol. 2013;43(2):133–142. doi:10.1016/j.ijpara.2012.10.022
8. Haddad JPA, Dohoo IR, Vanleewen JA. A review of Neospora caninum in dairy and beef cattle—a Canadian perspective. Can Vet J. 2005;46:230–243.
9. Duarte PO, Oshiro LM, Zimmermann NP, et al. Serological and molecular detection of Neospora caninum and Toxoplasma gondii in human umbilical cord blood and placental tissue samples. Sci Rep. 2020;25(7):1–8. doi:10.1038/s41598-020-65991-1
10. Pagmadulam B, Myagmarsuren P, Fereig RM, et al. Seroprevalence of Toxoplasma gondii and Neospora caninum infections in cattle in Mongolia. Vet Parasitol Reg Stud Rep. 2018;14:11–17. doi:10.1016/j.vprsr.2018.08.001
11. Lobato J, Silva DAO, Mineo TWP, et al. Detection of immunoglobulin G antibodies to Neospora caninum in humans: high seropositivity rates in patients who are infected by human immunodeficiency virus or have neurological disorders. Clin Vaccine Immunol. 2006;13(1):84–89. doi:10.1128/CVI.13.1.84-89.2006
12. Asmare K, Regassa F, Robertson LJ, Martin AD, Skjerve E. Reproductive disorders in relation to Neospora caninum, Brucella spp. and bovine viral diarrhoea virus serostatus in breeding and dairy farms of central and southern Ethiopia. Epidemiol Infect. 2013;141(8):1772–1780. doi:10.1017/S0950268812002191
13. Asmare K. Neospora caninum versus Brucella spp. exposure among dairy cattle in Ethiopia: a case control study. Trop Anim Health Prod. 2014;46(6):961–966. doi:10.1007/s11250-014-0599-0
14. Asmare K, Regassa F, Robertson LJ, Skjerve E. Seroprevalence of Neospora caninum and associated risk factors in intensive or semi-intensively managed dairy and breeding cattle of Ethiopia. Vet Parasitol. 2013;193(1–3):85–94. doi:10.1016/j.vetpar.2012.11.025
15. Jilo K, Tegegne D, Kasim S, Dabasa G, Zewdei W. Seroprevalence and public health significance of toxoplasmosis in small ruminants of pastoral community in Yabello District, Borana Zone, Southern Ethiopia. Vet Med Int. 2021;2021:1–11.
16. CSA. Federal Democratic Republic of Ethiopia. Central Statistical Agency. Agricultural Sample Survey, Volume II, Report on Livestock and Livestock Characteristics. Statistical Bulletin. CSA; 2016.
17. Thrusfield M. Veterinary Epidemiology.
18. Alvarado-Esquivel C, Romero-Salas D, García-Vázquez Z, et al. Seroprevalence and correlates of Toxoplasma gondii infection in domestic pigs in Veracruz State, Mexico. Trop Anim Health Prod. 2014;46(4):705–709. doi:10.1007/s11250-014-0551-3
19. Puhr R, Heinze G, Nold M, Lusa L, Geroldinger A. Firth’s logistic regression with rare events: accurate effect estimates and predictions? Stat Med. 2017;36(14):2302–2317. doi:10.1002/sim.7273
20. Kim J-H, Lee J-K, Lee B-C, et al. Diagnostic survey of bovine abortion in Korea: with special emphasis on Neospora caninum. J Vet Med Sci. 2002;64(12):1123–1127. doi:10.1292/jvms.64.1123
21. Mathew C, Klevar S, Løken T, et al. Reproductive infections in cattle in Tanzania – lessons for control priorities. SOJ Microbiol Infect Dis. 2017;5(2):1–9. doi:10.15226/sojmid/5/2/00169
22. Yadav GP, Manandhar S, Karna AK, Acharya KP, Maharjan D, Singh DK. Seroprevalence and risk factors associated with Neospora caninum in dairy cattle of western dairy pocket area in Chitwan District of Nepal. Bangladesh J Vet Med. 2016;14(2):215–220. doi:10.3329/bjvm.v14i2.31399
23. Koiwai M, Hamaoka T, Haritani M, et al. Nationwide seroprevalence of Neospora caninum among dairy cattle in Japan. Vet Parasitol. 2006;135(2):175–179. doi:10.1016/j.vetpar.2005.08.014
24. Vaclavek P, Koudela B, Modrý D, Sedlák K. Seroprevalence of Neospora caninum in aborting dairy cattle in the Czech Republic. Vet Parasitol. 2003;115(3):239–245. doi:10.1016/S0304-4017(03)00215-2
25. Dubey JP, Lindsay DS. Sarcocystosis in ruminants. Vet Clin Food Anim Pract. 2006;22(3):645–671. doi:10.1016/j.cvfa.2006.08.001
26. Bártová E, Sedlák K, Budíková M. A study of Neospora caninum and Toxoplasma gondii antibody seroprevalence in healthy cattle in the Czech Republic. Ann Agric Environ Med. 2015;22(1):32–34. doi:10.5604/12321966.1141365
27. Alexander H, Llano B, Sales M, Martins R, Polo G, Caetano A. Seroprevalence and risk factors for Neospora caninum infection in cattle from the Eastern Antioquia, Colombia. Vet Anim Sci. 2018;6:69–74. doi:10.1016/j.vas.2018.03.001
28. Cerqueira C, Calero B, Prakash D, Maria G. All about neosporosis in Brazil. Braz J Vet Parasitol. 2017;26(3):253–279. doi:10.1590/s1984-29612017045
29. de Aquino Diniz LV, Minutti AF, de Souza Lima Nino B, et al. Vertical transmission of Neospora caninum in bovine fetuses from a slaughterhouse in Brazil. Trop Anim Health Prod. 2019;51(6):1751–1755. doi:10.1007/s11250-019-01828-y
30. Olum M, Mungube E, Njanja J, et al. Seroprevalence of canine neosporosis and bovine viral diarrhoea in dairy cattle in selected regions of Kenya. Transbound Emerg Dis. 2020;67:154–158. doi:10.1111/tbed.13429
31. Fereig RM, AbouLaila MR, Mohamed SGA, et al. Serological detection and epidemiology of Neospora caninum and Cryptosporidium parvum antibodies in cattle in southern Egypt. Acta Trop. 2016;162:206–211. doi:10.1016/j.actatropica.2016.06.032
32. Marzieh N, Mehdi R, Maryam G, Reza S, Reza F. Seroprevalence of Neospora caninum infection and associated risk factors in cattle of Sistan Areas, Southeastern Iran in 2016. Iran J Parasitol. 2019;14(2):340–346.
33. Lefkaditis M, Mpairamoglou R, Sossidou A, Spanoudis K, Tsakiroglou M. Neospora caninum, a potential cause of reproductive failure in dairy cows from Northern Greece. Vet Parasitol Reg Stud Rep. 2020;19:100365. doi:10.1016/j.vprsr.2019.100365
34. Imre K, Marius I, Mirela I, Nicola F, Claudio G, Gheorghe D. Serological survey of Neospora caninum infection in cattle herds from Western Romania. J Parasitol. 2012;98(3):683–685. doi:10.1645/GE-3023.1
35. Bartels CJM, Arnaiz-Seco JI, Ruiz-Santa-Quitera A, et al. Supranational comparison of Neospora caninum seroprevalences in cattle in Germany, The Netherlands, Spain and Sweden. Vet Parasitol. 2006;137(1–2):17–27. doi:10.1016/j.vetpar.2005.12.016
36. Nasir A, Lanyon SR, Schares G, Anderson ML, Reichel MP. Sero-prevalence of Neospora caninum and Besnoitia besnoiti in South Australian beef and dairy cattle. Vet Parasitol. 2012;186(3–4):480–485. doi:10.1016/j.vetpar.2011.11.032
37. Calarco L, Ellis J. Species diversity and genome evolution of the pathogenic protozoan parasite, Neospora caninum. Infect Genet Evol. 2020;30(3):10–44.
38. Venturoso S, Venturoso J, Silva G, Maia O. Risk factor analysis associated with Neospora caninum in dairy cattle in Western Brazilian Amazon. Braz J Vet Parasitol. 2021;30(1):1–11.
39. Razmi GR, Mohammadi GR, Garrosi T, Farzaneh N, Fallah AH, Maleki M. Seroepidemiology of Neospora caninum infection in dairy cattle herds in Mashhad area, Iran. Vet Parasitol. 2006;135:187–189. doi:10.1016/j.vetpar.2005.09.004
40. Woodbine KA, Medley GF, Moore SJ, Ramirez A, Mason S, Green LE. A four year longitudinal sero-epidemiology study of Neospora caninum in adult cattle from 114 cattle herds in south west England: associations with age, herd and dam-offspring pairs. Vet Res. 2008;12:1–12. doi:10.1186/1746-6148-4-35
41. Macaldowie C, Maley SW, Wright S, et al. Placental pathology associated with fetal death in cattle inoculated with Neospora caninum by two different routes in early pregnancy. J Comp Pathol. 2004;131(2):142–156. doi:10.1016/j.jcpa.2004.02.005
42. Corbellini LG, Smith DR, Pescador CA, et al. Herd-level risk factors for Neospora caninum seroprevalence in dairy farms in southern Brazil. Prev Vet Med. 2006;74:130–141. doi:10.1016/j.prevetmed.2005.11.004
43. McDougall S, Heuer C, Morton J, Brownlie T. Use of herd management programmes to improve the reproductive performance of dairy cattle. Animal. 2014;8(SUPPL. 1):199–210. doi:10.1017/S1751731114000457
44. Salobir J, Frankič T, Rezar V. Animal nutrition for the health of animals, human and environment. Acta Agric Slov. 2012;100(SUPPL.3):41–49.
45. Crowe MA, Hostens M, Opsomer G. Reproductive management in dairy cows – the future. Ir Vet J. 2018;71:1. doi:10.1186/s13620-017-0112-y
46. Vanleeuwen JA, Haddad JP, Dohoo IR, Keefe GP, Tiwari A, Scott HM. Risk factors associated with Neospora caninum seropositivity in randomly sampled Canadian dairy cows and herds. Prev Vet Med. 2010;93(2–3):129–138. doi:10.1016/j.prevetmed.2009.11.013
47. Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW. Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine placenta but not after ingestion of colostrum spiked with Neospora caninum tachyzoites. Int J Parasitol. 2001;31(8):747–752. doi:10.1016/S0020-7519(01)00230-2
48. Bergeron N, Fecteau G, Pare J, Martineau R, Villeneuve A. Vertical and horizontal transmission of Neospora caninum in dairy herds in Québec. Can Vet J. 2000;41(6):464.
49. Basso W, Venturini L, Venturini MC, et al. Prevalence of Neospora caninum infection in dogs from beef-cattle farms, dairy farms, and from urban areas of Argentina. J Parasitol. 2001;87(4):906–907. doi:10.1645/0022-3395(2001)087[0906:PONCII]2.0.CO;2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.