Back to Journals » OncoTargets and Therapy » Volume 7
Serial changes of clinical parameters in a patient with advanced hepatocellular carcinoma with portal vein thrombosis achieving complete response after treatment with sorafenib
Authors Kee K , Hung C, Wang J, Lu S
Received 1 February 2014
Accepted for publication 11 March 2014
Published 27 May 2014 Volume 2014:7 Pages 829—834
DOI https://doi.org/10.2147/OTT.S61740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Kwong-Ming Kee,1,2 Chao-Hung Hung,1,2 Jing-Houng Wang,1,2 Sheng-Nan Lu1,2
1Division of Hepatogastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 2Chang Gung University College of Medicine, Kaohsiung, Taiwan
Abstract: The prognosis is usually poor in advanced hepatocellular carcinoma (HCC). Sorafenib is approved for Child-Pugh class A patients with unresectable and advanced HCC. We report here a rare case of a patient with advanced HCC with right portal vein thrombosis (PVT) who achieved a complete response after treatment with sorafenib. This 74-year-old man was a case of non-hepatitis B and C virus-related cirrhosis. Multiphase liver computed tomography showed an 8 cm tumor with early enhance, early wash out, and right PVT at segment 8 of the right lobe. A liver tumor biopsy confirmed the diagnosis of poorly differentiated HCC. Blood tests showed Child-Pugh class A cirrhosis and an alpha-fetoprotein level of 33,058 ng/mL. Sorafenib was initiated at 800 mg/day but was eventually reduced to 400 mg every other day because of a grade 3 hand-foot skin reaction. The alpha fetoprotein (AFP) level decreased rapidly with a linear trend after treatment. After log transformation, the calculated half-life of AFP was 6.84 days. There was no more tumor arterial enhancement, and tumor size was decreased to 3.7 cm on day 42. PVT shrank gradually and localized to the right anterior branch at month 9. There was no recurrence of tumor at the end of follow-up in month 19. Typical serial changes of clinical parameters were demonstrated in this patient.
Keywords: hepatocellular carcinoma, sorafenib, complete response, portal vein thrombosis
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most common cancer worldwide and is the third deadliest form of cancer overall.1 It is also one of the leading causes of cancer in Taiwan. The prognosis of advanced HCC is usually poor. Sorafenib (Nexavar®; Bayer HealthCare Pharmaceuticals Inc., Montville, NJ, USA/Onyx Pharmaceuticals Inc., South San Francisco, CA, USA) is a multiple tyrosine kinase inhibitor that is approved for Child-Pugh A patients with advanced HCC associated either with major vascular invasion or extrahepatic metastasis.2,3 In the Sorafenib HCC Assessment Randomized Protocol (SHARP)2 and Asian-Pacific studies,3 overall survivals improve significantly. However, no patient achieved a complete response (CR) in either the SHARP or Asian-Pacific trial.2,3 The current clinical practice guideline of the Barcelona Clinic Liver Cancer staging system4 recommends sorafenib as standard treatment for advanced HCC. The achievement of a CR after sorafenib treatment has rarely been reported,5–16 and the reduction of doses because of adverse effects is frequent.7,9,15 Our study reports that a patient with advanced HCC with right portal vein thrombosis (PVT) was treated with sorafenib. A serial change of clinical parameters was demonstrated, and the patient finally achieved CR.
Case presentation
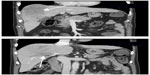
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. This 74-year-old male patient had non-hepatitis B and C virus (HBV and HCV)-related liver cirrhosis. He was referred from a local hospital, where a liver tumor was detected. Serum HBsAg (the surface antigen of the HBV), anti-HBs Ab (anti-HBV surface antibody), and anti-HCV antibody markers were all negative. Multiphase liver computed tomography (CT) showed an 8 cm liver tumor with early enhance, early wash out, and right PVT at segment 8 of the right lobe (Figure 1). Liver tumor biopsy confirmed the diagnosis of poorly differentiated HCC with a trabecular pattern (Figure 2). Blood tests showed that liver function was classified as Child-Pugh A, and an alpha-fetoprotein (AFP) level was 33,058 ng/mL. Sorafenib therapy was started at the standard dose of 800 mg/day on June 8, 2012 (Figure 3). A grade 3 hand-foot skin reaction happened at 11 days after the initiation of sorafenib treatment, and the dose was then decreased to 400 mg/day. Because of intermittent grade 2 hand-foot skin reaction, the dose was further reduced to 400 mg every other day on day 87.
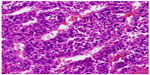  | Figure 2 Poorly differentiated hepatocellular carcinoma with trabecular pattern (hematoxylin and eosin, ×200). |
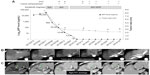
Figure 3 shows the serial changes of observable parameters in this patient with sorafenib treatment. The most rapid response to sorafenib was serum AFP level, which decreased to 666 ng/mL on day 21, 93.28 ng/mL on day 42, 14.85 ng/mL on day 65, 4.5 ng/mL on day 85, and less than 2 ng/mL after 144 days. The AFP level was then persistently less than 2 ng/mL until the end of follow-up in month 19. There exists a decreasing linear trend of AFP level after log transformation. We measured linear regression by using the first five decreasing time points of AFP level from 33,058 ng/mL to 4.5 ng/mL. The measured equation of linear regression was log10(AFP) =4.094−0.044× (day) (P=0.005; r2=0.949). According to the linear regression, we estimated that the half-life of decreasing AFP levels that respond to sorafenib is 6.84 days. The interval multiphase liver CT showed a continuing decrease in tumor size and PVT shrinkage without enhancement of the tumor. Tumor size decreased most rapidly in the first 2 months. Tumor size decreased from 8 to 3.7 cm on day 42, 2.9 cm on day 65, and slowly to 2.3 cm at the end of follow-up in month 19. The first follow-up liver CT on day 42 demonstrated no arterial enhancement of the the tumor; there was no more arterial enhancement of the tumor until the end of follow-up. Right PVT regression occurred gradually after the treatment, and was localized to the right anterior branch after 9 months of sorafenib. The last follow-up CT in month 19 still showed no tumor recurrence.
Discussion
Sorafenib is a multikinase inhibitor that inhibits angiogenesis and tumor growth. The efficacy of sorafenib has been shown in two Phase III clinical trials.2,3 The survival rates in these two clinical trials were all significantly higher in the sorafenib group compared with in the placebo group. However, no patients achieved a CR in these two clinical trials, in which tumor response rate was assessed by Response Evaluation Criteria in Solid Tumor (RECIST).17 In recent years, the modified RECIST was designed for HCC assessment. A CR corresponded to the disappearance of all contrast enhancement at the arterial phase in the modified RECIST, whereas disappearance of tumor was defined in the RECIST. Even after sorafenib was used in the last few years, still only a small number of HCC patients have been reported to achieve a CR after treatment. We report a case of advanced HCC with PVT that achieved CR, using the definition from the modified RECIST.
Table 1 shows clinical characteristics of 13 previously reported HCC patients who achieved CR after treatment with sorafenib. Among them, a total of nine patients had discontinued sorafenib after administration in a range of 11 days–28 months. Three of nine patients achieved CR within 6 to ~12 months and then received surgical resection after successful down-staging. Another six patients remained in CR status after follow-up from 8 to 22 months. Four patients who had continued sorafenib treatment achieved CR within 6 to ~23 months, and no recurrence was seen during the last follow-up.
The standard dose of sorafenib is 800 mg per day, and some patients have maintained the standard dose.10,12,14 However, the majority of patients have to reduce the dose because of hand-foot skin reaction.7,9,15 In our patient, sorafenib was started at 800 mg per day, and the dose was eventually reduced to 400 mg every other day because of grade 3 hand-foot skin reaction. This indicates that sorafenib is effective in some HCC patients despite a low-dose administration. In some patients, there has been no recurrence of HCC despite cessation of sorafenib.9,11
Our case study patient had right PVT, which indicated a high risk for extrahepatic metastasis. In some selected HCC patients with PVT, radiotherapy may be one treatment of choice. Some previously reported HCC cases also had PVT.10,12,15 Vascular endothelial growth factor plays an important role in the angiogenesis and progression of PVT, and because sorafenib may inhibit the vascular endothelial growth factor pathway and achieve a CR, vascular endothelial growth factor levels may be used to predict the efficacy of sorafenib for patients with PVT before treatment; this may need further study to clarify.
To date, there has been no useful biomarker to predict who will achieve CR after sorafenib treatment. In Figure 3, we observed that the earliest response to sorafenib was rapid decline of serum AFP levels. In addition, our case demonstrated a significant decreasing linear trend after log transformation. Some studies have also reported a rapid decrease in AFP levels, but this characteristic was not further analyzed.7–11,13–15 In the current case, the calculated half-life of AFP level was 6.84 days, which was similar to that reported in the previous studies.7–11,13–15 This implied that CR was achieved after initiation of sorafenib and that the result was comparable with that of tumor resection. This suggested that rapid decrease of AFP levels may be used as a surrogate to predict the treatment response of sorafenib.
In Figure 3, we observed that the first follow-up liver CT showed no arterial enhancement, which implied that rapid tumor response to sorafenib treatment showed a marked decrease of intratumoral vascularity. The treatment response was followed by a marked decrease in tumor size in the first 2 months. Finally, we found that PVT also shrank gradually over time. A quantitative imaging study may be included in the evaluation of HCC patients.18 The accurate segmentation of liver vessels and its internal tumors is important for the pretreatment and posttreatment evaluation of therapeutic liver treatments.
The etiologies of the majority of HCC reported cases were HBV,10 HCV,7,9,11,12,15 and hemochromatosis.10,14 In our patient, serum markers of HBV and HCV were both negative, which may indicate that HCC response to sorafenib might be related to the pathogenesis of HCC, and not to the etiology of HCC. In conclusion, the current study demonstrates that the serial changes of observable parameters in a case of advanced HCC with PVT achieved a CR, including rapid decrease of serum AFP level, followed by absent arterial enhancement of tumor, decreasing tumor size, and PVT shrinkage.
Author contributions
All of the authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. | |
Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. | |
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. | |
Bruix J, Sherman M, American Association for the study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022. | |
Abbadessa G, Rimassa L, Pressiani T, Carrillo-Infante C, Cucchi E, Santoro A. Optimized management of advanced hepatocellular carcinoma: four long-lasting responses to sorafenib. World J Gastroenterol. 2011;17(19):2450–2453. | |
Chelis L, Ntinos N, Souftas V, et al. Complete response after sorafenib therapy for hepatocellular carcinoma in an HIV-HBV co infected patient: Possible synergy with HAART? A case report. Med Oncol. 2011; 28 Suppl 1:S165–S168. | |
Curtit E, Thiery-Vuillemin A, Nguyen T, et al. Complete histologic response induced by sorafenib in advanced hepatocellular carcinoma: a case report. J Clin Oncol. 2011;29(12):e330–e332. | |
Gamstätter T, Weinmann A, Schadmand-Fischer S, et al. AFP measurement in monitoring treatment response of advanced hepatocellular carcinoma to sorafenib: case report and review of the literature. Onkologie. 2011;34(10):538–542. | |
Inuzuka T, Nishikawa H, Sekikawa A, et al. Complete response of advanced hepatocellular carcinoma with multiple lung metastases treated with sorafenib: a case report. Oncology. 2011;81 Suppl 1:152–157. | |
Irtan S, Chopin-Laly X, Ronot M, Faivre S, Paradis V, Belghiti J. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int. 2011;31(5):740–743. | |
Mizukami H, Kagawa T, Arase Y, et al. Complete response after short-term sorafenib treatment in a patient with lymph node metastasis of hepatocellular carcinoma. Case Rep Oncol. 2012;5(2):380–384. | |
Sacco R, Bargellini I, Gianluigi G, et al. Complete response for advanced liver cancer during sorafenib therapy: case report. BMC Gastroenterol. 2011;11:4. | |
Shiozawa K, Watanabe M, Ikehara T, et al. Sustained complete response of hepatocellular carcinoma with portal vein tumor thrombus following discontinuation of sorafenib: A case report. Oncol Lett. 2014;7(1):50–52. | |
So BJ, Bekaii-Saab T, Bloomston MA, Patel T. Complete clinical response of metastatic hepatocellular carcinoma to sorafenib in a patient with hemochromatosis: a case report. J Hematol Oncol. 2008;1:18. | |
Wang SX, Byrnes A, Verma S, Pancoast JR, Rixe O. Complete remission of unresectable hepatocellular carcinoma treated with reduced dose of sorafenib: a case report. Target Oncol. 2010;5(1):59–63. | |
Yeganeh M, Finn RS, Saab S. Apparent remission of a solitary metastatic pulmonary lesion in a liver transplant recipient treated with sorafenib. Am J Transplant. 2009;9(12):2851–2854. | |
Edeline J, Boucher E, Rolland Y, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118(1):147-156. | |
Conversano F, Franchini R, Demitri C, et al. Hepatic vessel segmentation for 3D planning of liver surgery experimental evaluation of a new fully automatic algorithm. Acad Radiol. 2011;18(4):461–470. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.