Back to Journals » Clinical Ophthalmology » Volume 13
Sentinel lymph node biopsy in the management of conjunctival melanoma: current insights
Authors Mor JM, Rokohl AC, Koch KR, Heindl LM
Received 26 March 2019
Accepted for publication 9 May 2019
Published 23 July 2019 Volume 2019:13 Pages 1297—1302
DOI https://doi.org/10.2147/OPTH.S187364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Joel M Mor,1 Alexander C Rokohl,1 Konrad R Koch,1 Ludwig M Heindl1,2
1Department of Ophthalmology, University of Cologne, Cologne, Germany; 2Center for Integrated Oncology (CIO) Aachen-Bonn-Cologne-Duesseldorf, Ophthalmic Oncology Unit, Cologne, Germany
Purpose: To evaluate the role of sentinel lymph node biopsy (SLNB) in conjunctival melanoma (CM).
Methods: This article provides a review of the literature from PUBMED.
Results: Data on SLNB in the management of CM are scarce and only two relatively large cohorts have been reported. Although indication criteria for SLNB vary slightly, positive findings can be expected in 11%–13% of CM cases, including small tumors. False negative SLNB findings are rare (<10%). Failure to identify SLNB has been attributed to the surgical learning curve and recurrent tumors with scar tissue impeding spread of the tracer material. Reported 5-year survival rate following CM management including SLNB, is up to 79%, but there are no comparative cohort studies proving the assumed benefit. Adverse events reported were non-severe and transient.
Conclusion: Patients can potentially benefit from SLNB and the procedure can be offered to eligible patients. However, there is not enough evidence to support SLNB as a mandatory part of CM management.
Keywords: sentinel, biopsy, conjunctival melanoma, lymphatic spread, lymph node
Introduction
With an annual incidence rate of 0.15–0.78/1,000,000, conjunctival melanoma (CM) (Figure 1) is the second most common malignancy of the ocular surface.1–3 Therapy encompasses wide local excision using minimal-touch technique and subsequent adjuvant therapy to the former tumor site in the hope of minimizing the risk of local recurrence.4,5 The many different approaches to adjuvant therapy comprise radiotherapy (proton beam radiotherapy, ruthenium-106 or strontium-90 brachytherapy), local chemotherapy (mitomycin C 0.04% eye drops) or topical immunotherapy (interferon α2b eye drops) for extermination of residual tumor cells.5–9
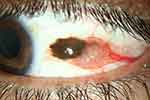 |
Figure 1 Melanoma of the temporal bulbar conjunctiva with a prominent feeder vessel in the left eye of a patient. |
Despite all these efforts, CM is characterized by high recurrence rates of approximately 26%, 51%, and 65% within 5, 10, and 15 years after primary therapy, respectively.10 Akin to the phenotypically related cutaneous melanoma, initial metastasis predominantly, though not exclusively, occurs to the regional lymph nodes (as opposed to uveal melanomas which typically initially metastasize to the liver) and can be observed in 16%, 26%, and 32% of patients within 5, 10, and 15 years, respectively. However, these rates tend to vary strongly between populations with one study reporting up to 41% lymph node metastasis within 6 years.10–13
Reported 10-year mortality rates range somewhere from 13%–32%.1,10,11,14 These data, however, stem from an era before the availability of targeted therapies and checkpoint inhibition, which have become regular components in the management of a multitude of tumors, including metastatic cutaneous melanoma, and hopes are high that patients with CM will be able to benefit from these as well.1,15,16
The goal of sentinel lymph node biopsy (SLNB), in general, is to detect putative subclinical micrometastases in the regional lymph nodes which otherwise escape detection by ultrasound imaging and clinical examination. These micrometastases are considered to be responsible for later tumor recurrence in the regional lymph nodes, ultimately resulting in morbidity and patient death.17–20 In theory, the sentinel lymph node (SLN) is the first lymph node (or lymph nodes, as there can be several) to be encountered by tumor cells during lymphatic metastatic spread and a positive SLNB warrants radical lymphadenectomy of the affected basin.18
SLNB has been tried successfully in the management of CM, but due to the low incidence rate of the tumor, obtaining large case numbers has not been possible so far. Aside from a handful of case reports from different ophthalmic centers, the only two relatively large case series have been reported by Esmaeli et al and Cohen et al.18,21,22 The former has been updated several times over the past decade and in the latest iteration reported on 31 CM patients undergoing SLNB.18 Other than these, clinical data are scarce. Still, SLNB holds the promise of a potential benefit for CM patients, reducing the rate of regional metastases, and providing the clinician with additional prognostic information. Moreover, though similar in concept, there is some degree of variation in surgical technique between different ophthalmic centers that is worth elucidating.
In this article, we aim to give an overview of SLNB techniques, reported results, and postoperative complications.
Treatment indications and techniques
Several criteria have been defined that render patients eligible for SLNB. While most centers reporting on SLNB generally have similar criteria, some variation can be found. In the largest study to date, requirements for SLNB were: no sign of metastasis, age >17 years, histological confirmation of CM, and tumor thickness of at least 1 mm or signs of ulceration.18 Other centers offered SLNB only in cases with a tumor thickness of >2 mm.22–25 Obviously, this requires correct handling of the tumor specimens. Cohen et al also included tumors in location associated with higher risk (forniceal, caruncular, tarsal melanomas) as well as recurrent melanomas after resection of primary acquired melanosis with atypia.22 Additionally, the presence of >1 mitotic figures per microscopic field has been suggested as a criterion.25 Recently, a CM management algorithm has been proposed based on four high risk features (non-limbal location, >2 mm thickness, ulceration, >1 mitotic figures per mm2), suggesting SLNB in cases where two or more of these features are present.23
Naturally, initial difficulties with the procedure have been attributed to surgical learning curve and most groups initially relied on a multimodal (preoperative lymphoscintigraphy, intraoperative radioactive mapping plus dye injection) approach to help with detection of SLNs.18,21,22
Reported by Nijhawan et al, preoperative lymphoscintigraphy was performed by local injection of technecium-99m (Tc-99m) nanocolloid and subsequent image acquisition using a gamma camera.18,21,26 Alternatively, other groups have used single photon emission computed tomography for imaging of the SLNs.23,24
During surgery, Tc-99m nanocolloid is injected subconjunctivally at the former tumor site, and a gamma probe is used for lymph node mapping.21,24–26 In comparison to cutaneous lymphatic mapping, smaller injection volumes have been suggested as spillage and scattering into the nasolacrimal system have led to difficulties in the past.27 Other centers have performed preoperative imaging and intraoperative mapping both following one single injection of Tc-99m nanocolloid.22 The additional concurrent injection of several different dyes has been described by multiple groups. Commonly, isosulfan blue, methylene blue, lymphazurin blue, and indocyanine green have been used, all to varying success.21–25,28 Several groups have since refrained from this practice however, since it often yielded inconsistent results (lack of visible lymph node staining) and did not apparently provide a significant additional benefit to Tc-99m injection.18,22
The classic teaching of ocular lymphatics is that the nasal adnexal regions drain via submandibular (and deep cervical) lymph nodes while the temporal adnexal regions drain via preauricular lymph nodes. However, clinical experience as well as SLNB results have shown that exceptions from this rule are not uncommon, and especially nasally located lesions may sometimes drain to the preauricular lymph nodes as well.22,29,30
Furthermore, attention has to be paid to the timepoint of SLNB relative to primary tumor resection. While the majority of groups report simultaneous primary tumor resection with SLNB in one combined procedure, Cohen et al in their study, performed SLNB within 6 weeks of tumor resection.18,22 The advantage of this approach is that tumor thickness and patient eligibility can be evaluated prior to SLNB. However, it also holds the risk of disrupting or changing lymphatic pathways which may ultimately result in biopsying a lymph node other than the “original” SLN.22
The excised lymph node is then examined by a pathologist via serial sectioning, H&E staining, and immunohistochemistry of common melanoma markers, eg, S100, Melan-A and human melanoma black 45.24
Given that reports of positive SLNB results are exceedingly rare, there is no standardized management following such results. Where possible, complete dissection of the nodal basin can be considered carefully.22 However, this is not standard practice in cutaneous melanoma management anymore, as it has not improved tumor-specific survival rates compared to lymph node observation.17 In frail patients, radiotherapy to the lymph node basin has been tried as well.
Published results
Published data seem to be not only scarce, but also hardly comparable, since study design varies substantially among reports. In particular, most cohorts comprise not only CM cases but oftentimes also other entities including eyelid skin melanoma, sebaceous cell carcinoma, conjunctival squamous carcinoma, and eyelid Merkel cell carcinoma.21,31 In fact, there are currently only two larger series of CM cases available (Table 1).18,22
In their 2017 update, Pfeiffer et al reported on 31 CM (and 20 eyelid melanoma) patients who underwent SLNB.18 Of those, four (13%) had positive biopsy results leading to dissection of the respective lymphatic basin. This rate of positive findings was lower than that in the subgroup of eyelid melanomas (20 patients), where 30% of sampled SLNs were positive. Similar rates have been found in cutaneous melanoma SLNBs.32 However, there were also two (7%) cases of false negative SLNB results in the CM cohort with patients developing late tumor recurrence in the regional lymph nodes. Since these were early cases, the authors attributed the false negative results to surgical learning curve and lack of experience at the time.18 The group reported a median follow-up of 61 months and combined 5-year overall survival of 79% of conjunctival and eyelid melanoma patients. Correlating American Joint Committee on Cancer staging with SLNB positivity was not possible due to low case numbers.18 Similarly, Cohen et al reported on their experience in a cohort of 22 CM patients.22 Intraoperative lymphatic mapping failed to identify an SLN in four (18%) cases and succeeded in 18 (82%). Failure to identify SLNs was attributed to prior surgery or radiotherapy in cases of recurrent CM, highlighting the role of scar tissue in the distribution of radiotracers. Of the successful biopsies, two (11%) were reported positive and 16 (89%) negative, with no false negative results recorded over the relatively short median 20 months follow-up period. Of note, six of those patients had recurrent CM (one of those had positive SLNB) and three had advanced CM. Survival rates were not reported in this study.22
Overall, neither study reported recurrence-free survival or metastasis-free survival.18,22
Apart from these two studies, a review of literature reveals a modest number of case reports and short case series of patients undergoing SLNB for CM, for a combined total of 18 CM cases.11,23–25,27,28,31,33–36 With the exception of one study reporting two positive SLNB findings in two patients with forniceal CM, all of the listed articles reported negative SLNB findings.28 Although all centers used Tc-99m based lymphoscintigraphy for lymphatic mapping, there was some variation in what dye was injected concurrently.18 Table 2 gives an overview of reported case numbers and lymphatic mapping techniques.
 |
Table 1 Comparison of the two largest cohort studies investigating sentinel lymph node biopsy (SLNB) in conjunctival melanomas (CMs) |
 |
Table 2 List of sentinel lymph node biopsy (SLNB) reports in conjunctival melanoma and applied lymphatic mapping technique |
Adverse events
In general, the rate of adverse events following SLNB is relatively low and severe complications requiring an intervention have not been described to date. Most commonly, transient facial nerve palsy lasting up to several weeks (6%, usually of the marginal mandibular branch) can occur, especially following biopsy of an SLN in the parotid region.18,22 Furthermore, dry mouth and facial edema have been reported.37
The injection of a dye can lead to prolonged staining of the conjunctiva and has been reported especially with isosulfan blue.18 Lymphazurin blue, which has been used more commonly in cutaneous melanoma SLNB, can permanently tattoo skin when injected, but is uncommon in SLNB for tumors of the ocular adnexa.28 In conclusion, SLNB, as reported so far, seems to be a relatively complication-free procedure.
Caveats and conclusions
Reports of CM biopsy cases are unfortunately even scarcer than would appear at first glance. This is because initially published case reports are often re-reported as part of case series. On top of that, these case series are updated every so often with the addition of a handful of new patients to the existing cohort.18,21,38 Obviously, these updates are important, especially given the extremely low incidence of the tumor, but one has to pay close attention to this when reviewing the literature. In total, there have been only eight positive SLNBs throughout the complete literature, spread over three reporting groups (Pfeiffer et al, 2017: four; Cohen et al, 2013: two; Baroody et al, 2004: two). It is interesting however, that SLN positivity was shown to be detectable even in cases of small T1 tumors, as well as recurrent melanomas staged for exenteration.18,22 These findings highlight the potential benefit of SLNB even in seemingly low grade cases where there is little suspicion of lymphatic spread. Considering that melanoma cells can be found in approximately 13% of biopsied lymph nodes, it is justifiable to offer this procedure to patients with CM. Exact criteria for SLNB will have to be reviewed in the future, but for now, most centers seem to agree on those cited in this article, tumor thickness being the primary factor. The procedure should be performed only in a specialized center in order to minimize false negative results and failure to find an SLN, especially in cases of recurrent melanoma, where scar tissue may complicate the procedure even further.22 Finally, it is unknown if lymph node dissection following positive SLNB for CM will actually benefit patients. Although evidence points in that direction, there is no study proving a survival benefit in CM.18,3,229
In conclusion, SLNB is a relatively safe procedure that can be offered to eligible patients with CM, but data are still too scarce to definitively accept SLNB as an obligatory procedure in the management of CM.
Acknowledgments
The authors would like to thank the German Research Foundation (DFG) FOR2240 “(Lymph)-angiogenesis and Cellular Immunity in Inflammatory Diseases of the Eye”, HE 6743/2-1, HE 6743/3-1, and HE 6743/3-2 to LMH; Brigitte and Dr. Konstanze Wegener Foundation to LMH; Ernst and Berta Grimmke Foundation to LMH; and GEROK program of the University of Cologne to JMM and LMH for their tremendous support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mor JM, Heindl LM. Systemic BRAF/MEK inhibitors as a potential treatment option in metastatic conjunctival melanoma. Ocul Oncol Pathol. 2017;3:133–141. doi:10.1159/000452473
2. Triay E, Bergman L, Nilsson B, All-Ericsson C, Seregard S. Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol. 2009;93:1524–1528. doi:10.1136/bjo.2009.157933
3. Norregaard JC, Gerner N, Jensen OA, Prause JU. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569–572.
4. Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B McMahan lecture. Arch Ophthalmol. 1997;115:808–815. doi:10.1001/archopht.1997.01100150810025
5. Heindl LM, Koch KR, Schlaak M, Mauch C, Cursiefen C. [Adjuvant therapy and interdisciplinary follow-up care of conjunctival melanoma]. Ophthalmologe. 2015;112:907–911.
6. Herold TR, Hintschich C. Interferon α for the treatment of melanocytic conjunctival lesions. Graefes Arch Clin Exp Ophthalmol. 2009;248:111–115. doi:10.1007/s00417-009-1189-0
7. Russell HC, Chadha V, Lockington D, Kemp EG. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. 2010;94:1316–1321.
8. Westekemper H, Meller D, Darawsha R, et al. Operative therapy and irradiation of conjunctival melanoma (in German). Ophthalmologe. 2015;112:899–906. doi:10.1007/s00347-015-0147-y
9. Glossmann JP, Skoetz N, Starbatty B, et al. [Conjunctival melanoma: standard operating procedures in diagnosis, treatment and follow-up care]. Ophthalmologe. 2018;115:489–498. doi:10.1007/s00347-018-0664-6
10. Shields CL, Shields JA, Gündüz K, et al. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch Ophthalmol. 2000;118:1497–1507.
11. Esmaeli B, Wang X, Youssef A, Gershenwald JE. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101–2105. doi:10.1016/S0161-6420(01)00782-5
12. Schlereth SL, Iden S, Mescher M, et al. a novel model of metastatic conjunctival melanoma in immune-competent mice. Invest Ophthalmol Vis Sci. 2015;56:5965–5973. doi:10.1167/iovs.15-17290
13. Refaian N, Schlereth SL, Koch KR, et al. Comparing the Hem- and lymphangiogenic profile of conjunctival and uveal melanoma cell lines. Invest Ophthalmol Vis Sci. 2015;56:5691–5697. doi:10.1167/iovs.15-16829
14. Werschnik C, Lommatzsch PK. Long-term follow-up of patients with conjunctival melanoma. Am J Clin Oncol. 2002;25:248–255.
15. Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi:10.1056/NEJMoa1210093
16. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi:10.1056/NEJMoa1011205
17. Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376:2211–2222. doi:10.1056/NEJMoa1613210
18. Pfeiffer ML, Ozgur OK, Myers JN, et al. Sentinel lymph node biopsy for ocular adnexal melanoma. Acta Ophthalmol. 2017;95:e323–e328. doi:10.1111/aos.13252
19. Heindl LM, Hofmann-Rummelt C, Adler W, et al. Prognostic significance of tumor-associated lymphangiogenesis in malignant melanomas of the conjunctiva. Ophthalmology. 2011;118:2351–2360. doi:10.1016/j.ophtha.2011.05.025
20. Heindl LM, Hofmann-Rummelt C, Adler W, et al. Tumor-associated lymphangiogenesis in the development of conjunctival melanoma. Invest Ophthalmol Vis Sci. 2011;52:7074–7083. doi:10.1167/iovs.11-7902
21. Ho VH, Ross MI, Prieto VG, Khaleeq A, Kim S, Esmaeli B. Sentinel lymph node biopsy for sebaceous cell carcinoma and melanoma of the ocular adnexa. Arch Otolaryngol Head Neck Surg. 2007;133:820–826. doi:10.1001/archotol.133.8.820
22. Cohen VM, Tsimpida M, Hungerford JL, Jan H, Cerio R, Moir G. Prospective study of sentinel lymph node biopsy for conjunctival melanoma. Br J Ophthalmol. 2013;97:1525–1529. doi:10.1136/bjophthalmol-2013-303671
23. Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Oncol Pathol. 2015;1:266–273. doi:10.1159/000381719
24. Wainstein AJ, Drummond-Lage AP, Kansaon MJ, et al. Sentinel lymph node biopsy for conjunctival malignant melanoma: surgical techniques. Clin Ophthalmol. 2014;9:1–6. doi:10.2147/OPTH.S71226
25. Rubinstein TJ, Perry JD, Korn JM, Costin BR, Gastman BR, Singh AD. Indocyanine green-guided sentinel lymph node biopsy for periocular tumors. Ophthalmic Plast Reconstr Surg. 2014;30:301–304. doi:10.1097/IOP.0000000000000096
26. Nijhawan N, Ross MI, Diba R, Ahmadi MA, Esmaeli B. Experience with sentinel lymph node biopsy for eyelid and conjunctival malignancies at a cancer center. Ophthalmic Plast Reconstr Surg. 2004;20:291–295.
27. Schwarz KA, Davison SP, Crane AE. Sentinel lymph node biopsy in the setting of conjunctival melanoma. Plast Reconstr Surg. 2008;121:212e–3e. doi:10.1097/01.prs.0000305377.95793.24
28. Baroody M, Holds JB, Kokoska MS, Boyd J. Conjunctival melanoma metastasis diagnosed by sentinel lymph node biopsy. Am J Ophthalmol. 2004;137:1147–1149. doi:10.1016/j.ajo.2004.01.014
29. Sherman DD, Gonnering RS, Wallow IH, et al. Identification of orbital lymphatics: enzyme histochemical light microscopic and electron microscopic studies. Ophthalmic Plast Reconstr Surg. 1993;9:153–169.
30. Echegoyen JC, Hirabayashi KE, Lin KY, Tao JP. Imaging of eyelid lymphatic drainage. Saudi J Ophthalmol. 2012;26:441–443. doi:10.1016/j.sjopt.2012.08.003
31. Maalouf TJ, Dolivet G, Angioi KS, Leroux A, Genin P, George JL. Sentinel lymph node biopsy in patients with conjunctival and eyelid cancers: experience in 17 patients. Ophthalmic Plast Reconstr Surg. 2012;28:30–34. doi:10.1097/IOP.0b013e31822fb44b
32. Chang JM, Kosiorek HE, Dueck AC, et al. Stratifying SLN incidence in intermediate thickness melanoma patients. Am J Surg. 2018;215:699–706. doi:10.1016/j.amjsurg.2017.12.009
33. Rubinstein TJ, Plesec TP, Singh AD. Sarcoid-like reaction in sentinel lymph node draining conjunctival melanoma. Ophthalmic Plast Reconstr Surg. 2015;31:e1–3. doi:10.1097/IOP.0000000000000018
34. Rubinstein TJ, Plesec TP, Singh AD. Desmoplastic melanoma of the eyelid and conjunctival melanoma in neurofibromatosis type 1: a clinical pathological correlation. Surv Ophthalmol. 2015;60:72–77. doi:10.1016/j.survophthal.2014.08.001
35. Motomura H, Sakamoto M, Maruyama Y, Harada T, Ishii M. Sentinel lymph node biopsy in conjunctival malignant melanoma at the lacrimal caruncle: a case report. Osaka City Med J. 2010;56:5–10.
36. Wilson MW, Fleming JC, Fleming RM, Haik BG. Sentinel node biopsy for orbital and ocular adnexal tumors. Ophthalmic Plast Reconstr Surg. 2001;17:338–344.discussion 344-5.
37. Vuthaluru S, Pushker N, Lokdarshi G, et al. Sentinel lymph node biopsy in malignant eyelid tumor: hybrid single photon emission computed tomography/computed tomography and dual dye technique. Am J Ophthalmol. 2013;156:43–49. doi:10.1016/j.ajo.2013.02.015
38. Savar A, Ross MI, Prieto VG, Ivan D, Kim S, Esmaeli B. Sentinel lymph node biopsy for ocular adnexal melanoma: experience in 30 patients. Ophthalmology. 2009;116:2217–2223. doi:10.1016/j.ophtha.2009.04.012
39. Klemen ND, Han G, Leong SP, et al. Completion lymphadenectomy for a positive sentinel node biopsy in melanoma patients is not associated with a survival benefit. J Surg Oncol. 2019. [Epub ahead of print]. doi:10.1002/jso.25444
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
