Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Sensory Integration and Perceptual-Motor Profiles in School-Aged Children with Autistic Spectrum Disorder
Authors Wuang YP, Huang CL, Tsai HY
Received 10 March 2020
Accepted for publication 29 May 2020
Published 6 July 2020 Volume 2020:16 Pages 1661—1673
DOI https://doi.org/10.2147/NDT.S253337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yee-Pay Wuang,1 Chien-Ling Huang,2 Hsien-Yu Tsai1
1Department of Occupational Therapy, Kaohsiung Medical University, Kaohsiung, Taiwan; 2Department of Rehabilitation Medicine, Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung, Taiwan
Correspondence: Yee-Pay Wuang
Department of Occupational Therapy, Kaohsiung Medical University, 100 Shih-Chuan 1st Road, Kaohsiung, Taiwan
Tel +11-886-7-3121101 ext2658
Fax +11-886-7-3215845
Email [email protected]
Purpose: This study aimed to investigate the sensory integration and perceptual-motor performances in elementary school children (5– 12 years) with autistic spectrum disorder (ASD) in Taiwan. The impacts of comprehensive body functions on activity participations in ASD were also investigated to provide evidence for clinical applications and further study.
Methods: One hundred and seventeen children with ASD (42 females; aged 5– 13 years, average age 8 years 3 months) were recruited. All participants were assessed with standardized measures of body functions and activity participations. The body function measures included Bruininks–Oseretsky of Motor Proficiency – Second Edition, Sensory Profile, Test of Sensory Integration Functions, and Test of Visual Perception Skills – Third Edition. The activity participation measures included the Chinese versions of both Vineland Adaptive Behavior Scale and School Function Assessment.
Results: School-aged children with ASD had different levels of impairments on body function measures. Most participant scores fell within the impairment range on 13 to 15 items out of the total 19 sensory and perceptual-motor measure subtests, with worst performance on coordination-related motor task and most sensory integrative dimensions. The results indicated a significant main effect for age and sex on some body functions and activity participations. Correlation analyses indicated strong associations between body function and activity participation across settings in ASD.
Conclusion: Our findings characterized the developmental continuum of body functions of school-aged children with ASD and showed their associations with adaptation and participation. While emphasizing the development of functional skills to facilitate age-appropriate activity participation in multiple scenarios, interventions aiming to improve body functions are indispensable.
Keywords: ASD, motor function, sensory processing, sensory integration, visual perception, activity participation
Introduction
Autism spectrum disorder (ASD) is one of the most common developmental disabilities, with an estimated prevalence of 13.4 per 1000 children.1 The main characteristics of ASDs include severe and complex impairments in social interaction and communication skills, repetitive stereotypical behaviors, as well as restricted interests and activities. The unusual combinations of sensory, cognitive, behavioral, and communication features seen with ASD might persist throughout life.2 Sleeping and eating difficulties, affective maladjustment, and difficulties with ideation and motor planning are often manifested.3
Sensory integration, visual perceptual skills, and motor functioning are the foundations for child’s overall development and adaptive social interactions. However, these fundamental components have less focus compared to social and communication issues for children with ASD. Early intervention for ASD is critical because rehabilitative treatments to enhance the development and functions for ASD children might be more efficient at younger age,4 and will result in substantial cost-savings to families of ASD children and the whole socio-medical system.5 In-depth understanding of early developmental profiles in sensory integration and perceptual-motor domains among children with ASD could help to improve the effectiveness of early interventions to the greatest extent.
Sensory Processing and Sensory Integrative Dysfunctions
Sensory processing disorders (SPD) include three main types of problems: sensory modulation disorder (ie, under-responsiveness, over-responsiveness, and sensory seeking), sensory-based motor disorder (ie, vestibular-bilateral functional problems), and sensory discrimination disorder (ie, dyspraxia).6–8 The new DSM-5 definition of autism9 as well indeed includes sensory issues as one of the four restricted and repetitive behavior features, and the sensory issues are defined as over- or under-responsivity to sensory stimulation or atypical attention on certain sensory elements in the environment. SPD are reported in a high proportion (92%) of ASD, and the levels of sensory dysfunction correlate to the autism severity and atypical behaviors but are not associated with cognitive levels (eg, intelligence quotient).10
Sensory integration was defined as the complicated neurological processes that categorize, modulate, and coordinate sensations from an individual’s body and from the surrounding environment, the end products of the functional sensory integration process are adaptive behaviors.11 The primary sensory systems involved in the sensory integration process are vestibular, proprioceptive, and tactile systems. Different kinds and degrees of problems in development, information processing, and behavior might occur when the sensory inputs from these primary sensory systems are not integrated or organized appropriately at the cortical and subcortical levels. Children with ASD often have sensory integrative dysfunction caused by poor sensory registration, motor incoordination, sequencing deficits, and specific verbal-motor dyspraxia.12
Perceptual-Motor Abilities
Children with ASD demonstrate difficulties in performing daily tasks requiring various visual perception functions such as visual figure ground, visual form constancy, and visual sequencing memory abilities.13 Besides limitations in both essential social communication and adaptive behaviors, children with ASD also exhibit delayed motor development and deficient perceptual-motor functions that influence multiple sensory and neuromotor systems.14 Research results suggest that children with ASD usually have co-existing motor dysfunctions and motor programming deficits.15 Motor coordination deficits are considered as a cardinal feature of ASD in addition to their featured social and behavioral difficulties, and the large effects from meta-analysis research provide compelling support for the ASD group having significant deficits in performing motor coordination tasks.16 These perceptual-motor deficits in ASD have great adverse impact on their qualitative and quantitative participation in various activities across settings.17
Activity Participation
The International Classification of Functioning, Disability and Health (ICF) model18 defines participation as “engagement in life events and contexts” resulting from interaction of individuals with their environments. Participation in daily activities is crucial and essential for fostering development and enriching life experience, and through these, children will be able to obtain skills and capabilities, establish inter-personal connections, and find meaningful goals of life.19 The concept of participation and function has become increasingly important in the field of childhood rehabilitations.20
The first study hypothesis is that children with ASD would have atypical or impaired sensory and perceptualmotor performances. On the basis of shifts of health paradigms, the second hypothesis is that body functions would have significant impacts on ASD children’s activity participations. The age and gender effects would be investigated as well. To address these study hypotheses, standardized instruments assessing different subdomains of body functions and activity participations were adopted. The results of a comprehensive assessment battery could provide thorough and in-depth understanding of the sensorimotor functions and activity participations in a wide age-ranged children with ASD.
Materials and Methods
Participants
Convenience sampling was used in this study to facilitate representative body functions from ASD participants across age range and different gender. The inclusion criteria were: (1) elementary school age (5–13 years); (2) physicians; and (3) no serious physical or behavioral problems. Participants who carried concurrent sensory impairments (eg, blindness, deafness) were also excluded. A total of 166 children met the study inclusion and exclusion criteria, with all children and parents consenting, and ultimately, 117 (75 males, 42 females) agreed to take part in this study. We divided the participants (mean age: 8 years 3 months; range: 5 years 1 month to 13 years 6 months) into three groups of different age bands since it seemed reasonable to expect greater differences in groups of children between the ages of 5–7 years and 8–10 years than in adolescents (11–13 years).21 These three age groups were of similar size: youngest (5–7 years) (n = 43; 18 females; mean age: 6 years 2 months); middle (8–10 years) (n = 44; 18 females; mean age: 8 years 11 months); and oldest (11–13 years) (n = 30; 8 females; mean age: 11 years 11months). Of this 117, 26 children (22.5%) had Asperger’s, 21 children (17.9%) had high-functioning autism, and 70 children had unspecified ASD. Sixty-seven participants attended the regular educational programs, and 40 were enrolled in special education programs.
Instruments
The body function measures included Bruininks–Oseretsky of Motor Proficiency – Second Edition, Sensory Profile, Test of Sensory Integration Functions, and Test of Visual Perception Skills – Third Edition. These measured were chosen to assess the motor functions, sensory processing, sensory integration, and visual perceptual functions, respectively. The activity participation measures included the Chinese versions of both Vineland Adaptive Behavior Scale and School Function Assessment.
Body Function Measures
The TVPS-3 consists of seven subtests: visual discrimination, visual memory, visual spatial relationship, visual form constancy, visual sequential memory, visual figure-ground, and visual closure.23 The TVPS-3 is appropriate for individuals aged 4–18 years 11 months. It was administered on an individual basis and the time for conducting the testing is about an hour. The TVPS-3 has good test–retest reliability (0.97),22 and moderately correlated with other standardized visual perception measures.24
The BOT-2 assesses proficiency for individuals aged 4–18, and consists of four motor-area composites. Fine manual control composite (FMC) measures the motor skills involved in handwriting and manipulative tasks acquiring accurate control of finger movements. Manual coordination composite (MC) assessed reaching, grasping, and object manipulation tasks relying heavily on motor control, speed, and coordination of upper extremities. Body coordination composite (BC) evaluates the balance and motor skills required for successful participation in PE class, sports, and leisure activities. Strength and agility composite (SA) assessed lower extremities muscle strength, running speed, and agility during locomotion. The BOT-2 had good test-retest (>0.95), inter-rater reliabilities (>0.92), and the internal consistencies of the four motor composites are from 0.78 to 0.97.25 The BOT-2 total composite correlated fairly well with other measures of motor performance, and its construct validity was supported as well.26,27
The SP is a parent-reported assessment of behaviors associated with deviant reactivity to different sensory stimulation for children aged 5–10 years. The SP (125 items) consists of three major domains: Sensory Processing, Modulation, and Behavioral and Emotional Responses. The total score of each section was used to indicate the sensory dysfunction tapped in different domain. Published cut-off scores are provided in the SP manual, and mean score for the typical reference sample was used to judge the sensory function (typical: ≥ mean; probable difference: 1 SD < below the mean; definite difference: 2 SD < the mean). The SP had sound reliabilities: the internal consistency ranged from 0.47 to 0.90, and the SEM ranged from 1.13 to 2.81.28 The validity of the SP was supported by its moderate correlations with the School Function Assessment supported as well.29
The TSIF is developed to diagnose the subtypes of sensory integrative dysfunction for children aged from 3 to 12 years. The six subtests in TSIF include postural movement, bilateral integration sequencing, sensory discrimination, sensory searching, attention and activity, and emotional–behavioral reactivity. These subtests consisted of different interactive activities that could tap the functions of the primary sensory systems (vestibular, proprioceptive, and tactile systems) involved in sensory integration process. The parents or teachers observe the targeted behaviors while children performing those interactive activities, and then rate the frequency of the behaviors during the whole activity. The rating score ranges from 1 (never) to 5 (always), and lower scores suggest better performance on sensory integration tasks. The TSIF has sound psychometric properties including high internal consistency, test–retest reliability, and sound construct validities.30
Activity Participation Measures
The Vineland Adaptive Behavior Scale32 was developed for use in populations aged from birth to 18 years 11 months, and the VABS-C was its translated version in Chinese. Adaptive behavior is defined as the age-appropriate performances of the everyday activities required for personal and social functioning, and this scale was designed to measure the construct of participation through its assessment of adaptive behaviors. The four domains are communication, daily living skills, socialization skills, and motor skills. We used the teacher-rated scale in the current study. The VABS-C measure had good reliabilities (split-half reliability: 0.91 to 0.99; and the test–retest reliability coefficient: 0.74 to 0.93). Sound discriminative and current validity were also supported well.31
The SFA-C is translated from the School Function Assessment.29 This questionnaire was developed to assess and monitor a child’s participation, support needs, and performance of functional (nonacademic) tasks and school-related activities. The SFA is comprised of three parts with 316 items total; these 3 parts are Participation, Task Support, and Activity Performance. The entire assessment can be administered or scales can be chosen depending on the child’s need. We selected the following sum scored to measure the overall school functions of the ASD; these are Participation (6 major school settings), Activity Performance-Physical Tasks (12 physical tasks, such as tool use, recreational games, and basic activities of daily living), and Activity Performance- Cognitive/Behavioral Tasks (9 tasks, such as comprehension, expression). These items were rated by the children’s teachers in the current study. The SFA-C had sound validities and reliabilities (internal consistency: 0.94 to 0.96; test– retest reliability: 0.87 to 0.99).34
Procedure
After obtaining approval from the Institutional Review Board of the Kaohsiung Medical University Hospital, this study was conducted from 2016 to 2019. Participants were recruited from educational (15 schools and 4 private agencies serving children with ASD) and clinical (departments of occupational therapy at three hospitals) sources at different areas. Written informed consent was obtained from the participant and his/her parents or guardian before the study. Participating teachers were consented as well. Information and diagnosis of each child were collected from medical or school records. The SFA-C, VABS-C, and the TSIF were sent to the child’s school teacher for rating and then scored by the PI. SP was filled out by one parent of each child. Four senior pediatric occupational therapists administered the BOT-2 and TVPS to the children according to standardized procedures provided by the test manuals. To improve the reliabilities and consistencies of the raters, the PI offered the four examiners 8-hour training sessions, and the training emphasized administration procedure and scoring standards of each assessment. It took about 2 hours to complete the testing, and all the assessments were conducted on an individual basis in quiet spaces either at the child’s classroom or OT room. This study was conducted in accordance with the Declaration of Helsinki.
Data Analysis
We used SPSS20 for all data analyses in the present study. Different types of scores were adapted to facilitate analyses. Raw scores of SP and SFA-C were used, while raw scores of BOT-2, TVPS-3, TSIF, and the VABS-C had to be converted to standard scores according to the administration manual. To evaluate whether children with ASD performed differently from normative samples for all the body functions and activity participation, a one-sample z-test was then computed. The percentage of children who scored on an individual subtest falling more than 1.5 standard deviations below the age-based normative mean was used to indicate the frequency of body function impairments.
Comparative Studies
The MANOVA (multiple analysis of variance) was performed to analyze the age and sex effects on all the sensorimotor and activity participation measures. We performed different 2 X 3 MANOVAs to assess the effects of age, sex, and age-sex interaction for the subtests of the body function and activity participation measures. Follow-up univariate F-tests were performed with Scheffe´ post-hoc comparisons if the multivariate test indicated a significant group effect (effect sizes f2: 0.02, small; 0.15, medium; 0.35, large).34 In light of the number of univariate analyses conducted, the significant level was set at 0.05/numbers of dependent variables for all follow-up analyses to maintain a family-wise error rate of less than 0.05. After adjusting for age, Pearson correlations were computed to assess the relations between body functions and overall activity participation. Correlation coefficients of 0.1, 0.3, and 0.5 were considered as small, medium, and large, and were used as a guideline to indicate the strength of correlation.35
Results
Profiles of Sensory Integration, Motor and Visual Perceptual Functions
The z-tests results demonstrated that 5- to 13-year-old ASD children had substantial impairment across all body function as shown in Table 1. All participants had deficient performance on at least 4–6 subtests out of 19 body function measures. More than half of the participants (71/117, 60.7%) had sensorimotor and visual perceptual impairments between 13 and 15 subtests, whereas 58/117 (49.6%) had results falling 1.5 SD below the mean on 16 or more subtests. Performance on sensory integration, motor functions, and visual perception tests is described as follows.
 |
Table 1 Z-Tests on the Standard Scores of the Sensorimotor Measures in Children with ASD (n=117) |
Sensory Integration Profile
Most participants scored in the impaired range on any of the three SP domains: the sensory modulation had the highest frequency of impairments (94.2%) and the behavioral and emotional response had the lowest (87.6%). All participants scored in the impaired range on the seven TSIF subtests with the best performance on postural movement task. Participants had the worst performance on the bilateral integration sequencing, sensory discrimination, and sensory searching subtests, and these tasks correlated with the overall somatosensory functions.
Motor Profile
On four BOT-2 composites, all participants met impairment criteria, and they had rather better performances on Strength and Agility composite (Table 1). All children (100%) scored in the impaired range on BOT-2 fine motor composite, with manual dexterity having the highest frequency (97.4%) of impairment. About 70% of children scored in the impaired range on BOT-2 gross motor composite, and the bilateral coordination had the highest frequency (63.2%) of impairment.
Visual Perceptual Functions
School-aged children with ASD in the present study had significantly impaired visual perceptual abilities, more than half (50%) of the participants were scored in the designated impaired range. In the seven visual perceptual tasks of the TVPS-3, the participants performed best on the visual–spatial relationship and the worst on constancy and visual figure-ground/visual closure tasks (88.5% of impairment).
Results of Comparative Studies
Body Functions
A MANOVA was performed to assess the effects of age group (three levels) and sex levels (two levels) on the four composites of the BOT-2. Main effect was only noticed for age (Wilk’s λ = 0.87, p = 0.04) on the BOT-2, and there was no significant effect for either sex (Wilk’s λ = 0.88, p = 0.10) or age x sex interaction (Wilk’s λ = 0.90, p = 0.17). Post-hoc analysis revealed the significant differences of the four BOT-2 composite scores in the middle (8–10 years) versus oldest (11–13 years) and youngest (5–7 years) versus oldest group (Table 2), and the effect sizes were all in the medium range (f2: 0.21–0.33).
 |
Table 2 Post Hoc Scheffé Multiple Comparison of Sensorimotor Measures Across Age and Sex in Children with ASD (N= 117) |
No significant effects for age (Wilk’s λ = 0.64, p = 0.66), sex (Wilk’s λ = 0.55, p = 0.13), or age x sex interaction (Wilk’s λ = 0.69, p = 0.35) for the seven TVPS-3 subtests except for the visual-spatial relationship subtest were noted. Significant differences of the visual-spatial relationship subtest were found in the middle versus oldest (f2 = 0.28; medium effect size) and youngest versus oldest (f2= 0.52, large effect size) group.
No significant main effects for sex or age x sex interaction effect on three sections of SP (sex: Wilk’s λ = 0.73, p = 0.54; age x sex: Wilk’s λ = 0.66, p = 0.21) and six subdomains of TSIF (sex: Wilk’s λ = 0.69, p = 0.69; age x sex: Wilk’s λ = 0.73, p = 0.66) measures were noted. Main effect was noticed for age group in the SP (Wilk’s λ = 0.73, p = 0.00) and the TSIF (Wilk’s λ = 0.45, p = 0.04). For the three SP subdomains, significant differences were noticed between the following age groups: middle versus oldest (Sensory Processing, Modulation), and youngest versus oldest group (Sensory Processing, Modulation, Behavioral, and Emotional Responses). The effect sizes were all in the medium range (f2: 0.25–0.34). For TSIF, significant differences of SP scores were found in the young versus middle (Postural Movement), middle versus oldest (Postural Movement, Bilateral Integration Sequencing), and youngest versus oldest group (all 6 subtests) (Table 2). The effect sizes were between the medium to large range (f2: 0.25–0.57).
Activity Participation
Regarding the four VABS-C domains, a significant main effect for age (Wilk’s λ = 0.99, p = 0.03) and sex was found (Wilk’s λ = 0.98, p = 0.04), and no significant age x sex interaction effect (Wilk’s λ = 0.95, p = 0.21) was demonstrated. The between-subject effects of the MANOVA model were adopted to further investigate the main effect of age and gender. For all the four domains, significant age effects with medium effect sizes (f2: 0.22–0.33) were found between the following age groups: middle versus oldest and the young versus oldest group (in daily living skills, socialization, and motor skills subdomains). The oldest group performed the best, and girls outperformed boys in daily living skills and socialization subdomains (Table 3).
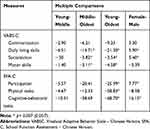 |
Table 3 Post Hoc Scheffé Multiple Comparison of Activity Participation Measures Across Age and Sex in Children with ASD (N= 117) |
After analyzing the effects of age group (three levels) and sex (two levels) on the three parts of SFA-C (participation, physical tasks, cognitive-behavioral tasks), the results showed significant main effects for age (Wilk’s λ = 0.95, p = 0.04) and sex (Wilk’s λ = 0.89, p = 0.03), but no significant age x sex interaction effect (Wilk’s λ = 0.99, p = 0.89) for the subdomains of this measure was found. The oldest group performed the best on all school functions, and girls significantly outperformed boys in participation and cognitive-behavioral tasks with definite difference (Table 3).
Correlational Studies
All correlation data are presented in Table 4. The TVPS-3 significantly correlated to VABS-C domains and SFA-C parts with large effect size mostly. The correlations between motor functions (BOT-2) and all the activity participation measure domains were in the moderate range except for the VABS-C Communication subdomain (small). All the sensory integration functions (measure by the SP and the TSIF) were strongly related to the activity participations (measured by the VABS-C, SFA-C) domains.
 |
Table 4 Correlations Between Sensorimotor Measures and Activity Participations in Children with ASD |
Discussion
Our study showed that school-aged ASD children were impaired on all body function measures (sensory integration, motor functions, and visual perceptual functions), and were strongly related to their activity participation. Numerous studies have assessed body function and its associations with activities; however, to our knowledge, no study has examined comprehensive body functions and activity participation across multiple scenarios in ASD children with wide age range.
Body Functions
Motor Functions in ASD
Early studies showed that most motor characteristics and development trajectories of children with ASD were similar to those of individuals with various developmental disabilities.36 However, most studies confirmed impairments in motor development primarily focused on ASD at younger ages.37,38 Our study results demonstrated that ASD children generally show some levels of impairment and atypical variations in both gross and fine motor skills compared to their typically-developing peers, and these impairments might last through adolescence and adulthood. Although the motor problems of ASD have been less emphasized compared to the social and communication deficits, assessment and remediation of comprehensive motor functions should be integrated into the complete rehabilitation plan and provided as early as possible.
Gross motor development is strongly associated with cognition, and consequently language in children.39,42 The study findings emphasize the necessity of early interventions that boost gross motor development since such interventions will also enhance cognitive development. Our research results echoed a previous meta-analysis study15 showing that motor coordination deficits are a predominant characteristic of ASD. To improve gross motor functions in ASD groups, therapists should particularly stress provision of therapeutic movements requiring various motor coordination abilities such as dynamic balance, and bilateral extremity functions. Although the individuals with ASD performed better on tasks requiring muscle strength and agility, rehabilitative interventions aiming to improve these two motor functions are strongly suggested since they are associated with general physical fitness, daily activities, and later vocational functions.40
The BOT-2 test results showed that ASD individuals had pervasive impairments in fine motor skills, and these findings were similar to earlier studies.41 We propose some possible reasons for the poor fine motor skills in ASD. The primary reason might be due to the impaired tactile-perceptual skills in ASD. Researchers have suggested that ASD individuals might have deficient stereognosis,43 one of the higher-level tactile-perceptual abilities, and such impairments will further exacerbate advanced fine motor performance. Another probable assumption is that subcomponents of fine motor skills require maturity and integration of the central nervous system networks, and the fronto-parietal pathway has been considered being responsible for most fine motor skills.44 This neural pathway involves the motor cortex, supplementary motor area, basal ganglia, and cerebellum, and early studies have identified differential activation in these cortical and subcortical areas in ASD.45 Lastly, weakness in grip strength explained by inadequate muscle tone might hamper functional fine motor tasks.43 Provision of preparatory activities could increase grip strength, while sensory integration intervention has been found effective in treating sensory modulation disorders related to hand functions and handwriting (eg, tactile defensiveness). To improve the tactile-perceptual functions in ASD individuals, occupational therapists usually offer a wide array of sensory training protocols and manipulative activities emphasizing speed, accuracy, and total efficiency.
Sensory Profile in ASD
Our study results showed that school-aged children with ASD had significant difficulties in sensory integration, and emotional and behavior regulations. The overall sensory processing abilities of ASD children seem to be still impaired compared to the referenced group as they reach adolescence.46
Low muscle tone, commonly observed in ASD populations, might be due to their sensory processing difficulties in regulating muscle endurance and tone. Hypotonia in ASD should be treated as early as possible to prevent further dysfunctions since low muscle tone is associated with significant limitations on multiple areas of attention levels, motor proficiency, and participation in academic and extracurricular activities.47 Awareness of the signs of hypotonia in young children might serve as an entrance to improve early diagnosis of ASD since a large-scale study supported the possible connections between infantile muscle tone and childhood autistic features.48
From our research results, children with ASD manifested various sensory integrative disorders (SID) including sensory modulation disorders, sensory discrimination and perceptual dysfunctions, vestibular-bilateral difficulties, dyspraxia, and sensory-seeking behaviors.49,50
Over-responsiveness in autism is pretty common and very often manifested as tactile defensiveness (TD) or auditory defensiveness (AD).50 Self-care activities and school functions are often affected by sensory defensiveness, and common social situations might be disturbing enough to lead their emotional outburst and maladaptive social behaviors.51 Somatodyspraxia refers to a sensory integrative dysfunction involving poor motor planning, deficient tactile discrimination, and proprioceptive processing,52 and it might be a significant area of concern for ASD since the participants in this study had poor tactile perception in conjunction with poor motor planning. Motor planning difficulties have been well documented in ASD populations,53 and ideation of motor planning might be affected and could be related to their repetitive and stereotyped behaviors that comprise one of the defining characteristics of ASD diagnosis.
The ASD participants also demonstrated significant impairments in postural movement and bilateral integration sequencing tasks, and thus indicated their vestibular and proprioceptive processing difficulties.52 A previous study that examined vestibular functions of children with ASD suggested that postural control in these children was underdeveloped,54 and delayed head lag at infancy served as a predictor of autism at later ages.55 Inefficient postural controls as well as poor bilateral coordination are likely to affect competence in motor activities. Bilateral coordination difficulties are associated with delayed midline skill development, such as hand preference and right-left discrimination.56 Proprioceptive problems in ASD cases of concern to occupational therapists are related to integration of proprioceptive information from the entire body to guide complex action, and proprioceptive insufficiencies might be related to their postural-motor and praxis difficulties.
Sensory-seeking behavior is prevalent among children with ASD, and the typical behaviors often involve visual, tactile, and auditory-seeking. It is convincing that sensory-seeking behaviors in this population serve arousal modulation functions,57 and it might be used to help dampen or override over-responsivity in a particular system.58 Some sensory-seeking behaviors in ASD children might not be socially appropriate and have even become a safety issue at home and schools.
Visual Perceptual Functions in ASD
The TVPS-3 results suggested that school-aged ASD children have below-average performances in various visual perceptual tasks. These visual perceptual impairments might contribute to some unique features in ASD; for example, impaired visual scanning and memory would affect their facial recognition of others.59
Our study results are opposite to some studies suggesting that ASD had superior visual search abilities (basic visual discrimination ability).60 The inconsistent finding might be caused by the different demographic contributes that our participants were much younger than those studies. Our ASD participants demonstrated challenges in performing the visual discrimination tasks of TVPS-3. These difficulties might be due to the unique information processing strategy utilized by ASD individuals, who, having discrepancies in visual attention strategy, are prone to concentrate their focus on detailed parts (local features) rather than the object as a gestalt (global shape) while processing visual stimulations.61 This information processing is different from those seen in typically developing children; for example, cognitive-impaired individuals demonstrated correct overall organization but made many mistakes on local details.62 The detail-oriented information strategies adopted by ASD individuals were well described,63 and this processing bias might hinder the generalizing skills of ASD children.64–66
Visual memory refers to the memory of an object, while visual sequential memory is more associated with memory of location. Research results regarding visual memory in ASD children are inconclusive, and the results suggest that ASD individuals have difficulty in performing visual memory tasks. A recent study showed that ASD participants had lower visual working memory; especially with having difficulties processing rapid visual information.67 Individuals with ASD possess rather intact visual object working memory than visual spatial working memory.67,68
The ASD children also had significant difficulties in performing the sequential memory tasks of TVPS-3. Deficits in sequential memory might hinder children’s memories for locations, and the level of the impairments was positively related to the memory loading. In addition, the impairments in visual memory might be age-related, and the relations become more noticeable from adolescence to adulthood.69 Both typically-developing and ASD populations show gradual improvements in visual memory as they grow up, but the extent of improvement is smaller in ASD individuals.70 Children with ASD have preserved associated visual memory and shape recognition. ASD individuals have been reported to have excellent recognition memory for visual stimulation and objects not carrying any social meaning (eg, colors, car brands),71 however, facial recognition still seems to be a particular impairment in ASD children in spite of the rather intact associative memory.
Spatial Relationship
The ASD participants had best performance on the visual-spatial relationship in the TVPS-3 domains, but it was still in the impaired range. Impairments in imitation abilities have been reported in ASD individuals,72,73 and these dysfunctions might hamper development of play skills and social functions. The mirror neuron system has been considered responsible for the imitation skills,74 and problems in the functioning of this system could be involved in social cognitive impairments commonly observed in ASD individuals.75 Deficient visual spatial processing in ASD children might make the mirror neuron system work harder, therefore more adversely affecting their imitation abilities.16,76
The participants had significant impairments on performing form constancy, figure ground and visual closure tasks. Both visual discrimination and mental rotation abilities are fundamental to form constancy, figure ground, and visual closure abilities.77 Mental rotation requires both the dorsal stream (spatial working memory) and ventral stream (object working memory) subsystem to operate together,78 and ASD individuals were found to have impaired neural correlates (prefrontal cortex network) in both spatial and object working memory.67,68 Among the TVPS-3 subtests, the ASD participants had the worst performance on Figure Ground tasks. This TVPS-Figure Ground subtest is established according to the visual processing hierarchy, and the individuals are asked to identify an image from a rival background. A previous study demonstrated a positive correlation between TVPS-Figure Ground subtest scores and autistic-like social features only seen in males; additionally, the figure-ground ability might be linked to facial recognition difficulties.79
Activity Participation
Our research results indicated that activity participation measures differed between young-oldest age groups; however, the impairments in adaptive behaviors that characterize ASD children might not diminish with age and maturity. The earlier studies showed that children with ASD had relatively stronger Daily Living Skills, Motor Skills, and poor Communication and Socialization skills among the adaptive functioning assessed.80 The present study had similar results, where the VABS scores demonstrated that both Communication and Socialization Skills were significantly worse than Daily Living and Motor skills. A previous study indicated that IQ is a significant predictor of adaptive behaviors in ASD,81 and the autism severity might partially explain the differences in Socialization and Daily Living Skills. In another study investigating adaptive behaviors in Taiwanese children with high-functioning autism, the results demonstrated that the social domain showed vulnerable adaptive functioning. Cognitive abilities were positively associated with adaptive functions, but autism severity was poorly related to the adaptive behaviors.82 Among the school functions, the results indicated that school-aged children with ASD have more problems in Participation and Cognitive-behavioral Tasks. Even the ASD individual had better performance on carrying out Physical Tasks, the commonly coexistent difficulties in body coordination and visual perception are still exhibited in the challenging features of most daily and school activities.
Correlations Between Body Functions and Activity Participation
Our finding highlighted a significant association (most are of large effect size) between body functions and activity participation measures, and these strong correlations support the possible contribution of sensory-perceptual-motor functions on substantial participation in children with ASD. Consequently, intervention aimed at treating the sensory-related issues is crucial for both clinicians and ASD individuals. Globally, occupational therapists have traditionally and widely used the Ayres Sensory Integration® frame of reference (SI-FOR) for children with ASD, and it has been validated in its effectiveness for improving both sensory integration function and other issues related to sensory processing difficulties in children with ASD.83
Gender Effects
The study results showed that gender did not have significant effects on body functions of children with ASD except for a few measures. Girls performed better on behavioral and emotional responses while boys had higher scores on visual-spatial relationship and most motor-related tasks. On activity participation, girls performed better on daily living skills, participation, and cognitive-behavioral domains while boys had higher scores on physical and motor-related tasks. These results are similar to the trend in typically developing children where males outperform females on most gross motor functions.84 Our research results indicated no gender effect in communication although some studies have demonstrated that ASD individuals with different gender had diverse impairments in social communication.84 Girls with ASD tend to use compensatory strategies (eg, keeping close to peers) to disguise their difficulties in social interactions according to the camouflage hypothesis,85 and the possible gender bias might contribute to the underestimation of the prevalence of ASD in females.
Conclusions
The school-aged with ASD had impaired sensory and perceptual-motor performances, and these impairments were significantly related to their activity participations. This study has several strengths. Firstly, the participants are representative since they included a wide range of school-aged children. Secondly, comprehensive and valid measurements were adopted to fully and reliably investigate the body functions and activity participations across multiple settings in Taiwanese school-aged children with ASD. There were some limitations with respect to the lack of control group and intellectual functioning measures. For further study, we recommend the completion of longitudinal studies with various subtypes (eg, Asperger’s, high-functioning autism) and intellectual functioning to determine the effects of maturation and individual differences on sensory integration and perceptual-motor skills in ASD school-aged individuals.
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Christensen DL, Bilder DA, Zahorodny W, et al. Prevalence and characteristics of autism spectrum disorder among 4-year-old children in the autism and developmental disabilities monitoring network. J Dev Behav Pediatr. 2016;37(1):1–8. doi:10.1097/DBP.0000000000000235
2. Wiggins LD, Levy SE, Daniels J, et al. Autism spectrum disorder symptoms among children enrolled in the Study to Explore Early Development (SEED). J Autism Dev Disord. 2015;45(10):3183–3194. doi:10.1007/s10803-015-2476-8
3. Baron-Cohen S. Autism and Asperger Syndrome. Cambridge: Oxford University Press; 2008.
4. Warren Z, McPheeters ML, Sathe N, Foss-Feig JH, Glasser A, Veenstra-Vanderweele J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatri. 2011;127:e1303–e1311. doi:10.1542/peds.2011-0426
5. Payakachat N, Tilford JM, Kovacs E, Kuhlthau K. Autism spectrum disorders: a review of measures for clinical, health services and cost–effectiveness applications. Expert Rev Pharmacoecon Outcomes Res. 2012;12(4):485–503. doi:10.1586/erp.12.29
6. Miller LJ, Anzalone ME, Lane SJ, Cermak SA, Osten ET. Concept evolution in sensory integration: a proposed nosology for diagnosis. Am J Occu Ther. 2007;61(2):135–140.
7. Miller LJ, Schosen SA, James K, Schaaf RC. Lesson learned: a pilot study of occupational therapy effectiveness for children with sensory modulation disorder. Am J Occu Ther. 2007;61:135–140.
8. Bundy AC, Shia S, Qi L, Miller LJ. How does sensory processing dysfunction affect play? Am J Occu Ther. 2007;61(2):201–208. doi:10.5014/ajot.61.2.201
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
10. Green D, Chandler S, Charman T, Baird G. Brief report: DSM-5 sensory behaviors in children with and without an autism spectrum disorder. J Autism Dev Disord. 2016;43:3597–3606. doi:10.1007/s10803-016-2881-7
11. Ayres AJ. Sensory Integration and the Child.
12. Lang R, O’Reilly M, Healy O, et al. Sensory integration therapy for autism spectrum disorders: a systematic review. Res Autism Spectr Disord. 2012;6(3):1014–1018. doi:10.1016/j.rasd.2012.01.006
13. Evers K, Noens I, Steyaert J, Wagemans J. Combining strengths and weaknesses in visual perception of children with an autism spectrum disorder: perceptual matching of facial expressions. Res Autism Spectr Disord. 2011;5(4):1327–1342. doi:10.1016/j.rasd.2011.01.004
14. Provost B, Heimerl S, Lopez BR. Levels of gross and fine motor development in young children with autism spectrum disorder. Phys Occu Ther Pediat. 2007;27:21–36. doi:10.1080/J006v27n03_03
15. Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007;29(9):565–570. doi:10.1016/j.braindev.2007.03.002
16. Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. 2010;40(10):1227–1240. doi:10.1007/s10803-010-0981-3
17. Shattuck PT, Orsmond GI, Wagner M, Cooper BP. Participation in social activities among adolescents with an autism spectrum disorder. PLoS One. 2011;6:e27176. doi:10.1371/journal.pone.0027176
18. King GA, Law M, King S, et al. Measuring children’s participation in recreation and leisure activities: construct validation of the CAPE and PAC. Child Care Health Dev. 2007;33:28–39. doi:10.1111/j.1365-2214.2006.00613.x
19. World Health Organization. International Classification of Functioning, Disability and Health. Geneva: World Health Organization; 2001.
20. Little LM, Ausderau K, Sideris J, Baranek GT. Activity participation and sensory features among children with autism spectrum. J Autism Dev Disord. 2015;45(9):2981–2990. doi:10.1007/s10803-015-2460-3
21. Memari AH, Pahahi N, Ranjbar E, et al. Children with autism spectrum disorder and patterns of participation in daily physical and play activities. Neurol Res Int. 2015;531906.
22. Martin N, Gardner MF. Test of Visual Perceptual Skills.
23. Brown T, Mullins E, Stagnitti K. The reliability of performance of health adults on three visual perception tests. Brit J Occu Ther. 2008;71:438–447. doi:10.1177/030802260807101007
24. Brown T, Mullins E, Stagnitti K. The concurrent validity of three visual perception tests used with adults. Occu Ther Health Care. 2009;23(2):99–118. doi:10.1080/07380570902773222
25. Bruininks RH, Bruininks BD. Bruininks–Oseretsky Test of Motor Proficiency.
26. Folio MR, Fewell RR. Peabody Developmental Motor Scales.
27. Gardner MF. Test of Visual–Motor Skills-Revised. Hydesville (CA): Psychological & Educational Publications; 1995.
28. Dunn W. Sensory Profile. San Antonio (TX): Psychological Corporation; 1999.
29. Coster W, Deeney T, Haltiwanger J. Haley S. School Function Assessment. San Antonio (TX): Therapy Skill Builders; 1998.
30. Lin JK. Test of Sensory Integration Function: User’s Manual. Taipei: Psychological Corporation; 2004.
31. Wu WT, Chang CF, Lu TH, Chiu SC. Vineland Adaptive Behavior Scale-Chinese Version. Taipei: Psychological Corporation; 2004.
32. Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scale. Minnesota (MO): American Guidance Service;; 1984.
33. Hwang JL. School Function Assessment-Chinese Version. Taipei: The Psychological Corporation; 2008.
34. Hwang JL. The reliability and validity of the school function assessment- Chinese version for cross-cultural use in Taiwan. Occu Ther Int. 2005;11(1):26–39. doi:10.1002/oti.195
35. Cohen J. Statistical Power Analysis for Behavioral Science.
36. Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatri. 2007;120(5):1183–1215. doi:10.1542/peds.2007-2361
37. Lloyd M, MacDonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism. 2013;17(2):133–146. doi:10.1177/1362361311402230
38. MacDonald M, Lord C, Ulrich DA. Motor skills and calibrated autism severity in young children with autistic spectrum disorder. Adapt Phys Activ Q. 2014;31:95–105. doi:10.1123/apaq.2013-0068
39. van der Fels IMJ, Te Wierike SCM, Hartman E, Elferink-Gemser MT, Smith J, Visscher C. The relationship between motor skills and cognitive skills in 4–16 year old typically developing children: a systematic review. J Sci Med Sport. 2015;18:697–703. doi:10.1016/j.jsams.2014.09.007
40. Gu X, Chang M, Solmon MA. Physical activity, physical fitness, and health- related quality of life in school-aged children. J Teach Phys Educ. 2016;35(2):117–126. doi:10.1123/jtpe.2015-0110
41. Alaniz ML, Galit E, Necesito CI, Rosario ER. Hand strength, handwriting, and functional skills in children with autism. Am J Occu Ther. 2015;69:245–251.
42. Houwen S, Visser L, van der Putten A, Vlaskamp C. The interrelationships between motor, cognitive, and language development in children with and without intellectual and developmental. Res Dev Disabil. 2006;53-53:19–33.
43. Abu-Dahab SMN, Skidmore ER, Holm MB, Rogers JC, Minshew NJ. Motor and tactile-perceptual skill differences between individuals with high-functioning autism and typically developing individuals ages 5–21. J Autism Dev Disord. 2013;43(10):2241–2248. doi:10.1007/s10803-011-1439-y
44. Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26(8):2260–2268. doi:10.1523/JNEUROSCI.3386-05.2006
45. Verhoeven JS, De Cock P, Lagae L, Sunaert S. Neuroimaging of autism. Neuroradiol. 2010;52:3–14. doi:10.1007/s00234-009-0583-y
46. Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J Autism Dev Disord. 2009;39:1–11. doi:10.1007/s10803-008-0593-3
47. Pope M, Liu T, Breslin CM, Getchell N. Using constraints to design developmentally appropriate movement activities for children with autism spectrum disorders. J Phys Educ Recreat Dance. 2012;83(2):35–41. doi:10.1080/07303084.2012.10598726
48. Serdarevic F, Ghassabian A, van Batenburg‐Eddes T, et al. Infant muscle tone and childhood autistic traits: a longitudinal study in the general population. Autism Res. 2017;5(5):757–768. doi:10.1002/aur.1739
49. Holland AJ. Atypical movement performance and sensory integration in Asperger’s syndrome. J Autism Dev Disabil. 2011;5:1301–1302.
50. Lanet CJ. Verification and classification of patterns of sensory integrative dysfunctions. Am J Occu Ther. 2011;65:143–151. doi:10.5014/ajot.2011.000752
51. Green SA, Ben-Sasson A. Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: is there a causal relationship? J Autism Dev Disord. 2010;40:1495–1504. doi:10.1007/s10803-010-1007-x
52. Mailloux Z, Mulligan S, Roley SS, et al. Progression of challenging behaviors in children and adolescents with autism spectrum disorders as measured by the Autism Spectrum Disorders-Problem Behaviors for Children (ASD-PBC). Res Autism Spectr Disord. 2010;4:400–404. doi:10.1016/j.rasd.2009.10.010
53. Siaperas P, Ring HA, McAllister CJ, et al. Atypical movement performance and sensory integration in Asperger’s syndrome. J Autism Dev Disord. 2011;42:718–725.
54. Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurol. 2004;63(11):2056–2061. doi:10.1212/01.WNL.0000145771.98657.62
55. Flanagan JE, Landa R, Bhat A, Baumanet M. Head lag in infants at risk for autism: a preliminary study. Am J Occu Ther. 2012;66:577–585. doi:10.5014/ajot.2012.004192
56. Bhat AN, Landa RJ, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys Ther. 2011;91:1116–1129. doi:10.2522/ptj.20100294
57. Baranek GT, Little LM, Parham LD, Ausderau KK, Sabitos-Devito MG. Sensory features in autism spectrum disorders. In: Volkmar F, Paul R, Pelphrey K, Rogers S, editors. Handbook of Autism.
58. Parham LD, Mailloux Z. Sensory integration. In: Case-Smith J, O’Brien JC, editors. Occupational Therapy for Children and Adolescents.
59. Snow J, Ingeholm J, Levy I, et al. Impaired visual scanning and memory for faces in high-functioning autism spectrum disorders: it’s not just the eyes. J Int Neuropsychol Soc. 2011;17(6):1021–1029. doi:10.1017/S1355617711000981
60. O’riordan MA. Superior visual search in adults with autism. Autism. 2004;8(3):229–248. doi:10.1177/1362361304045219
61. Brock J, Xu JY, Brooks KR. Individual differences in visual search: relationship to autistic traits, discrimination thresholds, and speed of processing. Percept. 2011;40(6):739–742. doi:10.1068/p6953
62. Wan YT, Chiang CS, Chen CJ, Wuang YP. The effectiveness of the computerized visual perceptual training program on individuals with Down syndrome: an fMRI study. Res Dev Disabil. 2017;66:1–15. doi:10.1016/j.ridd.2017.04.015
63. Frith U. Why we need cognitive explanations of autism. Q J Exp Psychol. 2012;65:2073–2092. doi:10.1080/17470218.2012.697178
64. Happ’e F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5–25. doi:10.1007/s10803-005-0039-0
65. Qian N, Lipkin RM. A learning-style theory for understanding autistic behaviors. Front Human Neurosci. 2011;5:77. doi:10.3389/fnhum.2011.00077
66. Sapey-Triomphe L, Sonié S, Hénaff M, Mattout J, Schmidt C. Adults with autism tend to undermine the hidden environmental structure: evidence from a visual associative learning task. J Autism Dev Disord. 2018;48(9):3061–3074. doi:10.1007/s10803-018-3574-1
67. Funabiki Y, Shiwa T. Weakness of visual working memory in autism. Autism Res. 2018;11(9):1245–1252. doi:10.1002/aur.1981
68. Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. J Autism Dev Disord. 2005;35:747–756. doi:10.1007/s10803-005-0021-x
69. O’Hearn K, Tanaka J, Lynn A, Fedor J, Minshew N, Luna B. Developmental plateau in visual object processing from adolescence to adulthood in autism. Brain Cog. 2014;90:124–134. doi:10.1016/j.bandc.2014.06.004
70. Chien Y, Gau S, Shang C, Chiu Y, Tsai W, Wu Y. Visual memory and sustained attention impairment in youths with autism spectrum disorders. Psychol Med. 2015;45(11):2263–2273. doi:10.1017/S0033291714003201
71. Semino S, Zanobini M, Usai MC. Visual memory profile in children with high functioning autism. Appl Neuropsychol Child. 2019;10:1–11.
72. Vivanti G, Trembath D, Dissanayake C. Mechanisms of imitation impairment in autism spectrum disorder. J Abnorm Child Psychol. 2014;42(8):1395–1405. doi:10.1007/s10802-014-9874-9
73. Williams JH. Action evaluation and discrimination as indexes of imitation fidelity in autism. In: Torres EB, Whyatt C, editors. Autism: The Movement-Sensing Perspective. Boca Raton (FL): CRC Press; 2018:89–103.
74. Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25(4):287–295. doi:10.1016/S0149-7634(01)00014-8
75. Wilkinson MR, Ball LJ. Why studies of autism spectrum disorders have failed to resolve the theory versus simulation theory debate. Rev Philos Psychol. 2012;3(2):263–291. doi:10.1007/s13164-012-0097-0
76. MacNeil LK, Mostofsky SH. Specificity of dyspraxia in children with autism. Neuropsychol. 2012;26(2):165–171. doi:10.1037/a0026955
77. Wan YT, Chiang CS, Chen CJ, Wang CC, Wuang YP. Profiles of visual perceptual functions in down syndrome. Res Dev Disabil. 2015;37:112–118. doi:10.1016/j.ridd.2014.11.008
78. Kravitz DJ, Saleem KS, Bajer CI, Ungerleid LG, Mishkin M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trend Cog Sci. 2013;17(1):26–49. doi:10.1016/j.tics.2012.10.011
79. DiCriscio S, Troiani V. Brief report: autism-like traits are associated with enhanced ability to dissembled visual forms. J Autism Dev Disord. 2017;47:1568–1576. doi:10.1007/s10803-017-3053-0
80. Perry A, Flanagan HE, Dunn Geier J, Freeman NL. Brief report: the Vineland adaptive behavior scales in young children with autism spectrum disorders at different cognitive levels. J Autism Dev Disord. 2009;39(7):1066–1078. doi:10.1007/s10803-009-0704-9
81. Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, Saulnier CA. The role of adaptive behavior in autism spectrum disorders: implications for functional outcome. J Autism Dev Disord. 2011;41(8):1007–1018. doi:10.1007/s10803-010-1126-4
82. Chang C, Lung F, Yen C, Yang P. Adaptive behaviors in high-functioning Taiwanese children with autism spectrum disorders: an investigation of the mediating roles of symptom severity and cognitive ability. J Autism Dev Disord. 2013;43:1347–1355. doi:10.1007/s10803-012-1684-8
83. Schaaf RC, Benevides T, Mailloux Z, et al. An intervention for sensory difficulties in children with autism: a randomized trial. J Autism Dev Disord. 2014;44:1493–1506. doi:10.1007/s10803-014-2111-0
84. Barnett LM, van Beurden E, Morgan PJ, Brooks LO, Beard JR. Gender differences in motor skill proficiency from childhood to adolescence. Res Q Exerc Sport. 2010;2:162–170.
85. Dean M, Harwood R, Kasari C. The art of camouflage: gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism. 2017;21(6):678–689. doi:10.1177/1362361316671845
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
