Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Sennoside A Induces GLP-1 Secretion Through Activation of the ERK1/2 Pathway in L-Cells
Authors Ma L, Cao X, Ye X, Ye J , Sun Y
Received 25 January 2020
Accepted for publication 7 April 2020
Published 29 April 2020 Volume 2020:13 Pages 1407—1415
DOI https://doi.org/10.2147/DMSO.S247251
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Li Ma,1 Xinyu Cao,2 Xiaotong Ye,2 Jianping Ye,2 Yongning Sun1,3
1Department of Traditional Chinese Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai 200233, People’s Republic of China; 2Shanghai Diabetes Institute, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai 201306, People’s Republic of China; 3Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200071, People’s Republic of China
Correspondence: Yongning Sun; Jianping Ye Tel +86-18930177579; +86-13818929364
Email [email protected]; [email protected]
Purpose: Glucagon-like peptide-1 (GLP-1) is secreted from the intestinal L-cells to stimulate insulin secretion in the blood glucose control. Our previous study indicates that Sennoside A (SA) can increase the plasma GLP-1 level in a mouse model of type 2 diabetes. However, the mechanism of SA activity remains largely unknown. This issue was explored in this study.
Materials and Methods: C57BL/6 mice were randomly divided into four groups: a control group without drug treatment, and the other groups with different SA dosages, respectively. Blood glucose was assayed by oral glucose tolerance test (OGTT). Plasma GLP-1 and insulin were investigated. Colon tissues were collected for mRNA or Western blot analysis. Immunofluorescence staining assays were performed to evaluate the number of β-cells and L-cells. In NCI-H716 cells, extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitors were employed to investigate the SA-induced GLP-1 secretion mechanism.
Results: In this work, the SA was found to improve OGTT in mice. Plasma GLP-1 and insulin were markedly elevated by SA at the dosage of 45 mg/kg/day. Meanwhile, the increased phosphorylation status of EKR1/2 and prohormone convertase 1/3 (PC1/3) proteins were observed in the colon of SA-treated mice. The number of L-cells exhibited no change in each group. In the NCI-H716 cells, GLP-1 secretion induced by SA was blocked by the ERK1/2 inhibitor.
Conclusion: The present study provides a direct evidence for the interaction between SA and L cells for induction of GLP-1 secretion. These data suggest that GLP-1 secretion induced by SA is dependent on the ERK1/2 signaling pathway. Therefore, the SA is a new drug candidate for the type 2 diabetes treatment by induction of GLP-1 secretion.
Keywords: Sennoside A, glucagon-like peptide, 1, GLP, 1, extracellular signal-regulated kinases 1/2, ERK1/2, L-cells, prohormone convertase 1/3, PC1/3
Introduction
Glucagon-like peptide-1 (GLP-1) is encoded by the proglucagon (GCG) gene in the intestinal L cells. GLP-1 secretion is induced by nutrients in the control of blood glucose and food intake.1 GLP-1 decreases blood glucose through multiple mechanisms, which includes the promotion of glucose-induced insulin secretion from the pancreatic β cells. It was reported that the decrease of GLP-1 was involved in the pathogenesis of type 2 diabetes (T2DM).2–4 The induction of GLP-1 activity is an effective strategy in the treatment of T2DM for various GLP-1 analogous. The GCG gene encodes the hormone glucagon in the pancreatic α-cells, a hormone elevated in the fasting condition for the induction of gluconeogenesis. The GCG gene products, GLP-1 and glucagon, are derived from alternative RNA posttranslational processing of prohormone by convertase PC1/3 in L-cells, and convertase PC2 in α-cells, respectively.5 The active form of GLP-1 (7–36 amide) is degraded into an inactive form (9–36 amide) by Dipeptidyl peptidase-4 (DPP4).6,7 The active form of GLP-1 is essential in the control of blood glucose.8,9
Sennoside A (SA), a major active constituent of Rhei Rhizoma, has been widely used in the treatment of constipation. SA activity was reported in the reduction of energy absorption through an acceleration of intestine transmits.10,11 It is reported that SA can restore GLP-1 level in the diet-induced obese (DIO) mice by an improvement of the gut microbiota profile, the short-chain fatty acids (SCFAs) levels, the mucosal structure and mitochondrial function in the colon of obese mice.12 Also, SA improved hepatic steatosis with a beneficial effect on mitochondrial structure and function.13 These studies demonstrate that SA may control obesity by means of the restoration of GLP-1 level in the obese mice. However, the mechanism of SA activity remains to be characterized in the regulation of GLP-1. The pharmacokinetic study showed that SA has a very low ability to enter the bloodstream.14 Therefore, it seems unlikely that SA works through the blood circulation.
In this work, SA activity was investigated in normal mice through examining body weight, oral glucose tolerance test (OGTT), plasma GLP-1 level and insulin level. Several signal molecules were studied, which included the phosphorylation status of extracellular signal-regulated kinases 1/2(ERK1/2) and the protein expression of PC1/3. In the NCI-H716 cells, ERK1/2 inhibitor (PD98059) was employed to test the role of ERK1/2 pathway for clarifying the SA-induced GLP-1 secretion mechanism.
Materials and Methods
Chemicals and Reagents
Sennoside A (98% in purity) was purchased from Desite biotechnology CO. Ltd. (Chengdu, China). The antibodies to GCG (ab200474, Abcam), PC1/3 (ab220363, Abcam), pERK1/2 (Thr202/Tyr204) (4370T, Cell Signaling Technology), ERK1/2 (4370S, Cell Signaling Technology) were obtained from the China Divisions of the corresponding companies. The ERK1/2 inhibitor PD98059 was got from MCE (HY-12,028, shanghai, China). Other chemicals were purchased from Sigma-Aldrich Co. Ltd. (Shanghai, China) unless stated otherwise.
Animals
Male C57BL/6 mice (6-week-old, SPF grade) were purchased from the Shanghai Xipuer-Bikai Laboratory Animal Co. Ltd. (Shanghai, China). The mice were kept in the animal facility of Shanghai Jiao tong University with controlled temperature (22 ± 2 ºC), humidity (60 ± 5%) and a 12-h dark/light cycle. They were fed on a standard chow diet (13.5% Kcal from fat, Shanghai Slac Laboratory Animal Co. Ltd) in the study. The mice were randomly divided into four groups at n = 12. Group one was employed for a control group without drug treatment. The other three groups were treated with Sennoside A (SA) by three dosages: a low dose (15 mg/kg/day), medium dose (30mg/kg/day) or high dose (45 mg/kg/day). SA was delivered to the mice through drinking water. The treatment was implemented for five weeks. At the end of treatment, the mice were subjected to tissue collection under the anesthesia following an intraperitoneal injection of sodium pentobarbital (35 mg/kg). The procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Shanghai Jiao tong University in accordance with the Guidelines for the Care and Use of Laboratory Animals (DWSY2014-068/DWLL2019-0275).
Oral Glucose Tolerance Test (OGTT), Plasma GLP-1 and Insulin Assay
OGTT was executed with overnight (12 h) fasting at a week before the mice was sacrificed. Glucose was administrated for the mice at 2 g/kg body weight by oral gavage in the OGTT assay. Plasma GLP-1 was determined in the mice through the ocular vein blood collected with heparin tubes containing dipeptidyl peptidase IV inhibitor (10 μL/mL), which was executed at 15 mins after oral administration of glucose. The plasma was obtained by centrifugation of the blood at 3000 g and stored at −80 ºC before the GLP-1 assay. Plasma GLP-1 and insulin were determined individually using the corresponding ELISA kits according to the manufacturer’s instructions. The biologically active form of GLP-1 (7–36) and insulin were measured with the GLP-1 ELISA Kit (BMS2194, Invitrogen, CA, USA) and insulin ELISA Kit (90,080, Crystal Chem, Downers Grove, USA).
Immunofluorescence Stainings
In the tissue collection, the proximal colon was excised, washed with ice-cold saline, and stored at −80 ºC. Pancreas and colon tissues were collected and fixed in 4% paraformaldehyde for analysis of β-cells and L-cells with immunofluorescence assays. The antibody-based staining was performed to determine the numbers of L cells in the colonic tissue and β-cells in the pancreas. Briefly, the tissue slides were deparaffinized, rehydrated, and experienced antigen retrieval with EDTA (pH 9.0). The slides were preincubated with 0.5% bovine serum albumin (BSA) to block the nonspecific interaction between antibody and samples. The GLP-1 antibody (diluted 1:800, Servicebio, Shanghai, China) and the insulin antibody (diluted 1:200, Servicebio, Shanghai, China) were applied to the slide for overnight at 4 ºC, respectively. The slides were incubated with a secondary antibody (diluted 1:400, Servicebio, Shanghai, China) at room temperature for 50 mins after washing out the first antibody. The nuclei were stained with DAPI for 10 mins at room temperature to evaluate the cell density. The fluorescent images were captured under a fluorescent microscope (Olympus, Tokyo, Japan).
Cell Culture
Human NCI-H716 cells were obtained from the Cell Bank of the China Science Academy (Shanghai, China). The cells grew in suspension at 37.8 ºC, 5% CO2. The culture medium was RPMI 1640 (11,875–093, Gibco, USA) supplemented with 10% FBS, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin. Cell adhesion and differentiation were induced by seeding the cells in dishes coated with Matrigel in high-glucose DMEM, 10% FBS, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin as shown in a previous study.15
Cell Viability Assay by CCK8
NCI-H716 cells (5.0×104) were seeded in a 96-well culture plate to induce cell differentiation for two days before the GLP-1 experiments. When the cells grew to 80% confluence, the culture medium was replaced with a serum-free DMEM supplemented with 0.2% BSA, and the cells were treated with SA at different concentrations for 24 h in the medium. Cell viability was measured using the cell counting kit - CCK8 assay Kit (Dojindo, Japan) (n = 6/group). The percentage of living cells was calculated, which were described in this research.16
GLP-1 Secretion in NCI-H716 Cells
The NCI-H716 cells (5.0×105/well) were seeded in a 24-well culture plate to induce cell differentiation. On the day of the experiment, medium was replaced with the Krebs–Ringer bicarbonate buffer (KRB,128 mmol/L NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 5 mM NaHCO3, and 10 mM HEPES, pH 7.4) containing 0.2% BSA and different concentrations of SA (0 μM, 1 μM, 10 μM or 100 μM) in the presence of 1 mM diprotin A. Following incubation at 37.8 ºC for 2 h, the supernatants were collected, treated with 50 mg/mL phenylmethylsulphonyl fluoride, and stored at −80 ºC for analysis of GLP-1 with the ELISA kit. In the study of the ERK pathway, NCI-H716 cells were pretreated with ERK1/2 inhibitors (PD98059, 50 μM) for 30 min in the presence of SA (100 μM). The cells were harvested by scraping, and the protein content of the cells was determined by the enhanced BCA protein assay kit. The GLP-1 content was normalized for the total protein of the cells.
mRNA of GLP-1
Total RNA was extracted from the colon tissues using TRIzol reagent (Invitrogen Life Technologies, CA, USA), and cDNAs were generated using a first-strand cDNA synthesis kit (Takara, Tokyo, Japan). The qRT-PCR was performed, which was described in detail.17 The primers of GLP-1 were listed below: GLP-1 forward, 5′-GAGGACCCTGATGAGATGAATG-3′; and reverse, 5′-GGAGTCCAGGTATTTGCTGTAG-3′.
Statistical Analysis
The results are expressed as the mean ± SEM to indicate the magnitude of the potential error when using existing samples in this study to infer the population. The statistical analyses were performed using one-way ANOVA. Tukey’s test for post-hoc analysis is performed after one-way ANOVA when there are significant differences between groups. All statistical analyses were performed using GraphPad Prism 8.0 (La Jolla, CA, USA) with a statistical significance set at P < 0.05.
Results
Sennoside A Improved OGTT in Mice
C57BL/6 mice were fed on Chow diet and treated with SA for 5 week at 3 dosages (15, 30, and 45 mg/kg/day) through the drinking water. The body weight markedly decreased in the group of 45 mg/kg/day compared with the untreated mice in the control group (Figure 1A). OGTT was conducted in the mice to test the influence of SA on GLP-1. Oral intake of glucose induces the GLP-1 secretion in the intestine. In OGTT, the blood glucose did not exhibit a important change at the basal condition in all groups of mice. The blood glucose significantly reduced in the mice of 45 mg/kg/day group after 15 mins of oral glucose administration. The glucose level was lower in the group at 30 mins and 60 mins than the control group (Figure 1B). No obvious change was observed for SA at the low or medium dosages (Figure 1B).
Sennoside A Induced Plasma GLP-1
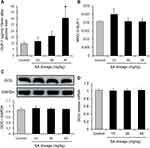
The plasma GLP-1 was examined in the mice to investigate the mechanism of improved OGTT by SA. The improvement of OGTT means that SA may promote the GLP-1 secretion in the lean mice. Blood GLP-1 was determined in the plasma at 15 mins of oral glucose challenge, which was often used to measure the GLP-1 secretion. GLP-1 showed a trend of up-regulation in the mice treated with SA, and the increase was in a dose-dependent manner (Figure 2A). An increase was displayed in the SA-treated group at dosage of 45 mg/kg/day (Figure 2A). The number of L-cells was examined in the large intestine for the mechanism of GLP-1 induction by SA with immunofluorescence staining against GLP-1. No apparent change was observed in the SA-treated groups as indicated by the mean value of optical density (MOD) of fluorescence (Figure 2B). The GCG protein was studied in the homogenization of large intestine, and no alteration was observed in the SA-treated group (Figure 2C). There was no alteration in GLP-1 mRNA in the large intestine of SA-treated mice (Figure 2D).
Sennoside A Induced Plasma Insulin
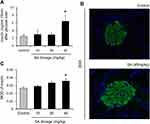
Plasma insulin was investigated in the mice to evaluate the GLP-1 function. An evidently increase was found in the plasma insulin in the group of 45 mg/kg/day (Figure 3A). To further analyze the number of islet β cells, the insulin immunofluorescence staining in mouse pancreatic tissue was performed. The number of β-cells increased in the pancreatic islet in the group of 45 mg/kg/day (Figure 3B and C). The increase in the biological activity of GLP-1 was supported by the assessment of plasma insulin and β-cell numbers.
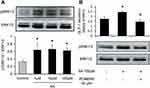
Sennoside A Induced Activation of ERK1/2 Pathway and Expression of PC1/3 in Colon Tissue
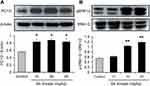
GLP-1 produced from PC1/3-mediated cleavage of proglucagon protein. The activity of PC1/3 was explored with its protein abundance. An obvious increase in PC1/3 protein was found in the colon of the SA-treated group (Figure 4A). In the research on the signaling pathway of SA in the induction of GLP-1 secretion, several signaling molecules were studied. An increase in ERK1/2 activity was observed with the change in phosphorylation status in the SA-treated mice (Figure 4B). The results indicate that SA may increase ERK1/2 activity in the intestinal L-cells to induce GLP-1 secretion.
Sennoside A Induced GLP-1 Secretion in NCI-H716 Cells
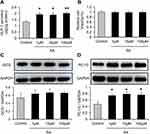
To ascertain the active role of SA in the inducing GLP-1, the cellular model of GLP-1 secretion was used. In the NCI-H716 cells, GLP-1 secretion was induced by SA in the culture supernatant, and a significant increase was found at the dosage of 1 μM and above of SA (Figure 5A). SA did not display any toxicity in the cell model for no reduction in the cell viability (Figure 5B), which was examined with the CCK8 assay in the 24 h SA-treatment. The protein level of GCG did not increased in the SA-treated cells (Figure 5C), which demonstrated that the SA did not induce GLP-1 expression in the cells. The PC1/3 expression was induced by SA in the cells (Figure 5D), which was in consistent with the intravital experiments.
Sennoside A-Induced GLP-1 Secretion Is Blocked by ERK1/2 Inhibitor (PD98059)
The above data suggested the protein level of GCG and L cell number did not increased in the SA-treated cells. To confirm the mechanism of SA-induced GLP-1 secretion, the protein expression of ERK1/2 by Western blot analysis was investigated in NCI-H716 cells. Similar to the findings in the vivo experiments, the protein expression of ERK1/2 phosphorylation significantly increased in the 2 h SA-treatment (Figure 6A). The in vivo and in vitro experiments showed that the ERK1/2 signaling pathway played an important role in SA-regulated GLP-1 secretion. To test the possibility, the NCI-H716 cells were pretreated for 30 min with media containing ERK1/2 inhibitor (PD98059, 50μM) in the presence of SA 100 μM. It was shown that the sole PD98059 did not affect the GLP-1 secretion compared with control (Figure 6B). However, the elevation of GLP-1 levels induced by SA was abolished by preincubation with PD98059 (Figure 6B). The data suggested that SA induced GLP-1 secretion from L-cells in an ERK1/2-dependent manner.
Discussion
This study demonstrates that SA may induce GLP-1 secretion through a direct interaction with L-cells. SA is a single compound derived from Chinese herbal medicine such as Rhei Rhizoma. SA is believed to reduce adiposity through inhibition of nutrient absorption in the intestine through the stimulation of intestine transition, which decreases the time of food digestion and nutrient absorption in the intestine. The influence of SA in GLP-1 was observed in obese mice in our earlier studies.12 Nevertheless, the SA activity remains to be characterized in the regulation of GLP-1 secretion, especially a direct effect of SA on L-cells. In the current study, SA was found to directly stimulate L-cells in the intestine to induce GLP-1 secretion with an improvement of OGTT in the nonobese mice. SA do not change the L-cell density in the large intestine, which supports that other mechanisms may be implicated in the increase of plasma GLP-1 levels by SA. Since the SA is not absorbed into the blood, it is unlikely that SA acts on L-cells through the blood circulation. SA increased the production of short-chain fatty acids (SCFAs) in the obese mice by the impact on gut microbiota.12 It is reported that the SCFAs can induce GLP-1 secretion.18 However, the cell culture data of the current study demonstrated that SA might act on L-cells directly without the involvement of SCFAs, which suggested that the increased level of plasma GLP-1 in the SA-treated mice was attributed to an enhanced secretion of L-cells upon SA stimulation. The influence of SA was observed at 45 mg/kg/day. The elevated GLP-1 was found with the enhanced β-cell function in insulin secretion and an increased number of β-cells in the pancreatic islet, which supported the increase in the biological activity of GLP-1.
The present data indicate that the SA may act through induction of protein abundance of PC1/3 in the L-cells. PC1/3 promotes the GLP-1 secretion by the processing of the proglucagon peptide. The SA effect on the PC1/3 was observed in the intestine tissue of SA-treated mice. The influence was confirmed in NCI-H716 cells, which showed that the direct interaction between SA and L-cells led to the PC1/3 elevation. The up-regulation of PC1/3 protein abundance by SA provides a molecular mechanism for the SA activity in the induction of GLP-1 secretion.
This work also suggests that the ERK1/2 pathway may mediate the SA activity in the induction of GLP-1 secretion. The ERK1/2 pathway involves in the cell growth, development, proliferation, and differentiation.19 ERK1/2 participates in the various physiological and pathological processes through the regulation of a variety of downstream molecules.20,21 Insulin was reported to induce GLP-1 secretion by the activation of ERK1/2 in NCI-H716 cells. An impairment of ERK1/2 activity made the cells secrete less GLP-1 upon insulin stimulation.22 SCFA also stimulated GLP-1 secretion via the GPR43, and GPR43 was also linked to activation of ERK1/2.18,23 In this study, the ERK1/2 activity was induced by SA, and the induction of GLP-1 secretion by SA was blocked by inhibition of ERK1/2. It is not clear that whether ERK1/2 is involved in the regulation of PC1/3.
It was reported that the cAMP-activating agents forskolin and IBMX promoted the GLP-1 secretion, suggesting that the cAMP/PKA signaling pathway may participate in the regulation of GLP-1 release.24 Additionally, an increase in the cytosolic Ca2+ concentration was another mechanism for the GLP-1 secretion.25 The cytosolic Ca2+ elevation was a result of an increase in the intracellular ATP levels, which leaded to the closure of KATP channels. Cell depolarization occurred after the closure of KATP channels, which caused the calcium influx.26,27 The Ca2+ elevation drives GLP-1 secretion by induction of exocytosis. However, it remains to be studied whether the cAMP signal and intracellular calcium ion flow are involved in the SA regulation of GLP-1.
In summary, the present study demonstrated that the SA directly acted on L-cells to induce GLP-1 secretion. The SA activity was observed with an elevation of GLP-1 in the plasma of the lean mice and the culture supernatant of the L-cell line. The SA improved protein expression of PC1/3 and induced activation of ERK1/2. The SA activity in the induction of GLP-1 secretion was blocked by the ERK1/2 inhibitor. The data indicated that SA may induce GLP-1 secretion in L-cells through activation of the ERK1/2 pathway. Nevertheless, the mechanism of ERK1/2 activation remains to be explored, and its effects on PC1/3 are not clear. The SA is a new bioactive component of Chinese herbal medicines that induces GLP-1 secretion through activation of the ERK1/2 pathway and PC1/3.18–20
Conclusion
The present study provides a direct evidence that SA interacts with L cells for GLP-1 secretion by activation of ERK1/2 and PC1/3. The effects of SA on the GLP-1 secretion is dependent on the ERK1/2 signaling pathway. Therefore, SA is a new drug candidate for the treatment of type 2 diabetes and inhibition of obesity by the induction of GLP-1 secretion.
Acknowledgments
The project was supported by the National Natural Science Foundation of China (NO. 81874377).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi:10.1016/j.cmet.2006.01.004
2. Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab. 2001;86(8):3853–3860. doi:10.1210/jcem.86.8.7743
3. Otten J, Ryberg M, Mellberg C, et al. Postprandial levels of GLP-1, GIP and glucagon after 2 years of weight loss with a paleolithic diet: a randomized controlled trial in healthy obese women. Eur J Endocrinol. 2019;180(6):417–427. doi:10.1530/EJE-19-0082
4. Vilsbøll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50(3):609–613. doi:10.2337/diabetes.50.3.609
5. Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95(2):513–548. doi:10.1152/physrev.00013.2014
6. D’Alessio DA. What if gut hormones aren’t really hormones: DPP-4 inhibition and local action of GLP-1 in the gastrointestinal tract. Endocrinology. 2011;152(8):2925–2926. doi:10.1210/en.2011-1385
7. Hjøllund KR, Deacon CF, Holst JJ. Dipeptidyl peptidase-4 inhibition increases portal concentrations of intact glucagon-like peptide-1 (GLP-1) to a greater extent than peripheral concentrations in anaesthetised pigs. Diabetologia. 2011;54(8):2206–2208. doi:10.1007/s00125-011-2168-7
8. Salehi M, Purnell JQ. The role of glucagon-like peptide-1 in energy homeostasis. Metab Syndr Relat D. 2019;17(4):183–191. doi:10.1089/met.2018.0088
9. Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(suppl 0.1):5–21. doi:10.1111/dom.13129
10. Rumsey R, Squires PE, Read NW. In vitro effects of sennoside on contractile activity and fluid flow in the perfused large intestine of the rat. Pharmacology. 1993;47(1):32–39. doi:10.1159/000139840
11. Kobayashi M, Yamaguchi T, Odaka T, et al. Regionally differential effects of sennoside A on spontaneous contractions of colon in mice. Basic Clin Pharmacol. 2007;101(2):121–126. doi:10.1111/j.1742-7843.2007.00088.x
12. Le J, Zhang X, Jia W, et al. Regulation of microbiota-GLP1 axis by sennoside A in diet-induced obese mice. Acta Pharm Sin B. 2019;9(4):758–768. doi:10.1016/j.apsb.2019.01.014
13. Le J, Jia W, Sun Y. Sennoside A protects mitochondrial structure and function to improve high-fat diet-induced hepatic steatosis by targeting VDAC1. Biochem Bioph Res Co. 2018;500(2):484–489. doi:10.1016/j.bbrc.2018.04.108
14. Li P, Lu Q, Jiang W, et al. Pharmacokinetics and pharmacodynamics of rhubarb anthraquinones extract in normal and disease rats. Biomed Pharmacother. 2017;91:425–435. doi:10.1016/j.biopha.2017.04.109
15. Reimer RA, Darimont C, Gremlich S, et al. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology. 2001;142(10):4522–4528. doi:10.1210/endo.142.10.8415
16. Hou W, Yin J, Alimujiang M, et al. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J Cell Mol Med. 2017;22(2):1316–1328. doi:10.1111/jcmm.13432
17. Fan H, Chen W, Zhu J, et al. Toosendanin alleviates dextran sulfate sodium-induced colitis by inhibiting M1 macrophage polarization and regulating NLRP3 inflammasome and Nrf2/HO-1 signaling. Int Immunopharmacol. 2019;76:105909. doi:10.1016/j.intimp.2019.105909
18. Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi:10.2337/db11-1019
19. Kong T, Liu M, Ji B, et al. Role of the extracellular signal-regulated kinase 1/2 signaling pathway in ischemia-reperfusion injury. Front Physiol. 2019;10:1–10. doi:10.3389/fphys.2019.01038
20. Dou M, Wu H, Zhu H, et al. Remifentanil preconditioning protects rat cardiomyocytes against hypoxia-reoxygenation injury via δ-opioid receptor mediated activation of PI3K/Akt and ERK pathways. Eur J Pharmacol. 2016;789:395–401. doi:10.1016/j.ejphar.2016.08.002
21. Kim J, Hong S, Wu P, et al. Raf/MEK/ERK can regulate cellular levels of LC3B and SQSTM1/p62 at expression levels. Exp Cell Res. 2014;327(2):340–352. doi:10.1016/j.yexcr.2014.08.001
22. Lim GE, Huang GJ, Flora N, et al. Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology. 2009;150(2):580–591. doi:10.1210/en.2008-0726
23. Seljeset S, Siehler S. Receptor-specific regulation of ERK1/2 activation by members of the “free fatty acid receptor” family. J Recept Sig Transd. 2012;32(4):196–201. doi:10.3109/10799893.2012.692118
24. Tian L, Jin T. The incretin hormone GLP-1 and mechanisms underlying its secretion. J Diabetes. 2016;8(6):753–765. doi:10.1111/1753-0407.12439
25. Kuhre RE, Holst JJ, Kappe C. The regulation of function, growth and survival of GLP-1-producing L-cells. Clin Sci. 2016;130(2):79–91. doi:10.1042/CS20150154
26. Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590(12):2917–2936. doi:10.1113/jphysiol.2011.223800
27. Reimann F, Habib AM, Tolhurst G, et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8(6):532–539. doi:10.1016/j.cmet.2008.11.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.