Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Self-microemulsifying drug-delivery system for improved oral bioavailability of 20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol: preparation and evaluation
Authors Cai S, Shi C, Zhang X, Tang X, Suo H, Yang L, Zhao Y
Received 31 October 2013
Accepted for publication 12 December 2013
Published 12 February 2014 Volume 2014:9(1) Pages 913—920
DOI https://doi.org/10.2147/IJN.S56894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Shuang Cai,1,* Cai-Hong Shi,2,* Xiangrong Zhang,2 Xiaojiao Tang,2 Hao Suo,2 Li Yang,2 Yuqing Zhao2
1Department of Pharmacy, The First Affiliated Hospital of China Medical University, 2Shenyang Pharmaceutical University, Shenyang, People's Republic of China
*These authors contributed equally to this work
Abstract: The objective of this study was to develop a self-microemulsifying drug delivery system (SMEDDS) to enhance the oral bioavailability of the poorly water-soluble compound 20(S)-25-methoxydammarane-3β;12β;20-triol (25-OCH3-PPD). Optimized SMEDDS formulations for 25-OCH3-PPD contained Cremophor® EL (50%) as the surfactant, glycerin (20%) as the cosurfactant, and Labrafil® M1944 (30%) as the oil. The SMEDDS were characterized by morphological observation and mean droplet size. The pharmacokinetics and bioavailability of the 25-OCH3-PPD suspension and SMEDDS were evaluated and compared in rats. The plasma concentrations of 25-OCH3-PPD and its main metabolite, 25-OH-PPD, were determined by ultra performance liquid chromatography-tandem mass spectrometry. The relative bioavailability of SMEDDS was dramatically enhanced by an average of 9.8-fold compared with the suspension. Improved solubility and lymphatic transport may contribute to this enhanced bioavailability. Our studies highlight the promise of SMEDDS for the delivery of 25-OCH3-PPD via the oral route.
Keywords: 25-methoxydammarane-3β;12β;20-triol (25-OCH3-PPD), 25-OH-PPD, self-microemulsifying drug delivery system, bioavailability, pharmacokinetics
Introduction
20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol (25-OCH3-PPD), a novel dammarane-type triterpene sapogenin (Figure 1), was isolated from total hydrolyzed saponins extracted from the leaves of Panax notoginseng using conventional and reverse-phase silica gel chromatography by Zhao et al.1 In a wide variety of cancer cells, it demonstrated the strongest cytotoxic effects among any of the known ginsenosides tested for tumor cells, particularly for lung cancer cells and prostatic cancer cells.2,3 25-OCH3-PPD decreased survival, inhibited proliferation, induced apoptosis, and led to G1 cell cycle arrest in both cell lines.2 It also decreased levels of proteins associated with cell proliferation (MDM2, E2F1, cyclin D1, and cyclin-dependent kinases 2 and 4) and increased or activated proapoptotic proteins (cleaved poly ADP ribose polymerase, caspase-3, caspase-8, and caspase-9).2 However, 25-OCH3-PPD had extremely low aqueous solubility, indicating low oral bioavailability. Low aqueous solubility is a common characteristic in biopharmaceuticals today, and according to some estimates, over 40% of new chemical entities show poor solubility, which is an obstacle to the use of new compounds.4–6
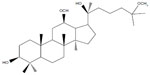  | Figure 1 Chemical structure of 20(S)-25-OCH3-PPD. |
A self-microemulsifying drug delivery system (SMEDDS) is an isotropic mixture of oil, emulsifiers, coemulsifiers, and drug substance, which forms an oil in water (o/w) emulsion with a nanometric droplet size less than 100 nm when exposed to an aqueous medium or gastrointestinal fluids.7–9 The small droplet size of a microemulsion can provide large interfacial surface areas, which is advantageous for drug release and absorption. A commercially available example of a SMEDDS preparation is Neoral® (Novartis, Basel, Switzerland) (cyclosporine A). Much more attention is now focused on SMEDDS due to its excellent efficiency in improving the solubility and oral absorption of poorly water-soluble drugs.10–14 Thus, SMEDDS could be a valuable approach in solving the delivery problems associated with 25-OCH3-PPD. The selection of a suitable self-emulsifying formulation requires assessment of the solubility of the compound in various components, the area of the self-emulsifying region as obtained in the pseudoternary phase diagrams, and the droplet size distribution of the subsequent self-emulsification.15
25-OH-PPD is the main active phase 1 metabolite of 25-OCH3-PPD,16,17 and was found to induce cell cycle arrest in G1 phase in H838 and H358 lung cancer cell lines in a dose-dependent manner.18 In that experiment, 25-OH-PPD was studied simultaneously with 25-OCH3-PPD.
The aims of the present study were to develop and characterize the optimal formulation of SMEDDS containing 25-OCH3-PPD, and to evaluate the enhancement of oral bioavailability by SMEDDS following oral administration to rats. An efficient self-microemulsifying vehicle for 25-OCH3-PPD was selected and optimized using solubility tests and construction of phase diagrams. The formulations were characterized according to mean particle size, emulsification time, color of the emulsion, and formulation stability studies.
Materials and methods
Materials
25-OCH3-PPD (99.0% pure) and 25-OH-PPD (99.0% pure) were prepared in our laboratory (Liaoning, People’s Republic of China), and the structures were determined by infrared, two-dimensional nuclear magnetic resonance, and Fourier transform mass spectrometry. Mifepristone (used as the internal standard, 98.8% purity) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Dalian Meilun Biology Technology Co., Ltd, Dalin, People’s Republic of China). Water, acetonitrile, and methanol (high-performance liquid chromatography grade) were purchased from Kangkede Technology Co, Ltd (Tianjin, People’s Republic of China). Medium-chain triglycerides (Labrafac® Lipophile WL1349), oleoyl macrogolglycerides (Labrafil® M1944), glyceryl monooleate (Peceol®) were gifted by Gattefosse (Saint-Priest Cedex, France). Macrogolglycerol ricinoleate (Cremophor® EL) was a gift from BASF (Ludwigshafen, Germany). Ethylis oleas was purchased from Qianwei Oil Science and Technology Co, Ltd (Shanghai, People’s Republic of China), Tween-80 from Shenyu Pharmaceutical and Chemical Co, Ltd (Shanghai, People’s Republic of China), PEG-400 from Tiantai Fine Chemical Co, Ltd (Tianjin, People’s Republic of China), and glycerin from Bodi Chemical Co, Ltd (Tianjin, People’s Republic of China). All other chemicals used were of analytical grade.
Solubility studies
The solubility of 25-OCH3-PPD was determined in various oils, surfactants, and cosurfactants. An excess amount of 25-OCH3-PPD was added into glass vials containing 2 mL of vehicle (oil, surfactant, cosurfactant), and the mixtures were placed in an air bath oscillator (THZ-82B, Medical Instrument Factory, Jiangsu, People’s Republic of China) for 48 hours at 37°C. After reaching equilibrium, the mixtures were centrifuged at 10,000 rpm for 5 minutes (TGL-16C, Fulgor Analysis Instrument Co, Ltd, Shanghai, People’s Republic of China), and the supernatants were filtered using a 0.45 μm membrane filter. The concentration of 25-OCH3-PPD in the filtrate was determined using a high-performance liquid chromatographic system (PUMP K-501, Unico Instruments Co, Ltd, Shanghai, People’s Republic of China) containing an evaporative light-scattering detector (Unimicro Technologies Inc, Shanghai, People’s Republic of China). The solvents of methanol-H2O (90:10, volume/volume [v/v]) were used as mobile phase for elution at a flow rate of 1.0 mL per minute. The accuracy and precision of the intraday and interday assay were within 10% of the relative error and coefficient of variation.
Screening of surfactants for emulsifying ability
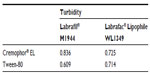
The various surfactants was screened for their emulsification ability.19 Briefly, 500 mg of surfactant was added to 500 mg of the selected oil phase. The mixture was gently homogenized at 37°C for 3 hours. Next, 200 mg of the isotropic mixture was accurately weighed and diluted with double distilled water to 40 mL to yield a fine emulsion. The resulting emulsions were observed visually for their relative turbidity. The emulsions were allowed to stand for 2 hours and their transmittance was assessed at 600 nm using a UV-2000 ultraviolet-visible spectrophotometer (Unico Instrument Co, Ltd, Shanghai, People’s Republic of China), using double distilled water as the blank.
Construction of pseudoternary phase diagram
Ternary diagrams of the surfactant, cosurfactant, and oil were plotted, each representing an apex of the triangle.15,20 A series of self-emulsifying systems was prepared in each of the two formulation systems with varying concentrations of oils (Labrafil M1944 and Labrafac Lipophile WL1349), surfactants (Tween-80 and Cremophor EL), and cosurfactants (glycerin). The concentration of surfactant was varied from 30% to 80% (weight/weight [w/w]), the oil concentration was varied from 10% to 70%, and the cosurfactant concentration was varied from 0% to 30% (w/w). For every mixture, the total surfactant, cosurfactant, and oil concentrations always added up to 100%. Eighty-four such mixtures with varying surfactant, cosurfactant, and oil concentrations were prepared for this investigation. The efficiency of nanoemulsion formation was assessed by adding 200 mg of each mixture to 40 mL of double distilled water, followed by gentle agitation using a magnetic stirrer. To assess the region for aqueous dispersion, the optical clarity of the aqueous dispersion of 25-OCH3-PPD-loaded SMEDDS was evaluated spectrophotometrically.
Characterization of 25-OCH3-PPD-loaded SMEDDS
Dispersibility test
The formulations were diluted 200 times with double distilled water at 37°C, with constant magnetic stirring at 50 rpm. The SMEDDS were observed for formation of stable nanoemulsions. The nanoemulsions formed were visually observed for phase clarity, self-emulsification time, and rate of emulsification.
Thermodynamic stability studies
The SMEDDS sample was subjected to stability studies at 4°C and 25°C, and a heating–cooling test using six refrigerator cycles at temperatures of 40°C and −4°C for 48 hours.
Droplet size
A SMEDDS sample was diluted with distilled water at 25°C under gentle agitation immediately before measurement. The droplet size distribution was determined by dynamic light scattering (Zetasizer, Nano ZS-90, Malvern Instruments, Malvern, UK). The detection range of the mean particle diameter was from 2 to 5,000 nm. The mean particle diameter analysis data were evaluated using the volume-weighting pattern.
Morphology
The SMEDDS morphology was observed by electron microscopy using negative staining with 1% weight/volume (w/v) neutral phosphotungstic acid. The prepared SMEDDS sample was diluted with deionized water (1:200) and mixed by gentle shaking. A drop of the sample obtained after dilution was placed on the surface of a copper grid, and then stained with 1% w/v phosphotungstic acid and dried in air. The microscopic appearance of the SMEDDS was examined under transmission electron microscopy (JEM-1200EX, JEOL Ltd, Tokyo, Japan).
In vivo absorption studies
Adult female and male Wistar rats (body weight 180–220 g) were purchased from the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, People’s Republic of China) for use in the pharmacokinetic study of 25-OCH3-PPD-loaded SMEDDS. All experimental procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Shenyang Pharmaceutical University in Shenyang. For the pharmacokinetic studies, 12 rats were randomly assigned to two groups. Each group contained six rats. Before the day of administration, the rats were fasted for 12 hours but allowed water ad libitum. The two treatment groups were separately treated with oral 25-OCH3-PPD suspension (control group) or 25-OCH3-PPD-loaded SMEDDS (treatment group) at a dose of 5 mg/kg, using a 5 mL oral feeding needle attached to a cannula. The comparator, ie, 25-OCH3-PPD suspension, was formulated by dissolving 25-OCH3-PPD with PEG 400-physiologic saline (30:70, v/v) and mixing well. The gavage volume was 10 mL/kg for each rat. Blood samples (150 μL) were collected into heparinized tubes from each rat by puncture of the retro-orbital sinus. This was performed at zero (predose), 0.25, 0.5, 1, 1.5, 2.5, 4, 6, 9, 12, and 24 hours after oral administration. The blood was immediately processed for plasma by centrifugation at 3,500 g for 10 minutes. Plasma samples were frozen and maintained at –20°C prior to analysis by liquid chromatography-mass spectrometry (LC-MS/MS).
Bioanalysis of 25-OCH3-PPD and 25-OH-PPD in rat plasma with LC-MS/MS
For analysis of 25-OCH3-PPD and 25-OH-PPD in rat plasma, 10 μL of the internal standard (500 μg/mL) were added to 50 μL of plasma. After addition of 100 μL of methanol for precipitation of protein, the sample was vortex-mixed for 30 seconds, followed by centrifugation at 15,000 g for 5 minutes at 4°C. The supernatant was transferred to a new tube, and a 10 μL aliquot of the solution was injected onto the ultra performance liquid chromatography-tandem mass spectrometry system for analysis.17
The LC-MS/MS system consisted of an API 4000 triple-quadrupole tandem mass spectrometer (Applied Biosystem/MDS SCIEX, Foster City, CA, USA) with an electrospray source, equipped with a turbo ion spray interface in positive ion mode. Table 1 shows the main parameters of the mass spectrometry conditions used for all analytes.
  | Table 1 Parameters of mass spectrometry conditions for analysis of the analytes |
Analyte concentrations were determined using Analyst 1.5 software (Applied Biosystem/MDS SCIEX). The total analytical run time was 15 minutes. Chromatographic separation was performed on an Acquity ultra performance liquid chromatography system (Waters, Milford, MA, USA) with a C18 column (150 mm × 4.6 mm, 5 μm, Agilent Technologies, Shanghai, People’s Republic of China). The solvents of 5 mM ammonium acetic acid (pH 7.8)-acetonitrile (65:35, v/v) were used as mobile phase for elution. The flow rate was 1.0 mL per minute.
Pharmacokinetic analysis
Pharmacokinetic parameters were calculated using a standard noncompartmental method. Maximum plasma concentration (Cmax) and time taken to reach maximum plasma concentration (Tmax) were determined by inspection of the plasma concentration-time curves. The area under the plasma concentration-time curve from zero to the last point (AUC0–t) was calculated using the linear-trapezoidal method. The AUC from zero to infinity (AUC0–∞) was calculated as follows:
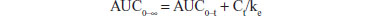
where Ct is the last measurable concentration. DAS 2.1.1 software (Mathematical Pharmacology Professional Committee of China, Shanghai, People’s Republic of China) was used for the pharmacokinetic analysis.
Results and discussion
SMEDDS formulation
Solubility studies were aimed at identifying a suitable oily phase, surfactants, and cosurfactants for development of 25-OCH3-PPD-loaded SMEDDS. Identifying the oil, surfactant, and cosurfactant having maximal solubilizing potential for a compound under investigation is very important to achieve optimum drug loading.9,21,22
The solubility of 25-OCH3-PPD in various oily phases, surfactants, and cosurfactants is presented in Table 2, respectively. The solubility studies clearly indicated that solubility of 25-OCH3-PPD in the tested oils, in decreasing order, was as follows: Labrafil M1944 > Labrafac Lipophile WL1349 > ethyl oleate. Solubility of 25-OCH3-PPD in the tested surfactants, in decreasing order, was as follows: Cremophor EL > Tween-80 > Peceol. Solubility of 25-OCH3-PPD in glycerin (cosurfactant) was markedly higher than in PEG-400. Based on these results, we selected Labrafil M1944 and Labrafac Lipophile WL1349 for the oil phase, Cremophor EL and Tween-80 as the surfactant, and glycerin as the cosurfactant for further investigation.
Selection of the surfactant was governed by emulsification efficiency (Table 3). The emulsification studies clearly indicated that both Tween-80 and Cremophor EL had a good ability to emulsify oil, Labrafil M1944, and Labrafac Lipophile WL1349. Thus, Tween-80 and Cremophor EL were selected as the surfactants for further investigation.
In our pseudoternary phase diagram study (Figure 2), systems consisting of Labrafil M1944 and Labrafac Lipophile WL1349 as oil phase, Cremophor EL and Tween-80 as surfactants, and PEG-400 as cosurfactants were diluted with double distilled water, and self-emulsifying formulations were selected from regions (in blue). In view of the current investigation, due to a larger nanoemulsion region and a greater capacity for incorporation of oily phase, which is most desirable for 25-OCH3-PPD, a Labrafil M1944-Cremphor EL-glycerin system was selected. As expected, compositions with lower absorbance showed the lowest droplet size because aqueous dispersions with small absorbance are optically clear and oil droplets are thought to be in a state of finer dispersion.23 The optimal self-microemulsion formula was Labrafil M1944, Cremophor EL, and glycerin in a ratio of 30:50:20.
Dispersibility test
The dispersibility test showed that the SMEDDS, after diluting with distilled water (1:200), formed a fine bluish-white nanoemulsion in less than one minute.
Thermodynamic stability studies
25-OCH3-PPD-loaded SMEDDS was stable at 4°C and 25°C and in the heating-cooling test at 40°C and −4°C for 48 hours in the thermodynamic stability studies. There was no change in mean particle size and self-emulsification time.
Droplet size
Droplet size distribution is a critical factor when evaluating a self-microemulsion system. Droplet size has an effect on drug absorption, as has been demonstrated in several papers. The smaller the droplet size, the larger the interfacial surface area provided for drug absorption.24,25 The mean particle diameter of the 25-OCH3-PPD-loaded SMEDDS in a solution diluted in distilled water (1:200) was 40.52±17.97 nm, confirming well separated emulsion droplets.
Morphology
The 25-OCH3-PPD-loaded SMEDDS became a microemulsion when diluted with distilled water (1:200, w/w). A transmission electron microscopy image is shown in Figure 3. The nanoemulsion droplets were observed to be spherical.
  | Figure 3 Transmission electron micrograph of 25-OCH3-PPD-loaded self-microemulsifying drug-delivery system. |
In vivo absorption studies
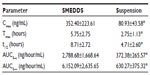
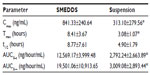
The basic structure of 25-OCH3-PPD is that of a tetracyclic triterpenoid, which showed extremely low aqueous solubility (Figure 1). The plasma concentration-time curves for 25-OCH3-PPD and 25-OH-PPD in rats following oral administration of 25-OCH3-PPD suspension and 25-OCH3-PPD-loaded SMEDDS at a dose of 5 mg/ are shown in Figures 4 and 5. The main pharmacokinetic parameters for 25-OCH3-PPD and 25-OH-PPD are presented in Tables 4 and 5. As can be seen, following oral administration of 25-OCH3-PPD-loaded SMEDDS and 25-OCH3-PPD suspension, the Cmax of 25-OCH3-PPD was 352.40±223.61 ng/mL and 80.93±43.58 ng/mL, respectively (the difference was statistically significant, P<0.05, Table 4). The Cmax of the 25-OH-PPD metabolite was 841.33±240.64 ng/mL and 313.10±279.56 ng/mL, respectively (this difference was also statistically significant at P<0.05, Table 5). The AUC0–∞ of 25-OCH3-PPD and 25-OH-PPD was 9.8 and 6.5 times greater, respectively, when 25-OCH3-PPD was administered as SMEDDS compared with the AUC0–∞ obtained for the suspension. These results show that formulation of 25-OCH3-PPD as SMEDDS resulted in significantly increased absorption of 25-OCH3-PPD compared with absorption from the suspension.
SMEDDS can improve oral bioavailability by enhancing permeation across the intestinal membrane, solubilization, a reduced or eliminated effect of food, droplet size reduction, lymphatic transport, and improvement of drug dissolution.26–28 Moreover, the enhancement of oral absorption of 25-OCH3-PPD-loaded SMEDDS compared with 25-OCH3-PPD suspension in our study was likely to be due to the effects of the surfactants and cosurfactants, including improved mucosal permeability, smaller droplets, and greater surface area.
Double Cmax peaks for 25-OCH3-PPD and 25-OH-PPD were observed after administration of SMEDDS (Figures 4 and 5). This may be because of the high lymphatic bioavailability of 25-OCH3-PPD, and the slow flow rate of lymph.14,29
Conclusion
A SMEDDS formulation was successfully developed for 25-OCH3-PPD, and the optimal formulation contained Cremophor EL (50%) as the surfactant, glycerin (20%) as the cosurfactant, and Labrafil M1944 (30%) as the oil. When diluted with distilled water, 25-OCH3-PPD-loaded SMEDDS could spontaneously form small particles with a mean droplet size of about 40 nm in less than one minute. In pharmacokinetic studies, SMEDDS enhanced the oral absorption of 25-OCH3-PPD and its active metabolite, 25-OH-PPD. Our studies indicate that a SMEDDS is a promising way to deliver 25-OCH3-PPD via the oral route.
Acknowledgments
This research was financially supported by the Major Scientific and Technological Specialized Project for “significant new formulation of new drugs” in the People’s Republic of China (N2009ZX09102-114) and The National Natural Science Foundation of China (81273389).
Disclosure
The authors report no conflicts of interest in this work.
References
Zhao Y, Wang W, Han L, et al. Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyl-dammarane-3, 12, 20-triol [20(S)-25-OCH3-PPD], a novel natural product from Panax notoginseng. Med Chem. 2007;3:51–60. | |
Wang W, Wang H, Rayburn ER, Zhao Y, Hill DL, Zhang R. 20(S)-25-methoxyl-dammarane-3, 12, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. Br J Cancer. 2008;98:792–802. | |
Zhang LH, Jia YL, Lin XX, et al. AD-1, a novel ginsenoside derivative, shows anti-lung cancer activity via activation of p38 MAPK pathway and generation of reactive oxygen species. Biochim Biophys Acta. 2013;1830:4148–4159. | |
Dahan A, Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release. 2008;129:1–10. | |
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. | |
Beig A, Miller JM, Dahan A. Accounting for the solubility-permeability interplay in oral formulation development for poor water solubility drugs: the effect of PEG-400 on carbamazepine absorption. Eur J Pharm Biopharm. 2012;81:386–391. | |
Patel D, Sawant KK. Self micro-emulsifying drug delivery system: formulation development and biopharmaceutical evaluation of lipophilic drugs. Curr Drug Deliv. 2009;6:419–424. | |
Spernath A, Aserin A. Microemulsions as carriers for drugs and nutraceuticals. Adv Colloid Interface Sci. 2006;128–130:47–64. | |
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11 Suppl 2:S93–S98. | |
Baek MK, Lee JH, Cho YH, Kim HH, Lee GW. Self-microemulsifying drug-delivery system for improved oral bioavailability of pranlukast hemihydrate: preparation and evaluation. Int J Nanomedicine. 2013;8:167–176. | |
Chen ZQ, Liu Y, Zhao JH, Wang L, Feng NP. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int J Nanomedicine. 2012;7:1115–1125. | |
Zhang P, Liu Y, Feng NP, Xu J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int J Pharm. 2008;355:269–276. | |
Liu WL, Tian R, Hu WJ, et al. Preparation and evaluation of self-microemulsifying drug delivery system of baicalein. Fitoterapia. 2012;83:1532–1539. | |
Sha XY, Wu J, Chen YZ, Fang XL. Self-microemulsifying drug-delivery system for improved oral bioavailability of probucol: preparation and evaluation. Int J Nanomedicine. 2012;7:705–712. | |
Kommuru TR, Gurley B, Khan MA, Reddy IK. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212:233–246. | |
Zhang X, Xu J, Zhang D, Gu J. Pharmacokinetics of 20(S)-25-methoxyl-dammarane-3, 12, 20-triol and its active metabolite after oral and intravenous administration in rat. Xenobiotica. 2009;39:457–464. | |
Shi CH, Zhang X, Suo H, et al. Simultaneous determination by LC-MS/MS of 25-methoxydammarane-3β, 12β, 20-triol epimers and active metabolites in rat plasma after intravenous administration. Xenobiotica. 2013;43:868–874. | |
Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. | |
Date AA. Lipid-based novel systems for drug delivery. Dissertation. University of Mumbai, India; 2006. | |
Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329:166–172. | |
Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25:47–58. | |
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: Development and optimization. Int J Pharm. 2010;391:203–211. | |
Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol Pharm Bull. 2004;27:1993–1999. | |
Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50:179–188. | |
Kang BK, Lee JS, Chon SK, et al. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm. 2004;274:65–73. | |
Rane SS, Anderson BD. What determines drug solubility in lipid vehicles: is it predictable? Adv Drug Deliv Rev. 2008;60:638–656. | |
Wasan EK, Bartlett K, Gershkovich P, et al. Development and characterization of oral lipid-based amphotericin B formulations with enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans. Int J Pharm. 2009;372:76–84. | |
Wang Z, Sun J, Wang Y, et al. Solid self-emulsifying nitrendipine pellets: preparation and in vitro/in vivo evaluation. Int J Pharm. 2010;383:1–6. | |
Wang L, Wu HF, Lu CH, Fan YN. [The pathway of absorption and conveying of puerarin microemulsion-in-oil]. Yao Xue Xue Bao. 2009;44:798–802. Chinese. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.