Back to Journals » International Journal of Nanomedicine » Volume 14
Self-Assembled Nanofibers Elicit Potent HPV16 E7-Specific Cellular Immunity And Abolish Established TC-1 Graft Tumor
Authors Li S, Zhang Q, Bai H, Huang W, Shu C, Ye C, Sun W, Ma Y
Received 10 May 2019
Accepted for publication 11 September 2019
Published 10 October 2019 Volume 2019:14 Pages 8209—8219
DOI https://doi.org/10.2147/IJN.S214525
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Sijin Li,1–3 Qishu Zhang,1–3 Hongmei Bai,1–3 Weiwei Huang,1–3 Congyan Shu,1–3 Chao Ye,1–3 Wenjia Sun,1–3 Yanbing Ma1–3
1Laboratory of Molecular Immunology, Institute of Medical Biology, Chinese Academy of Medical Science & Peking Union Medical College, Kunming, People’s Republic of China; 2Yunnan Key Laboratory of Vaccine Research & Development on Severe Infectious Disease, Kunming, People’s Republic of China; 3Yunnan Engineering Research Center of Vaccine Research and Development on Severe Infectious Disease, Kunming, People’s Republic of China
Correspondence: Yanbing Ma
Laboratory of Molecular Immunology, Institute of Medical Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, 935 Jiaoling Road, Kunming 650118, People’s Republic of China
Tel +86 871 6833 9287
Fax +86 871 6833 4483
Email [email protected]
Background: Vaccines are one of the most promising strategies for immunotherapy of HPV associated tumors; however, they generally lack significant clinical efficacy at present. This inefficacy might be due to inefficient generation of anti-tumor cellular immune responses.
Purpose: This study aimed to assess the potential of using self-assembled nanofibers as a new vaccine platform to elicit potent HPV antigen - specific anti-tumor immunity.
Methods: A HPV16 E744-62 peptide was chemically appended to the N terminus of self-assembling peptide Q11. The nanofibers were prepared and used to immunize mice through a preventive or therapeutic strategy in a TC-1 graft tumor model.
Results: Preventive immunization with nanofibers almost completely suppressed the growth of primarily grafted TC-1 tumors and even a re-challenge of tumor cells after a six-week rest. Therapeutic immunization significantly increased the levels of effector Th1 cells, CTLs and the cytokines IFN-γ and TNF-α in E7 peptide-stimulated splenocytes, and the immunization reduced Th2, MDSC and IL-4 contents compared to the controls. The nanofiber immunization significantly suppressed the growth of established tumors and achieved 66.7% and 50% tumor-free in mice carrying 2–3 mm tumors and even larger tumors with a diameter of 5–6 mm respectively. In addition, the nanofibers were more efficient than the corresponding unassembled peptides for the treatment of established larger size tumors.
Conclusion: The results indicated that self-assembling nanofibers could elicit robust HPV antigen -specific anti-tumor cellular immunity and are a potent antigen delivery system for HPV related tumor vaccines.
Keywords: self-assembling nanofibers, cellular immunity, tumor, vaccine, human papillomavirus, HPV
Introduction
Human papillomavirus (HPV) is the causative agent of cervical cancer, the second most common malignant tumor threatening women’s health.1,2 Nearly all cervical cancers are verified to be HPV-positive. HPV16 is considered the most important high-risk genotype of HPV, accounting for approximately 50% of cervical cancers.3 In addition, HPV is also related to many malignant tumor diseases, including penis, vulva, vagina, anus, oropharynx, head and neck cancers.4 Tumor vaccines represent a promising immunotherapy strategy for the treatment of cancers through triggering tumor antigen-specific cellular immunity. The expression of the HPV oncoproteins E6 and E7 results in the transformation of infected cells and the proliferation of malignant cells by promoting the cell cycle of infected cells, inhibiting apoptosis and increasing genomic instability. E6 and E7 are persistently expressed and present in infected and malignant cells, and therefore, they are often used as ideal antigen targets for therapeutic vaccines against HPV related tumors.5,6 At present, lots of therapeutic HPV vaccine candidates have been investigated pre-clinically and been shown to be quite effective in animal models.7,8 However, the current vaccines generally lack significant curative efficacy in clinical trials, and one of the possible reasons might due to the failure of generating powerful enough anti-tumor immune responses. The immunogenicity of E6 and E7 protein- or peptide-based subunit vaccines is limited in general, and therefore, exploring a potent antigen delivery carrier might represent a probable approach to booster the clinical efficacy of a tumor vaccine.9
Nanoparticle vaccines have attracted wide attention in recent years, since they have many advantages for being a vaccine platform, such as high efficiency in presenting antigen and encapsulating the immune enhancer, flexibility for functional modification, increasing the stability of the antigen and good biocompatibility.10–13 Nanoparticle vaccines include polymeric nanoparticles, inorganic nanoparticles, lipid-based nanoparticles, virus-like nanoparticles, bacteria-derived outer membrane vesicles, and self-assembling peptide nanoparticles or nanofibers.14 Self-assembling peptides are defined as the ability of some peptides to spontaneously arrange themselves into well-ordered nanostructures, which can be used as scaffolds for tissue engineering or nanomaterials for drug and vaccine delivery.15,16 Q11 is one of the widely used self-assembling peptides. It can easily assemble into unbranched nanofibers in salt solution, and the spectrum of circular dichroism analysis was consistent with a high degree of β-sheet or β-turn structure.17,18 It was indicated that the nanofiber assembly was mediated by β-sheet domain of Q11, and the forces maintaining nanofiber structure were non-covalent interactions including hydrophobic interaction, hydrogen bonding, and electrostatic interaction.19 The potentials and characteristics of Q11 nanofibers for delivering antigenic epitopes have been described in previous studies;20,21 however, the application of self-assembling nanofibers in a vaccine design is still preliminary and needs to be further explored, especially in the development of a tumor vaccine.
In this study, we generated a nanoscale vaccine employing the self-assembling peptide Q11 and assessed the potentials of self-assembled nanofibers as a powerful vaccine platform to elicit potent anti-tumor immunity. Our results indicated that Q11 nanofiber might be one of the alternative candidates of therapeutic HPV vaccines and provide a new vaccine approach for the immunotherapy of HPV associated cancers.
Materials And Methods
Peptides And Nanofibers Preparation
The peptides Q11 (Ac-QQKFQFQFEQQ-Am), E744-62 (QAEPDRAHYNIVTFCCKCD), and E744-62-Q11 (H2N-QAEPDRAHYNIVTFCCKCD-SGSG-QQKFQFQFEQQ-Am) were synthesized by GL Biochem Ltd. (Shanghai, People’s Republic of China). In peptide E744-62-Q11, epitope peptide (E744-62) was appended to the N terminus of self-assembling peptide Q11 through a flexible linker, Ser-Gly-Ser-Gly. Peptides were purified by reverse phase HPLC, lyophilized, and stored at −20 °C before use. nanofibers were prepared as previously reported with small modifications.22–24 Briefly, lyophilized peptides Q11 or E744-62-Q11 were dissolved in sterile water at 2 mmol/L and stored at 4°C overnight. Phosphate-buffered saline (PBS, 50 mmol/L PB plus 0.15 mol/L NaCl, pH 7.4) was used to dilute the peptides to 0.5 mmol/L, and the solution was incubated at room temperature for 4.5 h, allowing the peptides to assemble fully into nanofibers. The solution was diluted 4 times with PBS, and then 10 μL of the sample was dropped on a carbon-coated 400 mesh copper grids (Electron Microscopy Sciences) for 5 mins, negatively stained with 2% uranylacetate for 5 mins, and observed by electron microscopy.
Mice And Cell Lines
Female C57BL/6 mice (6–8 weeks; 16–18 g; SCXK[Beijing]2012-0001) were purchased from Vital River Laboratory Animal Technology Ltd. (Beijing, China). All mice were kept under specific pathogen-free conditions in the Central Animal Care Services of the Institute of Medical Biology, Chinese Academy of Medical Sciences & Peking Union Medical College (SCXK[Dian] 2011–0005). All animal experiments were approved by the Animal Ethics Committee of Institute of Medical Biology ([2017]16) and in accordance with the principles of “Guide for the Care and Use of Laboratory Animals” and “The Guidance to Experimental Animal Welfare and Ethical Treatment”. The TC-1 tumor cells, which were derived from the primary lung epithelial cells from C57BL/6 mice and co-transformed with the HPV-16 oncoproteins E6 and E7 and the c-Ha-ras oncogene, were purchased from the tumor Center of Chinese Academy of Medical Sciences. The cells were cultured in RPMI 1640 supplemented with 10% FBS.
Tumor Challenge And Mouse Immunization
TC-1 cells (1×105) mixed with Basement membrane matrix (BD Biosciences, San Jose, CA, USA) were injected subcutaneously (s.c.) into the right flank of the C57BL/6 mice to establish the HPV-associated grafted tumor model. A preventive immunization strategy was employed as described in Figure 2A. Mice were first immunized s.c. with 12.5 nmol of E744-62-Q11 or Q11 nanofibers or unassembled E744-62-Q11 peptides three times at an interval of 2 weeks (n = 5 mice per group) and then challenged with TC-1 cells 2 weeks after the last immunization. To assess the effective antitumor immune memory induced by nanofibers, the mice were rechallenged with TC-1 6 weeks after the first TC-1 cell inoculation (Figure 2A). In the therapeutic studies, the mice were first challenged with TC-1 cells. When the tumor diameter reached 2–3 mm or 5–6 mm, three immunizations were performed at an interval of 7 days (n =6 per group) (Figure 3A and 4A). The tumor growth was measured every 3–4 days using a micrometre caliper. Tumor volumes were calculated using the following formula: volume (mm3) = 0.5 × (width [mm])2 × length [mm]. Mice were euthanized when the largest tumor diameter reached 20 mm. At the end of each experiment, 4 mice were randomly selected from each group, and splenocytes were isolated for analyses on lymphocyte and cytokine responses.
Enzyme-Linked Immunospot (ELISPOT)
Splenocytes were isolated using lymphocyte separation medium. The antigen-specific IFN-γ-secreting cell was analysed using an ELISPOT kit (Dakewe Biotechnology Co., Ltd., Shenzhen, China), following the manufacturer’s instruction. Briefly, 3×105 cells/well were plated in a 96-well ELISPOT plate precoated with anti-mouse IFN-γ antibodies. The cells were stimulated with 5 μg/mL E744-62 peptide at 37 °C in 5% CO2 for 20 h. In addition, cells stimulated with PMA served as positive controls, and untreated cells were left as negative controls. Plates were imaged, and spots were counted using an ELISPOT reader (AID Diagnostika GmbH, Straβberg, Germany).
Enzyme-Linked Immunosorbent Assay (ELISA)
The culture supernatant of isolated splenocytes stimulated with E744-62 for 3 days was collected, and the levels of IFN-γ, IL-4 and TNF-α were measured by ELISA. The paired capture antibodies and biotinylated detection antibodies were purchased, and the assays were conducted following the manufacturer’s instructions (Affymetrix eBioscience, Inc., San Diego, CA, USA). Briefly, the capture antibodies were coated onto microplates at 4 °C overnight, and 100 μl sample of culture supernatant was loaded and incubated at 37 °C for 1 h, followed by 1 h incubation with biotinylated detection antibodies. Then, HRP-labelled streptavidin (Beyotime, Shanghai, China) was added and incubated for 45 mins at 37 °C. Finally, TMB Single-Component Substrate solution (Solarbio, Beijing, China) was used to develop at 37 °C, and 1 M HCL was added to stop the reaction. OD450 values were detected with an ELISA reader.
Flow Cytometry
Briefly, 1×106 splenocytes for each mouse were plated into 96-well plates and stimulated with 5 μg/mL E744-62 for 6 h, and 5 μg/mL Brefeldin A (Biolegend, San Diego, CA, USA) was added to allow the accumulation of intracellular cytokines. The cells were first stained with FITC-anti-CD4 (Biolegend, USA), FITC-anti-CD8α (Biolegend, USA), or PE-anti-CD11b (Biolegend, USA) and APC-anti-Gr1 (Biolegend, USA). Subsequently, fixation/permeabilization and intracellular staining for APC-anti-IFNγ (Biolegend, USA) or PE-anti-IL4 (Biolegend, USA) was performed. The samples were analysed by BD Accuri C6 (BD Biosciences, Germany), and the data were analysed using Flowjo software 7.6.
In Vivo Cytotoxic Assay
An in vivo assay for cytotoxic T lymphocytes was conducted as described elsewhere.25,26 Briefly, C57BL/6 mice were inoculated with TC-1 cells and then immunized with peptide E744-62, Q11 nanofiber, peptide E744-62 plus Q11 nanofiber, E744-62 -Q11 nanofiber, or PBS when the tumors reached 2–3 mm diameter. The immunization was performed three times at an interval of one week. Seven days after the 3rd immunization, the mice were intravenously challenged with 5×106 CFSE-labelled syngeneic splenocytes, which were prepared by 1:1 mixture of isolated splenocytes previously pulsed with 5 μg/mL E744-62 peptide for 1 h at 37 °C in the dark (labelled with 5 μM CFSE, CFSEhigh) or unpulsed (labelled with 0.5 μM CFSE, CFSElow). Fifteen hours later, lymphocytes were isolated from spleens and tumor-draining lymph nodes (inguinal nodes), and cells with different CFSE fluorescence intensities were analysed by flow cytometry.
Statistical Analysis
Data are presented as the means ± standard errors. Statistical analyses were performed using software GraphPad Prism 5.0. The significance of the differences between groups was analysed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test or unpaired Student’s t-test. The results with p < 0.05 were considered statistically significant.
Results
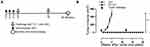
Nanofiber Characterization
The nanofiber structure is important for an antigenic peptide to elicit a strong immune response.23,27 A schematic of nanofiber self-assembly was shown (Figure 1A). The morphology of the prepared nanofibers in this study was visualized by electron microscopy, Peptides Q11 and E744-62-Q11 in PBS appeared as long and unbranched fibrils with widths of about 15 nm, which was identical to what reported by previous studies (Figure 1B).
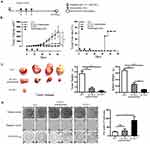
Preventive Immunization With Nanofibers Abrogated The Growth Of Grafted TC-1 Tumors In Mice And Produced Long-Term Immune Protection
In the preventive experiment, mice were immunized first with E744-62-Q11 nanofibers to establish anti-tumor immunity before facing the challenge of TC-1 cells. To determine the immune memory elicited by nanofibers, tumor-free immunized mice were re-challenged with tumor cells 6 weeks after primary TC-1 (Figure 2A). The results showed that there was no tumor growth found in E744-62-Q11-immunized mice, whereas the mice receiving the control Q11 nanofibers developed tumors (Figure 2B). After re-challenge, the E744-62-Q11-immunized mice remained tumor-free for at least 8 weeks (Figure 2B). The results indicated that the immunization with nanofibers could elicit potent anti-tumor immunity and long-lasting immune memory.
Therapeutic Immunization With Nanofibers Significantly Suppressed The Growth Of Established TC-1 Tumors With A Diameter Of 2-3 mm
To further investigate the potentials of using nanofiber as an antigen delivery carrier to elicit anti-tumor immunity and inhibit tumor growth, a therapeutic experiment was conducted. When the tumor size reached 2–3 mm in diameter, E744-62-Q11 nanofibers were used for immunization, and unassembled E744-62-Q11 peptides and Q11 nanofibers were also used for immunization as the controls (Figure 3A). Mice immunized with E744-62-Q11 nanofibers had significantly inhibited tumor growth compared to mice receiving Q11 or unassembled E744-62-Q11 (Figure 3B, left), and the percentage of tumor-free mice was up to 66.7% whereas all the control mice developed tumor (Figure 3B, right). It should be noted that immunization with unassembled E744-62-Q11 peptides also induced significant tumor suppression compared to the Q11 control, although not as significantly as the nanofibers.
At the endpoint of the experiment, the tumor mass and spleen of each mouse were collected and weighed, and the results showed that the mice immunized with E744-62-Q11 nanofibers had smaller tumor sizes and lighter spleen weights than the mice immunized with Q11 or unassembled E7-Q11 (Figure 3C). Furthermore, the splenocytes were isolated and antigen-specific IFN-γ-secreting splenocytes was measured by ELISPOT (Figure 3D). In comparison with the controls, the splenocytes form the mice immunized with E744-62-Q11 nanofibers had higher levels of IFN-γ-secreting lymphocytes responding to stimulation with the E744-62 peptide. The result indicated that E744-62-Q11 nanofibers were capable of promoting a potent anti-tumor cellular immune responses even under the setting of a tumor having been established.
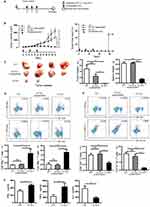
Therapeutic Immunization With Nanofibers Significantly Suppressed The Growth Of Established TC-1 Tumor With A Diameter Of 5-6 mm
Therapeutic immunization with E744-62-Q11 nanofibers demonstrated almost complete suppression of tumor growth in 2–3 mm-tumor-bearing mice. To more clearly display the potentials of E744-62-Q11 nanofibers, a therapeutic experiment employing mice bearing larger tumors was performed. When tumor sizes reached 5–6 mm, the immunization was initiated (Figure 4A). Mice immunized with E744-62-Q11 nanofibers had significantly suppressed tumor growth compared to mice immunized with Q11 and unassembled E744-62-Q11, and there was no significant difference between the Q11 and unassembled E744-62-Q11 groups (Figure 4B, left). Correspondingly, the percentage of tumor-free mice was still as high as 50% in the E744-62-Q11 nanofibers group but was zero in the control groups (Figure 4B, right).
In addition, the mice immunized with E744-62-Q11 nanofibers had significantly smaller tumor volumes and lighter tumor and spleen weights than the mice receiving either Q11 or unassembled E744-62-Q11, and there was no obvious difference between the Q11 or unassembled E744-62-Q11 groups (Figure 4C). It is known that CTL and Th1 cells are the important adaptive anti-tumor immune effector cells, whereas MDSC and Th2 cells might contribute significantly to tumor immunosuppression.28,29 Thus, the splenocytes stimulated by E744-62 in vitro were labelled and analysed by flow cytometry. The results indicated that the numbers of CD8+IFNγ+ T cells and CD4+IFNγ+ T cells in E744-62-Q11 nanofiber-immunized mice were significantly increased compared to those in the mice receiving Q11 or unassembled E744-62-Q11 (Figure 4D); in contrast, the numbers of Gr-1+CD11b+ MDSC and CD4+IL4+ Th2 cells in E744-62-Q11 nanofiber-immunized mice were significantly reduced compared to the controls (Figure 4E). Additionally, the culture supernatants of splenocytes stimulated by E744-62 were collected and analysed for cytokine content via ELISA. The splenocytes from E744-62-Q11 nanofiber-immunized mice produced higher levels of the Th1 cytokines IFN-γ and TNF-α and lower levels of the Th2 cytokine IL-4 (Figure 4F). The results indicated that E744-62-Q11 nanofibers were potent even against fully established tumors in mice, which reached diameters as large as 5–6 mm.
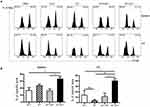
The Anti-Tumor Immunity Elicited By E744-62-Q11 Nanofibers Was Mediated By E7-Specific CTL Responses
To further explore whether anti-tumor effects produced by the immunization with E744-62-Q11 nanofibers were attributable to the production of specific cytotoxic lymphocytes, we performed an in vivo CTL assay. We used a high concentration of CFSE to label E744-62 peptide-pulsed splenocytes as target cells and a low concentration of CFSE to label unpulsed splenocytes as controls. Theoretically, nanofibers will trigger the production of E744-62-specific CD8+ cytotoxic T cells, which kill CFSEhigh splenocytes and ignore the CFSElow population. The reduction of the CFSEhigh splenocytes relative to the CFSElow population reflects the in vivo cytolysis of target splenocytes. The results showed that the mice immunized with E744-62-Q11 nanofibers had a significantly reduced populations of CFSEhigh cells in both spleens (Figure 5A) and tumor-draining lymph nodes (Figure 5B), whereas there was no significant change in the mice immunized with either Q11, E744-62 peptide, or E744-62 peptide mixed with Q11. The results indicated that E744-62 peptide alone or mixed with Q11 nanofibers was unable to elicit an effective antitumor CTL response, while a potent CTL response might account for the significant suppression of tumor growth found in E744-62-Q11 nanofiber-immunized mice.
Discussion
The effector mechanism of tumor vaccine is mainly to elicit tumor antigen-specific cellular immune responses and directly kill tumor cells. However, immunosuppression and immune escape will be developed through systemic and local mechanisms in cancer patients or tumor-bearing animals, leading to significant suppression of antitumor immunity induced by vaccine immunization. One possible approach is to modify and antagonize the tumor immunosuppressive microenvironment to promote the migration, survival, and functional maintenance of tumor-specific effector cells. Another approach is to develop a potent vaccine formula, including tumor antigen, antigen-delivery system, adjuvants, etc., that can get over immunosuppressive mechanisms and trigger robust anti-tumor cellular immunity. Recently, self-assembled nanofibers have drawn more and more attention for their possible application as a new vaccine platform due to their easy preparation, high immunogenicity, and causing little or no inflammation.19,30 It was reported that nanofibers elicited a long-lasting antibody response in a T cell-depend manner and were even efficient for use as a carrier to help break B cell tolerance and produce self-antibodies against a pathological self-protein. In addition to the powerful capability of eliciting humoural immune responses, nanofiber immunization was reported to be able to produce Th1/CTL-based T cell responses, indicating that nanofibers might be a promising antigen delivery platform for tumor vaccines.
In this study, we used a widely studied self-assembling peptide, Q11, to present HPV16 E744-62 peptide, which included a CTL and T helper epitope. We assessed the anti-tumor effects of the self-assembled nanofiber on TC-1 graft tumors through the use of both preventive and therapeutic immunization strategies. In the preventive study, immunization with nanofibers abolished the growth of subsequently grafted tumors and completely prevented the growth of re-challenged tumor after a 6-week rest. The results strongly indicated that nanofiber immunization elicited robust anti-tumor immunity and a long-lasting immune response and/or memory, and thus, nanofibers are capable of being explored as an effective antigen carrier for tumor vaccines. However, in the preventive study, immunization was fulfilled before tumor establishment, which means that the induction of the anti-tumor immunity was not inhibited by the immunosuppression developed during tumor growth. The study did not simulate a clinical setting very well; however, it helped us to easily assess the potency of the nanofiber vaccine for inducing anti-tumor immunity. Further, we conducted a therapeutic study to assess the immune capability of nanofibers facing fully established tumors. Once tumor immune suppression is established, the production, migration and anti-tumor activity of the vaccine-elicited effector cells, such as Th1/CTLs, might be significantly suppressed, leading to tumor growth without effective immunological surveillance. Thus, the therapeutic study mimicked the clinical situation better and is more challenging than the preventive experiment, and it may provide more useful information for evaluating the potentials of nanofibers as a tumor vaccine platform. Our results showed that immunization with nanofibers significantly suppressed the growth of established tumors with a diameter of 2–3 mm or larger tumors of 5–6 mm and even abolished tumors in 66.7% or 50% mice, respectively, in the two experiments. The nanofiber vaccine produced better effects for the treatment of smaller tumors. In addition, the immunization with assembled E744-62-Q11 nanofibers was more efficient than with unassembled E744-62-Q11 peptides, especially facing the larger established tumor. Actually, we are not sure that E7-Q11 can not be assembled in vivo. From our results that just E744-62 peptide didn’t show clear tumor-inhibition in our pilot studies (data not shown), and unassembled E7-Q11 did show significant suppression on tumor growth in the preventive study, we deduced that unassembled E7-Q11 in water might partly assembled into nanofibers in physiological salt and pH environment in vivo. However, considering unassembled E7-Q11 didn’t show as significant effects as E7-Q11 nanofiber in the preventive study and produced no significant tumor suppression in the treatment study, we can still conclude that nanofiber structure is important for E744-62-Q11 to elicit robust anti-tumor immunity even if our control of unassembled E7-Q11 was not so rigorous. In our previous studies, we used HBcAg VLPs to present the same E7 CTL epitope, and the vaccine based on VLP structure also produced significant anti-tumor effects; however, in comparison with the VLP, nanofiber presented better suppression on established tumors in similar experimental conditions. Although the comparison between nanofiber and VLP might not be strict, the results indicated a comparable or even better prospect of employing nanofibers to be a potent tumor vaccine carrier.
IFN-γ-producing Th1, CTL, NK, and γδ T cells are critical effector cells against cancer.31,32 Immunization with nanofibers elicited robust tumor-specific cellular immune responses in this study, as evidenced by an increased level of E7-specific IFN-γ-expressing splenocytes detected by ELISPOT and the higher frequency of key effector IFN-γ+CD4+ Th1 cells and IFN-γ+CD8+ CTL in isolated splenocytes in the vaccinated mice. However, the effector cells may lose their responsiveness due to tumor immune suppression developed through local and systemic mechanisms.33 The immunosuppressive tumor microenvironment, characterized by MDSC, Treg, and TAM, as well as their produced cytokines, chemokines, and other activity mediators, plays key roles in the formation of effector cell anergy and thus promotes tumor immune escape and tumor development.34,35 In this study, the levels of CD4+IL-4+ Th2 cells and CD11b+Gr-1+ MDSCs were found to be dramatically reduced. Additionally, the production of the key Th1 effector cytokines IFN-γ and TNF-α was found to be increased in the supernatants of E7 peptide-stimulated splenocytes, whereas levels of the Th2 cytokine IL-4 were reduced. Combining analysis on the elevated levels of IFN-γ-expressing splenocytes, Th1/CTL cells responses, and IFN-γ and TNF-α expression in cultured splenocytes, and on the other hand the reduced level of Th2 cell and IL-4 expression in the vaccinated mice, our results indicated that immunization with nanofibers generated Th1/CTL-biased T cell responses. Corresponding to the findings in tumor growth suppression, the results of effector cell production and cytokine expression showed that assembled E744-62-Q11 nanofibers were more efficient than unassembled E744-62-Q11 peptides, further supporting the importance of nanofiber structures for the vaccine.
In conclusion, we generated a nanofiber vaccine consisting of HPV16 E744-62 peptide and the self-assembling peptide Q11. Employing both preventive and therapeutic immunization strategies, we demonstrated that the nanofibers could elicit a potent antitumor cellular immunity and effectively suppress the development of graft tumors in mice. We thus revealed the potentials of nanofibers to be used as a new and promising vaccine platform for the treatment of cancers.
Acknowledgments
This work was financially supported by the CAMS Initiative for Innovative Medicine (2016-I2M-1–019), the National Natural Science Foundation of China (81773270), Applied Basic Research Projects of Yunnan Province (2016FA049), and Fundamental Research Funds for Institute of Pathogen Biology of PUMC (2014IPB107). The authors thank Mr Jingxian Zhou for excellent assistance in electron microscopy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
2. Steben M, DuarteFranco E. Human papillomavirus infection: epidemiology and pathophysiology. Gynecol Oncol. 2007;107(2):2–5. doi:10.1016/j.ygyno.2007.07.067
3. Hung CF, Ma B, Monie A, et al. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther. 2008;8(4):421–439. doi:10.1517/14712598.8.4.421
4. Bosch FX, Broker TR, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(8):1–31. doi:10.1016/j.vaccine.2013.07.026
5. Yang A, Jeang J, Cheng K, et al. Current State in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines. 2016;15(8):989–1007. doi:10.1586/14760584.2016.1157477
6. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. doi:10.1038/nrc2886
7. Einstein MH, Kadish AS, Burk RD, et al. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecologic Oncology. 2007;106(3):453–460. doi:10.1016/j.ygyno.2007.04.038
8. Hadeel K, Agnieszka G, Angelika R. Therapeutic vaccine strategies against human papillomavirus. Vaccines. 2014;2(2):422–462. doi:10.3390/vaccines2020422
9. Rosalia RA, Cruz LJ, Duikeren SV, et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015;40:88–97. doi:10.1016/j.biomaterials.2014.10.053
10. Irvine DJ, Hanson MC, Rakhra K, et al. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115(19):11109–11146. doi:10.1021/cr5004419
11. Dimierpoisson I, Carpentier R, N’Guyen TT, et al. Porous nanoparticles as delivery system of complex antigens for an effective vaccine against acute and chronic Toxoplasma gondii infection. Biomaterials. 2015;50(1):164–175. doi:10.1016/j.biomaterials.2015.01.056
12. Chen W, Huang L. Induction of cytotoxic T-lymphocytes and antitumor activity by a liposomal lipopeptide vaccine. Mol Pharm. 2008;5(5):464–471. doi:10.1021/mp700126c
13. Thomas C, Rawat A, Hope-Weeks L, et al. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm. 2011;8(8):405–415. doi:10.1021/mp200125j
14. Negahdaripour M, Golkar N, Hajighahramani N, et al. Harnessing self-assembled peptide nanoparticles in epitope vaccine design. Biotechnol Adv. 2017;35(5):575–596. doi:10.1016/j.biotechadv.2017.05.002
15. Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295(5564):2418–2421. doi:10.1126/science.1070821
16. Rad-Malekshahi M, Lempsink L, Amidi M, et al. Biomedical applications of self-assembling peptides. Bioconjug Chem. 2015;27(1):3–18. doi:10.1021/acs.bioconjchem.5b00487
17. Chesson CB, Huelsmann EJ, Lacek AT, et al. Antigenic peptide nanofibers elicit adjuvant-free CD8(+) T cell responses. Vaccine. 2014;32(10):1174–1180.
18. Restuccia A, Tian YF, Collier JH, et al. Self-assembled glycopeptide nanofibers as modulators of galectin-1 bioactivity. Cell Mol Bioeng. 2015;8(3):471–487. doi:10.1007/s12195-015-0399-2
19. Wen Y, Collier JH. Supramolecular peptide vaccines: tuning adaptive immunity. Curr Opin Immunol. 2015;35:73–79. doi:10.1016/j.coi.2015.06.007
20. Mora-Solano C, Wen Y, Han H, et al. Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers. Biomaterials. 2017;149:1–11. doi:10.1016/j.biomaterials.2017.09.031
21. Chen J, Pompano RR, Santiago FW, et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34(34):8776–8785. doi:10.1016/j.biomaterials.2013.07.063
22. Pompano RR, Chen J, Verbus EA, et al. Titrating T-Cell epitopes within self-assembled vaccines optimizes CD4+ Helper T cell and antibody outputs. Adv Healthc Mater. 2014;3(11):1898–1908. doi:10.1002/adhm.201400137
23. Rudra JS, Sun T, Bird KC, et al. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6:1557–1564. doi:10.1021/nn204530r
24. Sun T, Han H, Hudalla GA, et al. Thermal stability of self-assembled peptide vaccine materials. Acta Biomater. 2016;30:62–71. doi:10.1016/j.actbio.2015.11.019
25. Luci C, Hervouet C, Rousseau D, et al. Dendritic cell-mediated induction of mucosal cytotoxic responses following intravaginal immunization with the nontoxic B Subunit of cholera toxin. J Immunol. 2006;176(5):2749–2757. doi:10.4049/jimmunol.176.6.3635
26. Choi B, Moon H, Hong SJ, et al. Effective delivery of antigen–encapsulin nanoparticle fusions to dendritic cells leads to antigen-specific cytotoxic T cell activation and tumor rejection. ACS Nano. 2016;10(8):7339–7350. doi:10.1021/acsnano.5b08084
27. Si Y, Wen Y, Kelly SH, et al. Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8+ T cell responses. J Control Release. 2018;28:120–130. doi:10.1016/j.jconrel.2018.04.031
28. Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222(1):129–144. doi:10.1111/j.1600-065X.2008.00616.x
29. Wang S, Huang W, Li K, et al. Engineered outer membrane vesicle is potent to elicit HPV16 E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int J Nanomedicine. 2017;12:6813–6825. doi:10.2147/IJN.S143264
30. Karch CP, Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem Pharmacol. 2016;120:1–14. doi:10.1016/j.bcp.2016.05.001
31. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi:10.1126/science.1129139
32. Zhu Y, Ju S, Chen E, et al. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol. 2010;185(6):3174–3183. doi:10.4049/jimmunol.1000749
33. Zou W. Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi:10.1038/nri1806
34. Huang ZH, Shi L, Ma JW, et al. A totally synthetic, self-assembling, adjuvant-free MUC1 glycopeptide vaccine for cancer therapy. J Am Chem Soc. 2012;134(21):8730. doi:10.1021/ja211725s
35. Azmi F, Ahmad Fuaad AA, Giddam AK, et al. Self-adjuvanting vaccine against group A streptococcus: application of fibrillized peptide and immunostimulatory lipid as adjuvant. Bioorg Med Chem. 2014;22(22):6401–6408. doi:10.1016/j.bmc.2013.11.005
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.