Back to Journals » International Journal of Nanomedicine » Volume 12
Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycemic effect
Authors Yin J, Hou Y, Yin Y, Song X
Received 22 June 2017
Accepted for publication 14 October 2017
Published 6 December 2017 Volume 2017:12 Pages 8671—8680
DOI https://doi.org/10.2147/IJN.S144615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Juntao Yin,1 Yantao Hou,2 Yuyun Yin,3 Xiaoyong Song1
1Department of Pharmaceutics, Huaihe Hospital Affiliated to Henan University, 2Henan Vocational College of Applied Technology, Kaifeng, 3Henan Provincial Institute of Food and Drug Control, Zhengzhou, People’s Republic of China
Abstract: Diabetes mellitus is an incurable metabolic disorder that seriously threatens human health. At present, there is no effective medication available to defeat it. This work intended to develop selenium-coated nanostructured lipid carriers (SeNLCs) for enhancing the oral bioavailability and the curative effect of berberine, an antidiabetic phytomedicine. Berberine-loaded SeNLCs (BB-SeNLCs) were prepared by hot-melt dispersion/homogenization procedure followed by in situ reduction. BB-SeNLCs were characterized by particle size, morphology, entrapment efficiency (EE) and in vitro release. Pharmacokinetics of berberine solution, berberine-loaded NLCs (BB-NLCs) and BB-SeNLCs were studied in Sprague Dawley rats administered by oral gavage. The prepared BB-SeNLCs were around 160 nm in particle size with an EE of 90%. In addition, BB-SeNLCs exhibited a better sustained release of berberine compared to the plain NLCs. After oral administration, BB-SeNLCs greatly enhanced the oral bioavailability of berberine, which was approximately 6.63 times as much as that of berberine solution. The hypoglycemic effect of BB-SeNLCs was also significantly superior to that of BB-NLCs and berberine solution. It turned out that sustained drug release and good intestinal absorption, plus the synergy of selenium, were basically responsible for enhanced oral bioavailability and hypoglycemic effect. Our findings show that SeNLCs are promising nanocarriers for oral delivery of berberine to strengthen the antidiabetic action.
Keywords: berberine, diabetes mellitus, nanostructured lipid carriers, selenium, bioavailability
Introduction
The incidence of diabetes mellitus (DM) increases year by year due to improper diet and lifestyle, which causes a heavy burden to the society and individual family.1 Nowadays, the treatment of DM primarily depends on insulin injection and oral antidiabetic agents. However, insulin injection via the subcutaneous route has the shortcoming of pain and is occasionally associated with hypoglycemia, lipodystrophy and allergic reaction and even hyperinsulinemia.2 Likewise, oral antidiabetic agents such as metformin, repaglinide and glipizide possess the disadvantages of gastrointestinal upset, high chance of hypoglycemia, weight gain, secondary failure and so on.3 In recent years, stem cells technique has been actively examined to treat DM by reproducing the in vivo developmental processes of the pancreas.4 Islet xenotransplantation is becoming a clinical reality for DM therapy.5 Postprandial glucose control,6 C-peptide replacement7 and phytomedicines intervention8 have been explored for curing DM. Although these therapeutic approaches could alleviate the afflictions of patients with DM, they are unable to thoroughly cure DM. More effective medications are still required to be developed for DM treatment.
Berberine, an isoquinoline alkaloid from the herb of Coptis chinensis, is widely used for gastrointestinal infections and various inflammations in Asian countries. Recently, it has been shown to have potential therapeutic significance for type 2 DM.9–12 Berberine can attenuate oxidative stress occurring in DM through the miR-106b/SIRT1 pathway, thus affecting the function of islets. It has been documented that berberine can promote glucose and lipid metabolism of diabetic subjects by activating adenosine monophosphate-activated protein kinase and improving insulin sensitivity.13 In addition, berberine can promote α-glucosidase inhibition and regulate multiple signal pathways by targeting noncoding RNA to exert the antidiabetic effect.14,15 However, the clinical use of berberine is largely limited because of its poor intestinal absorption and inadequate plasma level after oral administration.16 Besides, oral administration, especially at a high dose, often causes gastrointestinal discomfort due to considerable exposure to free berberine.17 Therefore, it is necessary to develop advanced formulations of berberine to improve the oral delivery efficacy, thereby enhancing the in vivo antidiabetic effect.
Nanostructured lipid carriers (NLCs), the second generation of lipid nanoparticles, contain spatially incompatible liquid and solid lipids.18 NLCs are developed on the base of solid lipid nanoparticles (SLNs) in order to overcome the limitations of SLNs (eg, low drug loading and potential drug expulsion). The participation of liquid lipid creates an imperfect crystal matrix, resulting in high drug loading and physical stability.19 Of note, lipolysis of lipid-based formulations, including NLCs, is an ineluctable process when they transport through the harsh gastrointestinal tract, which tends to give rise to erratic drug release and absorption.20 Intensification of NLCs stability can diminish the in vivo transport variation and thus lead to a controllable drug release and high absorption percentage. Selenium coating may be a useful approach to upgrading the structure of NLCs and strengthening their gastrointestinal stability. Selenium is a trace element that the body has to depend on, which plays an important role in blood glucose homeostasis.21 However, to date, there is no report on selenium-coated NLCs (SeNLCs) for oral drug delivery.
In this study, SeNLCs were engineered for oral delivery of berberine, aiming to enhance its oral bioavailability and hypoglycemic effect through a synergy with selenium. We took advantage of the in situ reduction technique to achieve the coating of NLCs. Berberine-loaded SeNLCs (BB-SeNLCs) were characterized by particle size, entrapment efficiency (EE), morphology and in vitro release. The pharmacokinetics and hypoglycemic effect of BB-SeNLCs were investigated in rats through the oral route and compared with those of plain NLCs and solution formulations. The transcellular and hypoglycemic mechanisms of BB-SeNLCs were interpreted.
Materials and methods
Materials
Berberine chloride, oleic acid, sodium selenite (Na2SeO3) and vitamin C (Vc) were purchased from Aladdin Reagent (Shanghai, People’s Republic of China). Precirol® ATO 5 (glyceryl distearate) was kindly gifted by Gattefossé (Saint-Priest, France). Chlorpromazine, simvastatin, Filipin, Hoechst 33258 and 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) were provided by Sigma-Aldrich (St Louis, MO, USA). Deionized water was produced by Milli-Q water purification system (Merck Millipore, Billerica, MA, USA). High-performance liquid chromatography (HPLC)-grade acetonitrile was provided by Merck (Darmstadt, Germany). All other chemicals were of analytical grade and used as supplied.
Preparation of BB-SeNLCs
BB-SeNLCs were prepared by the hot-melt dispersion/homogenization procedure followed by in situ reduction.22,23 Briefly, berberine, oleic acid and Precirol® ATO 5 were dissolved in an appropriate amount of ethanol (90%, v/v) and then evaporated under vacuum to form a dry lipid film. The solid lipid film was subsequently heated to melt at 60°C. Meanwhile, an aqueous phase containing 1% Tween 80 (w/v) was prepared at the same temperature and introduced into the oil phase. After that, the mixture was subjected to high-speed shearing at 10,000 rpm for 5 minutes to produce coarse dispersions. The resulting coarse dispersions were further homogenized using a microfluidizer (Nano DeBee; BEE International, Waltham, MA, USA) to obtain fine berberine-loaded NLCs (BB-NLCs). On the base of BB-NLCs, in situ reduction was carried out to fabricate BB-SeNLCs, where Na2SeO3 and Vc were successively added into BB-NLC suspensions with a molar ratio of 1:5 and reacted for 4 hours at 45°C. BB-SeNLCs formed as Se4+ were reduced to Se which precipitated on the surface of nanoparticles. The residual unreacted species were removed by dialysis against deionized water.
Characterization of BB-SeNLCs
The particle size and ζ potential of BB-SeNLCs were measured using a Malvern analyzer (Zetasizer Nano ZS; Malvern Instruments, Malvern, UK) at 25°C. BB-SeNLCs were diluted appropriately with deionized water and placed in a cuvette. After equilibrium for 2 minutes, the sample was subjected to laser diffraction or Doppler velocimetry for particle size and ζ potential analyses. The morphology of BB-SeNLCs was visualized by transmission electron microscopy (TEM). Micrographs were taken at an acceleration voltage of 100 kV using an HT7700 transmission electron microscope (Hitachi, Toyota, Japan).
The EE of BB-SeNLCs was determined by a centrifugal ultrafiltration technique. BB-SeNLCs were centrifuged against a centrifugal filter device (Amicon® Ultra-0.5; Millipore) with a molecular weight cut-off (MWCO) of 10 kD to remove the unloaded berberine. Berberine in the filtrate was quantified by an HPLC system (Agilent 1100; Symmetry C18 column [5 μm, 4.6×150 mm]; flow phase: acetonitrile/0.02 M potassium dihydrogen phosphate solution =20/80; flow rate: 1.0 mL/min; detection wavelength: 345 nm; injection volume: 10 μL). The EE of BB-SeNLCs was calculated based on the equation: EE (%) = (1 − Cfre/Ctot) × 100, where Cfre and Ctot denote free and total berberine concentration in the nanosuspensions, respectively.
In vitro drug release
The in vitro drug release was studied using the dialysis bag method. In detail, aliquots of berberine solution, BB-NLCs or BB-SeNLCs equal to 50 mg of berberine were put into dialysis bags (MWCO 30 kD). The dialysis bags were fastened and then placed in 900 mL of pH 6.8 phosphate buffer solution (PBS). In addition, to examine the anti-digestive ability of SeNLCs, the release of BB-SeNLCs in the simulated intestinal fluid (SIF) containing pancreatic enzyme was performed. At the times of 0.25, 0.5, 1, 2, 4, 8, 12, 18 and 24 hours, 2 mL of release medium was withdrawn and replaced with isovolumic fresh medium. Berberine concentration in the release medium was determined by HPLC, and the release profiles of berberine from different formulations were plotted with accumulative release percentage versus time. Each experiment was performed in triplicate.
Pharmacokinetic study
All animal experiments were conducted according to the protocols issued by the Experimental Animal Ethical Committee of Huaihe Hospital Affiliated to Henan University and approved by the committee. Sprague Dawley (SD) rats (220±20 g) were fasted for 24 hours before administration but freely allowed access to water. The rats were randomly divided into three groups (berberine suspensions, BB-NLCs and BB-SeNLCs; n=5). The rats were respectively given three different formulations by gavage with the dose of 50 mg/kg. Approximately 0.25 mL of blood was collected via the tail vein at 0.5, 1, 2, 4, 6, 8, 12, 16 and 24 hours after administration and transferred into heparinized tubes. Then, plasma was prepared from the blood through centrifugation at 5,000× g for 5 minutes.
The plasma berberine was quantified by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS) (Xevo G2 QTOF; Waters, Milford, MA, USA). The plasma samples were treated with fivefold volume of acetonitrile and eddied completely. After centrifugation, the supernatant was evaporated to dry at 37°C using a vacuum concentrator (Eppendorf, Hamburg, Germany), and then the residues were reconstituted in 150 μL of 0.1% formic acid/acetonitrile (50/50, v/v). After vortexing and centrifuging again, the supernatant was injected into UPLC-QTOF/MS system for positive ion detection. The instrument and parameter configurations referred to the literature.24 Pharmacokinetic data were processed using PKSolver 2.0, a freely available add-in Excel program.
Hypoglycemic effect in diabetic rats
Streptozotocin-induced hyperglycemic rats were used to investigate the hypoglycemic effect of BB-SeNLCs. The induction method followed the reported procedure,25 based on male Wistar rats weighing 200±20 g. The hyperglycemic rats were randomized into six groups (n=6), namely saline, berberine solution, SeNLCs, BB-NLCs, BB-SeNLCs and insulin solution. Rats were fasted but freely accessible to water for 12 hours before administration. The rats were intragastrically (i.g.) administered with saline (negative control), berberine solution, SeNLCs (blank carrier), BB-NLCs and BB-SeNLCs, respectively, or subcutaneously (s.c.) injected with insulin solution (positive control). The oral dose of berberine-containing preparations was 50 mg/kg, and the insulin dose for injection was 1 IU/kg. After administration, approximately 0.25 mL of blood was withdrawn from the tail vein of each rat at predetermined intervals. The blood was immediately centrifuged at 5,000× g for 5 minutes to obtain the plasma. The blood glucose was determined using glucose assay kit (Abcam, Cambridge, UK) following the manufacturer’s instruction. The pharmacological effect (PE) of oral preparations relative to insulin (s.c.) was evaluated based on the area above the curve of blood glucose level versus time (AAC) using the equation: PE (%) = (AACi.g./AACs.c.) × 100.
Cellular uptake and trafficking
Caco-2 cells were purchased from American Type Culture Collection and cultured as per the reported protocol.26 Caco-2 cells were seeded in 24-well plates with a density of 1×105 cells/well. When the cells grew to an 80% confluence, they were ready for the cellular uptake. Before experiments, the cells were washed twice with pH 7.4 PBS. Then, berberine solution, BB-NLCs and BB-SeNLCs diluted to 25 μg/mL with culture medium were added into the wells. At the times of 0.5, 1, 2 and 4 hours, the medium was removed from the cells, and then the cells were fully rinsed with cold PBS. The washed cells were lysed with the radio-immunoprecipitation assay lysis buffer (0.1% phenylmethanesulfonyl fluoride). After centrifugation, the supernatant was prepared, in which the protein content was quantified using the bicinchoninic acid protein assay kit. Berberine concentration in the supernatant was determined by HPLC, which was corrected using the cell protein level. To visualize the cellular uptake, fluorescent BB-SeNLCs were prepared by loading DiO in BB-SeNLCs upon preparation. Caco-2 cells were incubated with DiO-labeled BB-SeNLCs for 30 minutes at 37°C followed by removal of medium. Then, the cells were rinsed with PBS, immobilized by 4% paraformaldehyde and subjected to inspection by confocal laser scanning microscopy (CLSM).
The cellular trafficking pathway of BB-SeNLCs was diagnosed by the transport inhibition technique.27 Caco-2 cells were preincubated with various inhibitors (hypertonic glucose, chlorpromazine, simvastatin and Filipin) at 37°C or treated under 4°C for 30 minutes. Then, DiO-labeled BB-SeNLCs were introduced into the cells and incubated for 2 hours. Afterward, the cells were washed, trypsinized and centrifuged to obtain cell pellets. The relative cellular uptake (shown as fluorescent intensity %) was analyzed by flow cytometry (FACS Aria; BD, San Jose, CA, USA).
Glucose utilization assay
To investigate the effect of BB-SeNLCs on glucose utilization, adipocytes were derived from adipose-derived stem cells. The construction of adipocytes using the inguinal fat tissue of SD rats referred to the reported method.23 The mature adipocytes were seeded in 24-well plates and cultured in 0.2% BSA culture medium with 10 mM of glucose at 37°C. Berberine solution, BB-NLCs or BB-SeNLCs was added into the cells with a final concentration of 2.5 μg/mL. In another group, 0.25 μg/mL of insulin served as a positive control. At 2 and 4 hours, the extracellular medium was withdrawn and assayed for the remaining glucose with the kit mentioned.
Results and discussion
Preparation and characterization of BB-SeNLCs
Berberine hydrochloride can only dissolve in hot water and is slightly soluble in the regular water and ethanol. In general, a compound in the form of hydrochloride possesses a low hydrophilicity, which tends to result in low drug loading in carriers. In this study, oleic acid as a counterpart was used to conjugate berberine via electrostatic interaction, thereby increasing the loading rate of berberine in NLCs. The hot-melt dispersion/homogenization technique is the commonest approach used for the production of NLCs. Other than the preparative process, it turns out that the factors affecting the formulation performance are the amounts of oleic acid and active ingredient used in NLCs. We investigated the effects of oleic acid and drug amounts to the lipid phase on the particle size and EE of BB-NLCs. Figure 1 presents the particle size and EE of BB-NLCs varying with oleic acid and drug concentrations. The ratios of oleic acid and drug had significant effects on the particle size and EE of BB-NLCs. High proportion of oleic acid resulted in smaller nanoparticles and high EE. Conversely, the increase in drug amount caused an increase in particle size and a decline in EE. This can be attributed to the contribution of oleic acid that disturbs the perfect crystal matrix of the lipid phase due to liquid property and facilitates encapsulation of berberine by solubilization and electrostatic adsorption.
BB-SeNLCs were prepared on the base of BB-NLCs by introducing Na2SeO3 and Vc into the system. The concentration of Na2SeO3 used for coating BB-NLCs was 0.28 mg/mL, which led to formation of a suitable coating layer that could be inferred from the particle size change before and after coating. The particle size of resultant BB-SeNLCs was determined to be 161.2 nm, whereas it was 116.4 nm for BB-NLCs (Figure 2). The ζ potential of BB-NLCs was −32.8 mV, which increased to −23.1 mV after Se coating. These phenomena indicate that Se has precipitated onto the surface of BB-NLCs upon reduction of Se4+. BB-NLCs and BB-SeNLCs appeared spherical in morphology as revealed by TEM (Figure 2). BB-SeNLCs took on a high surface electron density as seen from the TEM micrograph compared with BB-NLCs, demonstrating the success of Se coating. Furthermore, our developed BB-SeNLCs possessed a relatively high EE (90.26%), which largely reduced the gastrointestinal exposure of free berberine, and hence the mucosal irritation. Se level was approximately 50 μg/mL in BB-SeNLCs as assessed using the stoichiometric ratio and chemical dosage, assuming a complete reaction occurred.
Release characteristics of berberine formulations
The release profiles of berberine solution, BB-NLCs and BB-SeNLCs in pH 6.8 PBS or SIF are shown in Figure 3. Berberine solution exhibited a quicker drug release in pH 6.8 PBS. Normally, berberine release from the solution formulation would be instant. However, berberine release took several hours, indicating that certain resistance was provided by the dialysis bag. In pH 6.8 PBS, BB-NLCs exhibited a slow drug release with the accumulative release less than 35% within 24 hours. However, of note, BB-SeNLCs presented a slower drug release compared with BB-NLCs. The accumulative release was just 18.89% at 24 hours, demonstrating a sustained-release effect. We assume that the sustained-release effect of BB-SeNLCs is concerned with Se coating that thickens the shell of NLCs and consolidates the structure. To further confirm the reinforced inflexibility of nanocarriers, we investigated the release of BB-SeNLCs in lipase-containing medium (SIF). It was found that BB-SeNLCs still exhibited an excellent sustained release in the digestive medium, even slower than BB-NLCs in pH 6.8 PBS. The finding suggests that Se coating can protect NLCs from lipolysis to some extent, thereby reducing free drug exposure and absorption variation after oral administration.
Improved oral bioavailability
The pharmacokinetic profiles of berberine solution, BB-NLCs and BB-SeNLCs following oral administration are presented in Figure 4. The solution formulation resulted in limited berberine absorption and lower oral bioavailability. In comparison with berberine solution, BB-NLCs significantly enhanced the drug absorption both in the rate and extent. BB-SeNLCs exhibited a similar pharmacokinetic profile to BB-NLCs, but higher plasma drug concentration and duration. The main pharmacokinetic parameters are presented in Table 1. For berberine solution, the maximum plasma concentration (Cmax) and the area under the plasma concentration-versus-time curve (AUC0–t) were merely 45.06 ng/mL and 173.74 ng·h/mL, respectively. Otherwise, BB-NLCs brought about greater Cmax and AUC0–t, up to 148.21 ng/mL and 689.54 ng·h/mL, respectively. The results show that plain NLCs can promote the oral absorption of berberine. However, BB-SeNLCs were more effective and significant in enhancing the bioavailability. BB-SeNLCs yielded higher plasma berberine concentration and maintained it for a long duration after the peak time. The Cmax and AUC0–t reached 172.88 ng/mL and 1,107.80 ng·h/mL, respectively. The relative oral bioavailability of BB-NLCs and BB-SeNLCs, calculated based on the non-compartmental model, were 396.87% and 663.65%, respectively, compared with berberine solution. BB-NLCs and BB-SeNLCs had similar in vivo half-life (T1/2), but exhibited different mean residence time (MRT). BB-SeNLCs possessed a longer MRT than BB-NLCs, indicating that Se-plated NLCs can prolong the circulation time of berberine due to the sustained-release effect.
NLCs belong to the scope of lipid-based formulations, which can optimize the oral delivery of lipophilic drugs by solubilizing the drug in the intestinal milieu, recruiting intestinal lymphatic drug transport and altering enterocyte-based drug transport and disposition.28 Meanwhile, lipolysis of lipid nanoparticles and reconstruction into smaller mixed micelles can also facilitate the payload transport across the intestinal epithelia, thus increasing the oral absorption.22,29 These advantages render NLCs rather suitable for oral drug delivery. Our engineered SeNLCs were developed from NLCs that possess better physiological stability and sustained drug release capacity. Therefore, they are supposed to further enhance the oral bioavailability of berberine.
Enhanced hypoglycemic effect
The blood glucose level-versus-time curves following administration of different formulations in diabetic rats are shown in Figure 5. Berberine solution produced a feeble hypoglycemic effect. The maximal blood glucose reduction was merely 18.63% relative to the saline group. BB-NLCs resulted in more marked blood glucose decline compared with the solution formulation, indicating transport of more berberine molecules into the body through NLCs. In addition, BB-NLCs achieved a more durable pharmacological effect than berberine solution. It was noteworthy that SeNLCs alone brought forth a certain hypoglycemic effect close to the efficacy of berberine solution. The hypoglycemic activity of Se nanoparticles has been critically documented by other research teams.23,30,31 By comparison, BB-SeNLCs caused more significant hypoglycemic effect, where the maximal blood glucose decline was 33.7%. Of course, the potency of BB-SeNLCs in lowering the blood glucose was inferior to that of subcutaneous insulin. Nevertheless, the PE of oral BB-SeNLCs containing 50 mg of berberine corresponded to 113.96% when compared to subcutaneous insulin (1 IU), according to the AAC. As for berberine solution and BB-NLCs, the PE values were 70.93% and 17.87%, respectively. Obviously, BB-SeNLCs are more effective in blood glucose regulation, which circumvent the sharp fluctuation of blood glucose and achieve a long-lasting pharmacological action. It is shown that there exists a synergic effect between berberine and selenium in lowering blood glucose.
Cellular uptake and trafficking
Figure 6A presents the intracellular berberine concentration at different times after incubation with berberine solution, BB-NLCs and BB-SeNLCs. There exists a significant difference in the cellular uptake between free and NLCs-laden berberine. Free berberine failed to be adequately assimilated into Caco-2 cells, which may be attributed to the high hydrophilicity of berberine hydrochloride. However, when encapsulated in NLCs or SeNLCs, the cellular uptake of berberine was strikingly quick compared to the free form. The cellular uptake of BB-NLCs was approximately three times the uptake of free berberine at 0.5, 1 and 2 hours. In the first 2 hours, there was no significant difference in cellular uptake between BB-NLCs and BB-SeNLCs. However, after that, BB-SeNLCs achieved higher berberine uptake, showing some advantages in facilitating berberine transport toward cells. This may be connected with the high-density nature of SeNLCs that results in nonspecific phagocytosis.32 The reduced blood glucose level seemed to be not in line with the large differences in cellular uptake in terms of free berberine, BB-NLCs and BB-SeNLCs. It should be noted that transcytosis and redistribution occur after cellular uptake, during which some nanoparticles fail to be delivered to the target site (eg, the liver). NLCs can be transported into the circulation system through the lymphatic route that circumvents the portal vein, which may be the cause of diluted in vitro–in vivo correlation.
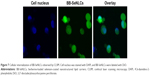
The cytosolic delivery of BB-SeNLCs can be featured by the cellular internalization. CLSM imaging revealed that intense cellular internalization took place on BB-SeNLCs (Figure 7). There was apparent SeNLCs-associated fluorescence present in the cytoplasm, even transporting into the nucleus. Internalization of inflexible nanoparticles, such as quantum dots, silicon nanoparticles and mesoporous carbon, into various cells has been verified by researchers.33–35 The excellent transcellular ability of SeNLCs confers them great suitability for oral delivery of berberine.
The relative cellular uptake of BB-SeNLCs in the presence of endocytosis inhibitors or under 4°C is presented in Figure 6B. The cellular uptake of BB-SeNLCs was apparently inhibited by hypertonic sucrose and chlorpromazine, and limited under low temperature, whereas simvastatin and Filipin posed less effects on the cellular uptake. Hypertonic and chlorpromazine treatment reduced the cellular uptake by 20.2% and 27.4%, respectively, in comparison with the control. Low temperature reduced the cellular uptake by 41.8%. These results indicate that clathrin-mediated endocytosis is involved in the process of cellular trafficking of BB-SeNLCs.36
Effect of BB-SeNLCs on glucose utilization
The extracellular glucose concentrations of adipocytes treated with various berberine preparations are shown in Figure 8. Free berberine exhibited 15.3% and 41.5% reduction of extracellular glucose at 2 and 4 hours, respectively. This indicates that glucose can be continually utilized by the adipocytes in the presence of berberine. BB-NLCs resulted in a 54.5% reduction of extracellular glucose compared with free berberine, especially at the point of 4 hours. Relative to the two former formulations, BB-SeNLCs showed more significant glucose reduction in the extracellular medium both at 2 and 4 hours. In terms of glucose utilization, the potency of 2.5 μg/mL of BB-SeNLCs was close to that of 0.25 μg/mL of insulin. In our study, BB-SeNLCs demonstrated an exceptional facilitation effect on glucose utilization by the adipocytes that may be the underlying mechanism of enhanced hypoglycemic effect.
Conclusion
In this work, we successfully developed an SeNLCs formulation for oral delivery of berberine to ameliorate the in vivo bioavailability and hypoglycemic effects of berberine. We found that the designed SeNLCs possessed a sustained-release effect and high intestinal stability. SeNLCs significantly improved the pharmacokinetics of berberine after oral administration, thereby enhancing the hypoglycemic effect. Cell uptake and trafficking experiments revealed that SeNLCs could be easily transported into the enterocytes. In addition, SeNLCs had a positive effect on glucose utilization which promoted the extracellular glucose to be efficiently assimilated by the adipocytes. These results support the notion that SeNLCs are a suitable nanocarrier for oral berberine delivery. This work provides insight into the use of functionalized lipid nanoparticles to orally deliver therapeutics with low bioavailability and potentiate their curative effect.
Acknowledgments
This work was financially supported by the Administration of Traditional Chinese Medicine Project of Henan Province (2014ZY02088) and the Social Development Research Project of Kaifeng City Technology Bureau (105).
Disclosure
The authors report no conflicts of interest in this work.
References
Karthikeyan R, Marimuthu G, Spence DW, et al. Should we listen to our clock to prevent type 2 diabetes mellitus? Diabetes Res Clin Pract. 2014;106(2):182–190. | ||
Wong CY, Martinez J, Dass CR. Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. J Pharm Pharmacol. 2016;68(9):1093–1108. | ||
Arai M, Maeda K, Satoh H, Miki A, Sawada Y. Creation new patient information leaflets with diabetes by pharmacists and assesment conducted by patients. Yakugaku Zasshi. 2016;136(10):1449–1454. | ||
Kondo Y, Toyoda T, Inagaki N, Osafune K. iPSC technology-based regenerative therapy for diabetes. J Diabetes Investig. Epub 2017 Jun 13. | ||
Matsumoto S, Tomiya M, Sawamoto O. Current status and future of clinical islet xenotransplantation. J Diabetes. 2016;8(4):483–493. | ||
Madsbad S. Impact of postprandial glucose control on diabetes-related complications: how is the evidence evolving? J Diabetes Complications. 2016;30(2):374–385. | ||
Pinger CW, Entwistle KE, Bell TM, Liu Y, Spence DM. C-Peptide replacement therapy in type 1 diabetes: are we in the trough of disillusionment? Mol Biosyst. 2017;13(8):1432–1437. | ||
Prabhakar PK, Doble M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin J Integr Med. 2011;17(8):563–574. | ||
Tian CM, Jiang X, Ouyang XX, Zhang YO, Xie WD. Berberine enhances antidiabetic effects and attenuates untoward effects of canagliflozin in streptozotocin-induced diabetic mice. Chin J Nat Med. 2016;14(7):518–526. | ||
Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55(8):2256–2264. | ||
Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294(1):E148–E156. | ||
Chen DL, Yang KY. Berberine alleviates oxidative stress in islets of diabetic mice by inhibiting miR-106b expression and up-regulating SIRT1. J Cell Biochem. 2017;118(2):4349–4357. | ||
Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm Sin B. 2012;2(4):327–334. | ||
Jhong CH, Riyaphan J, Lin SH, Chia YC, Weng CF. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. BioFactors. 2015;41(4):242–251. | ||
Chang W. Non-coding RNAs and berberine: a new mechanism of its anti-diabetic activities. Eur J Pharmacol. 2017;795:8–12. | ||
Liu CS, Zheng YR, Zhang YF, Long XY. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia. 2016;109:274–282. | ||
Wu SJ, Don TM, Lin CW, Mi FL. Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier. Mar Drugs. 2014;12(11):5677–5697. | ||
Poonia N, Kharb R, Lather V, Pandita D. Nanostructured lipid carriers: versatile oral delivery vehicle. Future Sci OA. 2016;2(3):FSO135. | ||
Andrade LM, de Fatima Reis C, Maione-Silva L, et al. Impact of lipid dynamic behavior on physical stability, in vitro release and skin permeation of genistein-loaded lipid nanoparticles. Eur J Pharm Biopharm. 2014;88(1):40–47. | ||
Qi J, Zhuang J, Lu Y, Dong X, Zhao W, Wu W. In vivo fate of lipid-based nanoparticles. Drug Discov Today. 2017;22(1):166–172. | ||
Ogawa-Wong AN, Berry MJ, Seale LA. Selenium and metabolic disorders: an emphasis on type 2 diabetes risk. Nutrients. 2016;8(2):80. | ||
Zhou X, Zhang X, Ye Y, et al. Nanostructured lipid carriers used for oral delivery of oridonin: an effect of ligand modification on absorption. Int J Pharm. 2015;479(2):391–398. | ||
Deng W, Xie Q, Wang H, Ma Z, Wu B, Zhang X. Selenium nanoparticles as versatile carriers for oral delivery of insulin: insight into the synergic antidiabetic effect and mechanism. Nanomedicine. 2017;13(6):1965–1974. | ||
Xue M, Zhang L, Yang MX, et al. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int J Nanomedicine. 2015;10:5049–5057. | ||
He H, Sheng J, David AE, et al. The use of low molecular weight protamine chemical chimera to enhance monomeric insulin intestinal absorption. Biomaterials. 2013;34(31):7733–7743. | ||
Natoli M, Leoni BD, D’Agnano I, Zucco F, Felsani A. Good caco-2 cell culture practices. Toxicol In Vitro. 2012;26(8):1243–1246. | ||
Zhang X, Qi J, Lu Y, He W, Li X, Wu W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine. 2014;10(1):167–176. | ||
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–248. | ||
Li W, Zhang T, Ye Y, Zhang X, Wu B. Enhanced bioavailability of tripterine through lipid nanoparticles using broccoli-derived lipids as a carrier material. Int J Pharm. 2015;495(2):948–955. | ||
Al-Quraishy S, Dkhil MA, Abdel Moneim AE. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine. 2015;10:6741–6756. | ||
Ahmed HH, Abd El-Maksoud MD, Abdel Moneim AE, Aglan HA. Pre-clinical study for the antidiabetic potential of selenium nanoparticles. Biol Trace Elem Res. 2017;177(2):267–280. | ||
Rudt S, Wesemeyer H, Müller RH. In vitro phagocytosis assay of nano- and microparticles by chemiluminescence. IV. Effect of surface modification by coating of particles with poloxamine and Antarox CO on the phagocytic uptake. J Control Release. 1993;25(1):123–132. | ||
Cepeda-Perez E, Lopez-Luke T, Plascencia-Villa G, et al. SERS and integrative imaging upon internalization of quantum dots into human oral epithelial cells. J Biophotonics. 2016;9(7):683–693. | ||
Cao Z, Peng F, Hu Z, et al. In vitro cellular behaviors and toxicity assays of small-sized fluorescent silicon nanoparticles. Nanoscale. 2017;9(22):7602–7611. | ||
Zhang X, Zhang T, Ye Y, et al. Phospholipid-stabilized mesoporous carbon nanospheres as versatile carriers for systemic delivery of amphiphobic SNX-2112 (a Hsp90 inhibitor) with enhanced antitumor effect. Eur J Pharm Biopharm. 2015;94:30–41. | ||
Bewersdorff T, Vonnemann J, Kanik A, Haag R, Haase A. The influence of surface charge on serum protein interaction and cellular uptake: studies with dendritic polyglycerols and dendritic polyglycerol-coated gold nanoparticles. Int J Nanomedicine. 2017;12:2001–2019. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.