Back to Journals » Cancer Management and Research » Volume 9
Selective serotonin reuptake inhibitors and the risk of hepatocellular carcinoma in hepatitis B virus-infected patients
Authors Chang CM, Hsieh MS, Yang TC , Hsieh VC , Chiang JH, Huang HH, How CK, Hu SY , Yen DH
Received 2 August 2017
Accepted for publication 25 October 2017
Published 28 November 2017 Volume 2017:9 Pages 709—720
DOI https://doi.org/10.2147/CMAR.S148097
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leylah Drusbosky
Chia-Ming Chang,1–3 Ming-Shun Hsieh,1–4 Tsung-Chieh Yang,3,5 Vivian Chia-Rong Hsieh,6 Jen-Huai Chiang,7 Hsien-Hao Huang,1,3 Chorng-Kuang How,1,3 Sung-Yuan Hu,8 David Hung-Tsang Yen1,3
1Department of Emergency Medicine, Taipei Veterans General Hospital, 2Institute of Occupational Medicine and Industrial Hygiene, National Taiwan University College of Public Health, 3College of Medicine, National Yang-Ming University, Taipei, 4Department of Emergency Medicine, 5Division of Gastroenterology, Department of Internal Medicine, Taipei Veterans General Hospital, Taoyuan Branch, Taoyuan, 6Department of Health Services Administration, 7Management Office for Health Data, China Medical University, 8Department of Emergency Medicine, Taichung Veterans General Hospital, Taichung, Taiwan, Republic of China
Background: This study aimed to investigate the association between the use of selective serotonin reuptake inhibitors (SSRIs) and the risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B virus (HBV) infection.
Methods: We conducted a population-based cohort study by using claims data from the Taiwan National Health Insurance Research Database (NHIRD). The study cohort comprised 1380 newly diagnosed HBV-infected patients with SSRI use who were frequency matched by age, sex, liver cirrhosis, and index year with HBV-infected patients without SSRI use in the comparison cohort. Each patient case was followed from 2000 to 2012 to identify incident HCC cases. Cox proportional hazards regression was performed to evaluate the association between SSRI use and HCC risk. The further sensitivity analysis used case-control study design. A total of 9070 HCC subjects retrieved from NHIRD, and equal non-HCC subjects were analyzed after matching for age and sex.
Results: We identified 9 and 24 HCC cases in the study and comparison cohorts during the follow-up period of 7056 and 6845 person-years, respectively. The incidence rate of HCC was 1.28 and 3.51 per 1000 person-years for SSRI and non-SSRI users, respectively. After adjusting for potential confounders, the adjusted hazard ratio (HR) for SSRI use was 0.28 (95% confidence interval [CI], 0.12–0.64; p = 0.0027). For SSRI users with a cumulative defined daily dose (cDDD) of 28–89, 90–364, and ≥365, the adjusted HRs were 0.51, 0.22, and 0.12, respectively, (95% CI, 0.21–1.25, 0.05–0.94, and 0.02–0.90, respectively) compared with non-SSRI users (<28 cDDD). The sensitivity analysis showed that the SSRI presented with a dose-response protective effect for HCC in the multivariate analysis.
Conclusion: SSRIs use may possibly reduce the risk of HCC in HBV-infected patients in a dose-responsive manner.
Keywords: selective serotonin reuptake inhibitors, SSRI, hepatitis B virus, HBV, hepatocellular carcinoma, HCC, depression
Introduction
Serotonin, also known as 5-hydroxytryptamine (5-HT), is a biogenic amine derived from tryptophan that functions as a ligand for a large family of 5-HT receptors.1 Serotonin is critical for neurotransmission in the central nervous system, thus controlling mood, behavior, sleep, and anxiety. Selective serotonin reuptake inhibitors (SSRIs) are the most widely prescribed medication for treating depression because they are considered safe and well-tolerated compared with earlier generation antidepressants, such as tricyclic antidepressants and monoamine oxidase inhibitors.2,3
Hepatitis B virus (HBV) infection typically leads to chronic hepatitis, often following a progressive course over decades, finally resulting in cirrhosis and hepatocellular carcinoma (HCC). In Taiwan, the prevalence of viral hepatitis has been high, and the prevalence of chronic infections was 15–20%. HBV carriers are at a substantially increased risk of HCC and liver-related death compared with people without HBV infection.4–6
Depression is a common disorder in patients with chronic hepatitis C virus (HCV) infection undertaking interferon therapy.7 Certain SSRIs have been reported to be safe and effective in treating such patients.8,9 In a study involving 783 HCV-infected patients, 63% had depression and 38% were on antidepressants, mostly SSRI.10 In addition, patients with cirrhosis were depressed more frequently, and were more likely to take antidepressants.11 A large section of the hepatitis population in Taiwan was possibly exposed to SSRI-based antidepressants; therefore both low and high risks associated with serious adverse effects such as HCC development should be evaluated with care. Current studies on SSRI use in HBV-infected patients, particularly on the association between SSRI use and the risk of HCC, are lacking. Furthermore, although many preclinical studies have been conducted, no definite conclusion has been reached on the association between serotonin and HCC. As we know, the use of tricyclic antidepressants and SSRIs was related to the lower risk for HCC.12 A recent larger case-control study showed the association between SSRI and decreased risk of HCC in a dose-dependent manner.13 However, no present study showed the association between HBV-infected patients and SSRI use.
For the present study, we aimed to determine the association between SSRI use and the risk of HCC in patients with chronic HBV infection in Taiwan by using the Taiwan National Health Insurance Research Database (NHIRD).
Methods
Data sources
We conducted a nationwide population-based cohort study by using data from the NHIRD. The National Health Insurance program in Taiwan was launched on March 1, 1995, by the National Health Insurance Administration (NHIA), and provided coverage to more than 23.03 million residents in Taiwan (approximately 99.2% of the population). The NHIA released identification-encrypted data to the National Health Research Institute (NHRI) to establish the NHIRD. The Longitudinal Health Insurance Database 2000 (LHID2000), used in this study, contains medical information of 1 million beneficiaries randomly sampled from the registry of all beneficiaries in 2000. Claims data in the LHID2000 were extended from January 1, 1996 to December 31, 2011. Age- and sex-related distributions in the original claims data and the sampled data do not differ significantly. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used for diagnoses.
The NHIRD contains admission and outpatient visit data, including information on patient characteristics, such as age; sex; date of admission, discharge, and outpatient visits; and 5 main discharge or 3 outpatient visit diagnoses (according to ICD-9-CM codes). In addition, the data files contain information on drug prescriptions, including the names of prescribed drugs and dosage. The data in the NHIRD are of high quality, and their validity has been proved in several studies.14–16
The NHRI scrambles patient identification and replaces it with surrogate numbers to ensure privacy. Furthermore, data confidentiality is maintained in accordance with NHIA and NHRI data regulations. Because the NHIRD contains de-identified secondary data for research, our study was exempted from the informed consent of participants. This study was approved by the Institutional Review Board of China Medical University (CMUH104-REC2-115).
Study population and outcomes
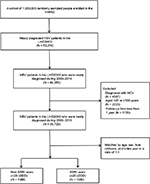
Patients aged ≥20 years and <100 years newly diagnosed with HBV infection (ICD-9-CM: 070.2, 070.3, and V02.61) between January 1, 2000 and December 31, 2010 were recruited. Patients with concurrent HCV infection (ICD-9-CM: 070.41, 070.44, 070.51, 070.54, 070.7, and V02.62), those with newly diagnosed HCC (ICD-9-CM: 155.0) within 1 year of HBV infection, those with a history of malignancy diseases including HCC, and those with a follow-up duration <1 year or with missing data were excluded. HCC cases were identified and confirmed through the registration in the Catastrophic Illness Patient Database, a subset of the NHIRD.17–19
The initiation date of SSRI use in HBV-infected patients was defined as the index date for the study cohort. For comparison, a random date was assigned to the patients in the comparison cohort for matching, with the same period in years between the new diagnosis of HBV infection and index date.
SSRI exposure
The SSRIs of interest in this study were citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline. We identified patients who received SSRI prescriptions in the outpatient visit from January 1, 2000 to the date of HCC diagnosis or the end of follow-up. The defined daily dose (DDD) recommended by the World Health Organization is a unit for measuring a prescribed drug amount. DDD is the assumed average maintenance dose per day of a drug consumed for its main indication in adults. We can compare SSRIs while maintaining the same standard by using the following formula: (total amount of drug) / (amount of drug in a DDD) = number of DDDs. Cumulative DDD (cDDD), indicating the total exposed dosage, was used to estimate the sum of dispensed DDD of any SSRI. Tsan et al used this definition to investigate the association between statins and HCC risk in patients with HBV and HCV infections.15,16 To examine the dose-responsive relationship, we categorized the cumulative dose of SSRI into 4 groups (<28, 28–89, 90–364, and ≥365 cDDD) because the duration of the refill card in Taiwan was 3 months. Patients with <28 cDDD SSRI use during the study period were defined as non-SSRI users. In this study, the SSRI cumulative doses were categorized into 3 groups (28–89, 90–364, and ≥365) and put into the linear regression model as continuous variables to estimate the p-value for trend.
Potential confounders
We systematically identified the potential confounders for HCC by referring to the ICD-9-CM codes in the claims data: alcohol-related disease (ARD) (ICD-9-CM: 291, 303.0, 303.9, 305.0, and 571.0–571.3), liver cirrhosis (ICD-9-CM: 571.2, 571.5, 571.6, 572.2–572.4, 572.8, and 573.0), hypertension (HTN) (ICD-9-CM: 401–405), hyperlipidemia (ICD-9-CM: 272.0–272.4), biliary stones (ICD-9-CM: 574), chronic kidney disease (ICD-9-CM: 581–584, 586–588, 403, 404, and 285.21), diabetes mellitus (DM) (ICD-9-CM: 250, 357.2, 362.01, 362.02, and 366.41), ischemic heart disease (IHD) (ICD-9-CM: 411–414), congestive heart failure (CHF) (ICD-9-CM: 428), and peripheral arterial occlusive disease (PAOD) (ICD-9-CM: 440–444). Diagnoses given ahead of or in concurrence with the diagnosis of HBV infection were considered as underlying comorbidities. Many agents have exhibited positive results in chemoprevention for HCC.16,19,20 These potential drug confounders included antivirals (adefovir, entecavir, lamivudine, telbivudine, and interferon), statins, metformin, and aspirin.
Statistical analyses
Differences in demographic characteristics and comorbidities between the study and comparison cohorts were examined using the chi-square and 2-sample t-tests. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated for each variable using Cox proportional hazards regression. Differences in the incidence of HCC development between different SSRI consumption groups (<28, 28–89, 90–364, and ≥365 cDDD) were estimated using Kaplan-Meier curves by performing the log-rank test. Statistical analyses were conducted using the SAS 9.4 statistical package (SAS Institute Inc., Cary, NC, USA), and p<0.05 was considered statistically significant.
Results
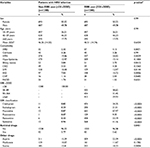
We recruited 2760 patients (1374 men and 1386 women) newly diagnosed with HBV infection during the study period. The study cohort comprised 1380 SSRI users who met the inclusion criteria. After frequency matching by age, sex, liver cirrhosis, and index date, an equal number of non-SSRI users comprised the comparison cohort. Figure 1 shows the selection process of participants in the study and comparison cohorts. The mean age of patients was 46.51 ± 14.74 years and 46.26 ± 14.33 years in the study and comparison cohorts, respectively. The basic demographic characteristics of the study and comparison cohorts are listed in Table 1. The percentage of comorbidities including ARD, HTN, DM, and IHD was higher in the study cohort compared with the comparison cohort. The incidence rate of HCC was 1.28 and 3.51 per 1000 person-years for the study cohort and comparison cohort, respectively. The median duration between the first diagnosis of HBV and HCC was 9.52 and 8.25 years for the study and comparison cohorts, respectively.
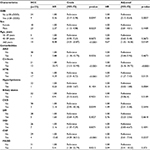
In univariate analyses, covariates such as the male sex, age ≥60 years, ARD, cirrhosis, HTN, IHD, CHF, and PAOD increased the risk of HCC (Table 2). Conversely, SSRI use reduced HCC risk (HR = 0.36; 95% CI, 0.17–0.78; p = 0.0097). In multivariate analyses using Cox proportional hazards regression, SSRI use exerted a significant protective effect on HCC after adjusting for the potential confounders of age, sex, and all comorbidities (adjusted HR = 0.28; 95% CI, 0.12–0.64; p = 0.0027).
Dose-responsive relationship
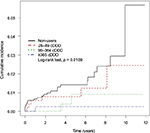
A dose-responsive relationship was noted between SSRI use and HCC risk. For SSRI users with a cDDD of 28–89, 90–364, and ≥365, the adjusted HRs were 0.51, 0.22, and 0.12, respectively, (95% CI, 0.21–1.25, 0.05–0.94, 0.02–0.90, respectively) compared with non-SSRI users (<28 cDDD). Kaplan-Meier curves of the 4 cDDD groups for the study and comparison cohorts, estimated by conducting the log-rank test, differed significantly (Figure 2). Furthermore, the difference was directly proportional to the follow-up duration in the 4 groups. The sensitivity analysis adjustments had little effect on the estimates of the association between SSRI use and HCC risk in different models (Table 3). The SSRI effect was dependent on the dose-responsive relationship in each cDDD subgroup within different adjustment models for metformin, statins, and aspirin (Table 4).
Sensitivity analysis
Because of the small number of HCC events in the cohort study, we further conducted a sensitivity analysis by using case-control study design. The HCC subjects were retrieved from the catastrophic illness database of NHIRD, which had been certificated in many studies.16 A total of 9070 HCC subjects and an equal number of control non-HCC subjects were analyzed after matching for age and sex. The demographic and baseline comorbidities are shown in Table S1, which includes the medications including SSRI, antiviral drugs, statins, and metformin.
In the logistic regression model, univariate analysis showed an odds ratio (OR) of 0.70 (95% CI 0.59–0.82, p<0.0001) for SSRI use in HCC occurrence. After adjusting for age, sex, comorbidities, and medications, the adjusted OR was 0.60 (95% CI 0.48–0.75, p<0.0001), as shown in Table S2. The further analysis showed that the SSRI presented with a dose-response protective effect for HCC in the multivariate analysis (28 cDDD, adjusted OR=0.73 [95% CI 0.51–1.05, p=0.0904]; 28–89 cDDD, adjusted OR=0.58 [95% CI 0.38–0.88, p=0.0099]; 90–364 cDDD, adjusted OR=0.43 [95% CI 0.27–0.69, p=0.0004]; ≥365 cDDD, adjusted OR=0.63 [95% CI 0.39–1.03, p=0.0654]), and p for trend <0.0001, as showed in Table 5.
Discussion
The SSRIs exerted a protective effect on HCC development in HBV-infected patients in a dose-responsive manner after adjusting for potential confounders, including underlying comorbidities and miscellaneous medication (antiviral drugs, metformin, statins, and aspirin), no matter if in cohort study or case-control study designs.
Some studies discussed the relationship between cancer and SSRI use. Coogan et al reported that SSRI exposure reduced the risk of colorectal cancer.21 One nationwide study in Finland reported that SSRI use with high cumulative dose resulted in higher risk of breast cancer. But there was no proved association between SSRI use and HCC development.22 However, in our study, we found a protective effect of SSRI on HCC development and this presented in a dose-responsive manner.
In the cell line studies, the effect of SSRIs on HCC is highly controversial. Several studies have reported the protective effect of SSRIs on HCC development. Chen et al indicated that sertraline induced apoptosis in HepG2 cells via the tumor necrosis factor-mitogen-activated protein 4 kinase 4-Jun N-terminal kinase signaling pathway.23 Mun et al reported that fluoxetine exhibited apoptotic effects against Hep3B cells through the loss of matrix metalloproteinase, reactive oxygen species (ROS) formation, and the modulation of mitogen-activated protein kinase activities.24 Kuwahara et al reported on the anti-tumor effects of SSRIs in human HCC HepG2 cells.25
By contrast, several studies have reported the association between SSRIs and HCC. Two major mechanisms contribute to the pathogenesis of SSRI-related HCC development. First, SSRIs exert direct carcinogenic effects on the liver. Second, SSRIs accelerate liver cirrhosis through fibrosis and steatohepatitis formation, and therefore exacerbate HCC development indirectly.
Regarding the direct effects, Liang et al reported that serotonin promoted the proliferation of serum-deprived HCC cells through the up-regulation of fork head box O3a.26 Soll et al also reported that serotonin promoted the growth of human HCC.27 Regarding the indirect effects of SSRIs, Ruddell et al reported that serotonin fostered liver fibrosis by stimulating stellate cells.28 Ebrahimkhani et al indicated that serotonin exacerbated fibrosis by promoting transforming growth factor β1 production.29 In a murine model of diet-induced steatohepatitis, Nocito et al reported that serotonin increased the production of ROS and lipid peroxides, leading to inflammation and mitochondrial damage, and ultimately, hepatocyte damage.30
Strengths
This study had several strengths. The study cohort was collated using data from a computerized database on randomly sampled HBV-infected patients from all the HBV-infected patients in Taiwan, thus eliminating the possibility of selection bias. In addition, because the data on SSRIs and other medication use were obtained from a historical database from which all the prescription information for the study period were available, the possibility of recall bias can be eliminated. We also clarified possible confounders from different medications. Furthermore, we conducted a sensitivity analysis by using case-control study design. The results showed that SSRI use had a potential protective effect on HCC development in a dose-responsive manner.
Limitations
Our study had potential limitations. First, we did not obtain any detailed information such as liver ultrasound examination reports or laboratory data such as viral loads. Several unmeasured confounders, including body mass index, alcohol intake, and over-the-counter drug use, which are associated with HCC, are unavailable in the database. However, we used hyperlipidemia and ARD to substitute for obesity and alcohol consumption, respectively, for the adjustment of such potential confounders. Third, we could not verify the exact dosage that the study participants actually took. We presumed that all medications were taken by patients as prescribed, and this may result in overestimating the actual ingested dosage because some degree of non-compliance is always expected. Finally, the sample seems small because of the strict inclusion criteria and matching methods between the study and comparison cohorts. Therefore, we conducted a sensitivity analysis by using case-control study design for the few events of HCC in the cohort study. The results were similar to our cohort study. However, the limited number of patients in the study and comparison cohorts made the documented protective effect of statins and metformin in other studies appear non-significant.15,16 If this study could be conducted using a cohort of all HBV-infected patients from the NHIRD, in a similar manner to other studies, instead of random samples of 1 million beneficiaries, the protective effect of SSRIs on HCC development could become more evident.15
Conclusion
SSRI use may possibly reduce the risk of HCC in HBV-infected patients in a dose-responsive manner. A further understanding of the underlying mechanisms and studies with a large sample size are warranted.
Acknowledgment
This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW106-TDU-B-212-113004), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10601010036), Taiwan Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in the study design, data collection, analysis, decision to publish, or manuscript preparation. No additional external funding was received for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.