Back to Journals » Open Access Journal of Clinical Trials » Volume 10
Selective enhancement of focused attention by Alpinia galanga in subjects with moderate caffeine consumption
Authors Srivastava S
Received 3 February 2018
Accepted for publication 11 May 2018
Published 6 September 2018 Volume 2018:10 Pages 43—49
DOI https://doi.org/10.2147/OAJCT.S164450
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Arthur E. Frankel
Video abstract presented by Shalini Srivastava.
Views: 889
Shalini Srivastava
Enovate Biolife, Wilmington, DE, USA
Introduction: The purpose of the secondary analysis of the data from the clinical trial “A Study to Evaluate Efficacy of IP on Alertness and Mental Fatigue” (ClinicalTrials.gov NCT02816827) was to investigate the effect of the Alpinia galanga proprietary extract E-AG-01 (EnXtra) on focused attention, in comparison with caffeine and placebo in moderate caffeine habitués.
Participants and methods: A total of 59 caffeine-habituated healthy young adults (aged 18–40 years) with body-mass index of 18.5–25.0 kg/m2 were crossed over in four interventional groups: placebo, E-AG-01, caffeine, and a combination of caffeine and E-AG-01. All participants completed the attention-network test, which measures the accuracy and speed factors of the alerting, orienting, and executive-control networks.
Results: The results of accuracy parameters in terms of percentage-error rate showed a remarkable difference between E-AG-01 and the other treatment groups, wherein the error rate dropped by 1.63% (1 hour), 1.32% (3 hours), and 0.78% (5 hours) from baseline. The caffeine group demonstrated a decrease of 0.37% (1 hour) and 0.44% (3 hours), followed by an increase of 0.2% (5 hours), whereas the error rate of subjects in the caffeine + E-AG-01 group decreased by 0.24% (1 hour) and 0.26% (3 hours), followed by an increase of 0.2% at 5 hours. The placebo group exhibited an increase of 0.14% (3 hours) and 0.77% (5 hours).
Conclusion: These results show that E-AG-01 exhibited selectively enhanced focused attention to a higher extent in comparison with caffeine and placebo.
Keywords: accuracy, ANT, dietary supplement, error rate, energy drink, EnXtra, clinical trial
Introduction
In the modern world, with its myriad electronic and nonelectronic distractions, paying attention to the task at hand becomes extremely difficult. Maintaining focus, especially on something monotonous, often seems to require an inordinate amount of brainpower. These lapses in focus can have negative consequences, resulting in accidents and hampered productivity. As such, the use of attention-enhancing supplements to achieve a vital mental edge is hitting the ceiling.
In psychology, attention is defined as a behavioral and cognitive process of concentrating selectively on a discrete aspect of information, whether deemed subjective or objective, while ignoring other perceivable information.1 Personal capacity of focused attention on pertinent information, while ignoring that deemed irrelevant, has become a necessity in everyday life. Attention networks are specifically and vitally involved in the regulation of focused attention. Alertness, which is a prime component of the attention network, is defined by the American Psychological Association as achieving and maintaining a state of high sensitivity to incoming stimuli. To state it in simple terms, it is the state of being awake, aware, attentive, and prepared to act or react.
Alpinia galanga, a common culinary spice, has been traditionally used for the treatment of various diseases, due to its anti-inflammatory, analgesic, hypoglycemic, antiallergenic, antimicrobial, gastroprotective, antioxidant, antiplatelet, anticancer, and immunomodulatory properties. The nootropic effect of A. galanga has also been well explored;2 however, EnXtra, a proprietary extract of A. galanga (E-AG-01), has been proven in a clinical study to be an efficient psychostimulant.3 In this randomized, placebo-controlled crossover study, effect of EnXtra on the attention network was studied using Fan et al’s attention-network test (ANT).4 The ANT is a widely used tool in psychophysiology to understand attention processes (alerting, orienting, and executing effects). The study demonstrated that administration of caffeine (200 mg) led to a rise in mental alertness; however, the effect was observed to decline within 3 hours, whereas E-AG-01 demonstrated prolonged alertness, lasting throughout the duration of the study, ie, 5 hours. However, the paper did not explain the role of EnXtra in improving mental acuity by using error rate as a parameter for the participants.
American Psychological Association has defined focus as “the attention concentrated on certain stimuli in the environment and not others, enabling important stimuli to be distinguished from peripheral or incidental ones.” For example, it is really important for participants engaged in sports to concentrate on performance-enhancing cues and avoid irrelevant cues. To elucidate further by an analogy, a tennis player should focus primarily on the position of the ball and be capable of avoiding other irrelevant cues, such as hanging placards and banners.
ANT helps to analyze an individual’s ability to distinguish important stimuli, and its results help identify correctly placed cues, which otherwise results in error. Based on this fact, we decided to evaluate the effect of E-AG-01 on the accuracy factor of attention via a supplemental data analysis on error rate of the same study, which was not included in the designed statistical analysis plan of the study. The intention was to explore the role of E-AG-01 in improving mental sharpness by focusing on these relevant cues and decreasing the number of errors during the test.
Participants and methods
This was a randomized, double-dummy, double-blind, placebo-controlled, crossover study that was designed, conducted, analyzed, and reported in accordance with laid ethical guidelines (Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice [ICH-GCP]), in order to maintain the authenticity and credibility of the data. The study was reviewed by an independent ethics committee (Aditya Ethics Committee, Ahmedabad, India; registered with the Office for Human Research Protections, US Department of Health and Human Services; IRB00006475) and approved on February 9, 2016. This clinical trial was registered in the trial registry before enrollment of the participants (ClinicalTrials.gov NCT02816827). The study was initiated on March 2, 2016, and the last subject’s last visit occurred on August 24, 2016.
Study population
Potential volunteers were contacted through multiple sources, including an in-house “healthy volunteers” database and a consumer-group survey agency. Informed consent to participate in the study was signed by each participant screened. The signed informed consent also authorized the study investigator to release relevant protected health information collected during the study. Privacy rights of participants were observed throughout the study. All subjects were screened for demographic parameters (age, sex, height, weight, and body-mass index) at the screening visit to confirm compliance with the protocol. Baseline characteristics of the population are presented in Table 1. Healthy nonsmoking caffeine-habituated male and female teetotalers 18–40 years old with at least minimal computer literacy were enrolled in the study. Caffeine-consumption history was recorded to ensure that participants were acquainted with caffeine’s stimulant effects and were not caffeine-sensitive. Subjects with body-mass index 18.50–25.0 kg/m2, resting blood pressure ≤140/90 mmHg, and alertness score (ANT version 1.3.0) of 50±20 ms at screening visit and subsequent study visits were considered eligible. Subjects with a Generalized Anxiety Disorder (GAD)-7 score ≥7 and Patient Health Questionnaire (PHQ)-9 score ≥14 were excluded, as higher scores are associated with mood disturbances and clinical conditions known to impact study end points.5,6 Pregnant or breast-feeding females were excluded, and those currently in their menstrual period were included only after the last day of menstrual flow. Females consuming oral contraceptives were included in the study only after switching to barrier contraception and a washout period of 7 days from the last dose of oral contraception. Any concomitant therapy was strictly prohibited during the course of the study. All subjects were provided with a demonstration of study procedures and relevant instructions. Informed consent to participate in the study and authorization for release of relevant protected health information to the study investigator were obtained from each of the enrolled subjects.
  | Table 1 Baseline demographic characteristics (n=59) Abbreviations: BMI, body-mass index; PHQ, Patient Health Questionnaire; GAD, Generalized Anxiety Disorder (scale). |
Interventions
Subjects were divided into four treatment arms and randomized (based on the randomization chart in SPSS version 10.0) for allocation to one of the interventional products (IPs) on each study visit, and a similar trend was followed for subsequent visits. The remaining interventions on consecutive visits were administered only after a sufficient washout period of not less than 5 half-lives of caffeine in the blood to avoid a carryover effect. IPs included placebo, A. galanga proprietary extract (E-AG-01), caffeine, and a combination of E-AG-01 with caffeine (composite). All treatments were administered to subjects in the form of capsules that were identical in appearance and packed in duly labeled high-density polyethylene bottles. Blinding codes were secured at the site in tamper-evident sealed envelopes with no access to the study team. As such, the double-blind nature of the study was ensured and strictly followed. Subjects receiving only A. galanga, caffeine, or placebo were coassigned to an additional placebo capsule to achieve a double-dummy design identical to the caffeine + E-AG-01 regime. The composition details of all IPs are listed in Table 2. Gastronomic use of A. galanga has generally been well documented in the form of spice. It has also been reported to improve cognitive performance in animals; however, the current study aimed to explore the role of this A. galanga rhizome extract in decreasing errors made during high-demand cognitive tasks. The study used a water-soluble, methyl eugenol-free extract standardized for polyphenols, flavonoids, polysaccharides, and pyrocatecollic-type tannins. Pure anhydrous caffeine (99.7%) was supplied by Shri Ahimsa Mines and Minerals (Jaipur, India).
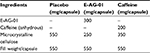  | Table 2 Composition of treatments |
Test-visit procedure
Subjects reported to the clinic during morning hours, with testing beginning early in the day (8–9 am) for each visit to avoid the influence of daily challenges (related to mental and physical stress) on study outcomes. Also, the time of day was matched for all four visits to reduce variability in response due to diurnal patterns. Subjects reported to the clinic following 24-hour abstinence from caffeine-containing products or any psychostimulants prior to all study visits. Subjects were also instructed to obtain sufficient sleep during the night prior to testing, which was confirmed by an updated sleep diary. Upon arrival at the clinic, subjects were asked to relax for 15–20 minutes, after which vital parameters were measured and a standardized meal of ~200 ca was provided to control variations from possible confounding dietary factors. Baseline data were collected at 30 minutes postbreakfast, followed by the administration of an IP, wherein one dose of the product was administered to the subject by a trial coordinator at the investigational site.
The study tool was administered at 1, 3, and 5 hours post-IP administration and data collected. No other food or calorie-containing beverages were provided during this period. Subjects were allowed to drink water as desired and relax in an isolated room at a comfortable temperature with free access to a computer or magazines during the clinic stay. Any psychostimulating activity was strictly prohibited during this period. To analyze the effect of the IP on sleep architecture, a second dose of the corresponding IP was supplied in a labeled bottle to be taken before dinner during the same night of the visit, and subjects were asked to record all details pertaining to sleep quality and duration in the sleep diary. To ensure treatment compliance, product accountability was monitored by a clinical research coordinator on the following visit. Vital parameters (heart rate and pulse rate) were monitored for each subject to explore the safety of the intervention in the study population. Any adverse event encountered during the study was planned to be reported as per the ICH-GCP guidelines.
Study tool
The ANT was developed in 2002 by Fan et al, and is based on a well-developed neural network model of the human attention system.6 The test is a measure of attention designed to quantify the efficiency of vigilance, orienting, and executive-control networks through the combination of a cued reaction-time task7 and a flanker task.8
As a speed-choice task, ANT provides two measures of performance: (a) response time, which is indicative of response speed and, (b) error rate, which suggests task accuracy. Based on collective information from these scores, inter-network and within-network correlations can be established to examine network performance for the presence of a speed–accuracy tradeoff. The ANT has been widely used to assess mental performance in both healthy and diseased populations.5,9 Participants were seated in a silent and secluded room. All external distractions were avoided, and participants were asked to give complete attention to the task at hand. They were instructed to press a button as accurately and quickly as possible to identify the direction of the target, which was a leftward or rightward arrow at the center and flanked on either side by two arrows in the same direction (congruent condition) or the opposite direction (incongruent condition). The target and flankers were presented until the participant made a response or 2,000 ms elapsed. A cue (an asterisk) was presented for 200 ms before the target appeared. The task used three cue conditions: no cue (baseline), center cue (at the fixation for alerting), and spatial cue (at the target location for alerting and orienting).
Statistical analysis
A supplemental poststudy analysis was performed on the ANT data, wherein the effect of E-AG-01 on the second performance measure, ie, accuracy was analyzed in terms of change in error rate. It was calculated based on percentage correct and incorrect hits, and percentage error rate was determined from the average wrong responses registered at 1, 3, and 5 hours postingestion of E-AG-01, caffeine, a combination of the two, or placebo. Student’s paired t-test was used for intergroup comparisons at different time points using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA). The effect of IPs on accuracy factors of attention, ie, focused and selective attention, served as the ultimate aim for this analysis.
Results
Of 124 participants screened for the study, 59 meeting the protocol-defined inclusion criteria were enrolled in the study. The study flowchart is depicted in Figure 1. Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Screening (GAD-7) scores indicated that the subjects did not have any mental health concerns, such as anxiety or depression. The error rate per second in E-AG-01 group declined from 4.48 (0.85%) at baseline to 2.85 (0.36%), exhibiting a decrease of 1.63% at 1 hour. The error rate remained low: 1.32% and 0.77% from baseline at 3 and 5 hours, respectively. This decrease in error rate was remarkable and consistent for a period of 5 hours compared to placebo, which demonstrated almost no change at 1 hour and an increase in error rate at 3 (0.14%) and 5 hours (0.77%). The caffeine group demonstrated a decrease of 0.37% (1 hour) and 0.44% (3 hours) and subsequently an increase of 0.2% (5 hours), whereas the error rate of subjects in the caffeine + E-AG-01 group decreased by 0.24% and 0.26% at 1 and 3 hours, respectively, followed by an increase of 0.2% at 5 hours. Changes in error rate are presented in Table 3. Therefore, the reduction in error rate in response to neutral, incongruent, and congruent flanker stimuli led to an increase in facilitation after the consumption of E-AG-01. These observations revealed higher mean accuracy and a significant reduction in error rate in the E-AG-01 group in comparison with the remaining study groups.
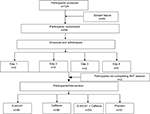  | Figure 1 Participant flowchart. Abbreviation: ANT, attention-network test. |
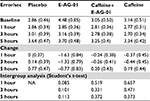  | Table 3 Error rate Abbreviation: NA, not applicable. |
Discussion
The present placebo-controlled clinical trial3 evaluated the psychostimulant effect of E-AG-01 alone and in combination with caffeine. Based on the results of predetermined efficacy outcomes, it was concluded that the stimulant effect of E-AG-01 was indeed superior to caffeine, with a novel finding of its capacity to relieve the caffeine-withdrawal effect. Attention is a vast paradigm that involves several subcomponents, such as arousal (activation level and level of alertness), focused attention (ability to focus attention on a stimulus), sustained attention (ability to attend to a stimulus or activity over a long period), selective attention (ability to attend to a specific stimulus or activity in the presence of other distracting stimuli), alternating attention (ability to change focus between two or more stimuli), and divided attention (ability to attend to different stimuli or attention at the same time).10 The most enduring outcome of focused attention is the extent to which distractions can be prevented, resulting in lower error rate and increased accuracy.
When treated with placebo, participants showed a rise in error rate, a pattern that is commonly observed with increasing time on tasks. As expected, this group had a persistent error rate at 1 hour and gradually increased, reaching 27.3% over baseline at the end of 5 hours. We observed that after ingestion of 300 mg E-AG-01, subjects were consistently able to reduce the error rate for the next 5 hours compared to baseline. The decrease in error rate was 29.46% and 17.4% from baseline at 3 and 5 hours, respectively. The error rate in the caffeine group initially decreased at 1 hour, but then following the phenomenon of caffeine “crash”, error incidence almost tripled in magnitude (–14.0% to +6.4%) by 5 hours, owing to the impending caffeine crash. When combined, EnXtra + caffeine did not impact the error rate as significantly as stand-alones (decrease of 7.9% [1 hour] and 8.6% [3 hours], with an increase of 6.6% by 5 hours); however, the combination remained effective in averting the caffeine crash, in a similar way as that demonstrated by the previous published study.3 Figure 2 summarizes the percentage change in error rate from baseline.
  | Figure 2 Change in error rate. |
The observation on the caffeine arm is in coherence with that reported by Foxe et al,11 who analyzed the effect of caffeine and theanine on vigilance during sustained attention via a response-inhibition task.
Comparing with theacrine, which has been extensively analyzed for its mild stimulant and calming effects, a similar study assessed the effect of theacrine on reaction time in comparison with caffeine. The findings concluded that neither theacrine nor caffeine improved reaction time in a statistically significant manner, though subjective improvement in alertness and focus was observed. In addition, it has been reported that such positive effects on response time and accuracy can favorably influence complex decision-making and ability to solve problems.12,13 As such, applying the same analogy, it can be postulated that E-AG-01 can be beneficial in improving speed and accuracy during performance tasks that demand quick judgment and problem resolution.
Also, caffeine has been studied extensively with electroencephalography to prove that it is capable of improving vigilance by reducing tonic α-band activity in the frontoparietal region up to 85 minutes after its ingestion.14,15 Similarly, it can be postulated that EnXtra might be enhancing focused and selective attention by reduction in α-band power in the associated cerebral regions. This postulation can be confirmed by a prospective imaging-based investigation.
Generally, evaluation of a study outcome should be done in terms of both clinical relevance and statistical significance. Where the end point for interventional and placebo arms lacks sufficient statistical difference (as in the current analysis), the physiological relevance of the result should be considered as to whether there is a meaningful impact of the intervention on day-to-day life. Taking this fact into account, it can be inferred that the changes in error rate in the E-AG-01 group (–29.46% [3 hours] and –17.4% [5 hours]) and the placebo group (4.9% [3 hours] and 26.9% [5 hours]) are certainly clinically relevant. As the error rate correlated inversely with mental performance, these results further confirm the superior effect of E-AG-01 over placebo.
As mentioned in the previous publication of our pilot screening study (Sivanandan and Pimple, unpublished data, 2018), E-AG-01, a water-soluble A. galanga extract, has shown capability to improve mental alertness. Also, as evident from the current data, enhanced focused attention following EnXtra administration may lead to better information-processing speed, which could be due to increased stimulating-neurotransmitter levels16 and enhanced cell signaling by the water-soluble phytoconstituents of A. galanga. Therefore, it can be postulated that EnXtra may help enrich an individual’s cognitive functions as well. This indeed serves as an efficacy outcome for prospective studies.
Conclusion
This supplemental analysis clearly demonstrates an improvement in task accuracy, as evidenced by the sustained attenuation of error rate after consumption of E-AG-01. Also, this beneficial effect was exhibited without any response speed–accuracy tradeoff (highest increase in alertness score proven in earlier published results). Therefore, it can be inferred that the consumption of E-AG-01 leads to faster and more focused processing of relevant information with an improvement in the brain’s ability to concentrate for longer periods of time, rendering it efficient during demanding tasks.
Data sharing statement
Data is confidential, however, can be provided upon request. Enovate Biolife grants permission to the journal to share minimal raw data (raw-data sheet) with interested researchers after an indication to the corresponding author.
Acknowledgments
We are thankful to Mr Jin Fan4 for granting us the permission to use the standardized ANT software for our research purpose. We thank Dr Mark S Mennemeier for providing his scientific input on study conceptualization and design. We further extend our thanks to the study team of Vedic Life Sciences for their work in the study and assistance in data collection. We also thank Mr Jayesh Chaudhary and Mrs Rekha Patel for their interest in this study and for granting permission to publish. The authors are grateful to Enovate Biolife for financial support. We would also like to thank Dr Surekha Pimple for her substantial involvement in manuscript preparation.
Author contributions
By submitting this manuscript, the author takes full responsibility for the integrity and accuracy of research, adherence to ethics standards, including the approval of the study by the ethics committee, respect of copyright, and absence of plagiarized ideas, text, and graphics. The study was conceptualized and designed by SS. The manuscript was prepared and refined through the efforts of the listed author. SS agrees to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are investigated appropriately and resolved. SS read and approved the final manuscript for publication.
Disclosure
Enovate Biolife funded the trial, provided the investigational products, and approved the manuscript submission to this journal. E-AG-01 is an A. galanga proprietary extract from Enovate Biolife, commercially available as EnXtra. Enovate Biolife provides support in the form of a salary to SS who holds a full-time position with the organization. The author reports no other conflicts of interest in this work.
References
Anderson JR. Cognitive Psychology and Its Implications. 6th ed. Basingstoke, UK: Worth Publishers; 2004. | ||
Saha S, Banerjee S. Central nervous system stimulant actions of Alpinia galanga (L.) rhizome: a preliminary study. Indian J Exp Biol. 2013;51(10):828–832. | ||
Srivastava S, Mennemeier M, Pimple S. Effect of Alpinia galanga on mental alertness and sustained attention with or without caffeine: a randomized placebo-controlled study. J Am Coll Nutr. 2017;36(8):631–639. | ||
Fan J, Mccandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–347. | ||
Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev Cogn Neurosci. 2014;7:13–22. | ||
Najmi S, Amir N, Frosio KE, Ayers C. The effects of cognitive load on attention control in subclinical anxiety and generalised anxiety disorder. Cogn Emot. 2015;29(7):1210–1223. | ||
Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. | ||
Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Atten Percept Psychophys. 1974;16(1):143–149. | ||
Xiao M, Ge H, Khundrakpam BS, et al. Attention performance measured by attention network test is correlated with global and regional efficiency of structural brain networks. Front Behav Neurosci. 2016;10:194. | ||
Macleod JW, Lawrence MA, Mcconnell MM, Eskes GA, Klein RM, Shore DI. Appraising the ANT: psychometric and theoretical considerations of the attention network test. Neuropsychology. 2010;24(5):637–651. | ||
Foxe JJ, Morie KP, Laud PJ, Rowson MJ, de Bruin EA, Kelly SP. Assessing the effects of caffeine and theanine on the maintenance of vigilance during a sustained attention task. Neuropharmacology. 2012;62(7):2320–2327. | ||
Habowski SM, Sandrock JE, Kedia AW, Ziegenfuss TN. The effects of Teacrine, a nature-identical purine alkaloid, on subjective measures of cognitive function, psychometric and hemodynamic indices in healthy humans: a randomized, double-blinded crossover pilot trial. J Int Soc Sports Nutr. 2014;11 Suppl 1:P49. | ||
Kuhman DJ, Joyner KJ, Bloomer RJ. Cognitive performance and mood following ingestion of a theacrine-containing dietary supplement, caffeine, or placebo by young men and women. Nutrients. 2015;7(11):9618–9632. | ||
Dager SR, Friedman SD. Brain imaging and the effects of caffeine and nicotine. Ann Med. 2000;32(9):592–599. | ||
Dimpfel W, Schober F, Spüler M. The influence of caffeine on human EEG under resting conditions and during mental loads. Clin Investig. 1993;71(3):197–207. | ||
Spencer JP. The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev. 2009;38(4):1152–1161. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
