Back to Journals » Biologics: Targets and Therapy » Volume 10
Selective biologics for ulcerative colitis and Crohn's disease - clinical utility of vedolizumab
Received 20 October 2015
Accepted for publication 23 November 2015
Published 9 March 2016 Volume 2016:10 Pages 33—52
DOI https://doi.org/10.2147/BTT.S71679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wei-Qun Ding
Jill MV Petkau, Bertus Eksteen
Snyder Institute for Chronic Diseases, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Abstract: Inflammatory bowel disease (IBD) encompasses a cluster of different disease phenotypes which are broadly classified into ulcerative colitis and Crohn's disease. Disease pathogenesis is driven by abnormal host immune responses to their resident gut microbiome in genetically susceptible individuals. Clinical disease features and outcomes are heterogenous and not unexpected as over 163 genetic loci are associated with disease susceptibility, and there are great variability in environmental exposures. Despite this variability, there has been relatively few efficacious therapies for particularly moderate-to-severe IBD. Treatment has been dominated by antitumor necrosis alpha agents with significant success but equally potentially serious adverse events. Therapeutic targeting of leucocyte trafficking has emerged as a viable alternative therapy, with vedolizumab being the lead compound. This review focuses primarily on its biological function as a selective gut immunotherapy, its safety and efficacy, and its emerging role as a mainstream therapy in managing IBD.
Keywords: adhesion molecule antagonist, anti-α4β7 integrin, inflammatory bowel disease, leukocyte trafficking, monoclonal antibody, selective gut immunotherapy, tumor necrosis factor alpha
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC), collectively referred to as inflammatory bowel disease (IBD), are chronic inflammatory conditions of the gastrointestinal (GI) tract resulting from an inappropriate immunological response to trigger(s) in the genetically susceptible host’s environment and microbiome. The inflammation of CD is transmural and discontinuous, affecting any portion of the digestive tract, whereas UC is characterized by superficial colonic inflammation that progresses in a continuous manner proximally from the rectum. Diarrhea, blood in the stool, abdominal cramping and pain, fatigue, weight loss, and malnutrition are hallmark symptoms of IBD, often associated with extra-intestinal manifestations involving the joints, skin, eyes, and liver.
IBD affects more than 233,000 Canadians (equating to one in 150 Canadians), with more than 10,000 new cases diagnosed each year;1 it is as common as type 1 diabetes and epilepsy, and twice as common as multiple sclerosis and Parkinson’s disease.2 The incidence and prevalence rates of CD and UC in Canada are reported to be among the highest in the world,1,3 and the rate of pediatric-onset IBD diagnoses continues to significantly increase.1 Canadian statistics align with global trends of increasing incidence and prevalence of IBD in developed and developing countries.3 IBD affects approximately 1.7 million Americans (estimated 780,000 with CD; estimated 907,000 with UC), with an incidence rate of 10.7 per 100,000 persons for CD and 12.2 per 100,000 persons with UC.4,5 The annual incidence of CD and UC in Europe was reported as 12.7 per 100,000 person-years and 24.3 per 100,000 person-years, respectively, with a reported CD prevalence of 322 per 100,000 persons and UC prevalence of 505 per 100,000 persons.3 The incidence and prevalence rates of both CD and UC in Asia reflect a rising trend, correlating with an increase in industrialization in this area of the world that historically has not experienced significant IBD.3 The financial burden of IBD is substantial, with direct medical costs in excess of CAD $1.2 billion and indirect costs approximating CAD $1.6 billion in 2012.6 Health-related quality of life scores are reproducibly decreased in patients living with IBD compared with individuals without IBD.1,7–9
Given the significant global burden of IBD, there exists a need for safe and potent biological therapy options to effective treat individuals with moderate-to-severe phenotypes of UC and CD. There has been much speculation as to the potential role of vedolizumab (VDZ) for the treatment of UC and CD when the drug was still development.10,11 This review is intended to persuasively demonstrate that VDZ, an anti-α4β7 integrin antagonist, is an efficacious and safe alternative to tumor necrosis factor alpha (TNFα) antagonist treatment options for both UC and CD without the serious complication of progressive multifocal leukoencephalopathy (PML) previously associated with natalizumab.
Current IBD therapy paradigm
The goal of medical treatment in IBD is primarily focused on interrupting or modifying the aberrant immune response to induce and sustain mucosal remission so as to 1) minimize complications leading to hospitalizations and surgery; 2) modify extra-intestinal disease; and most importantly, 3) optimize quality of life.12,13
Mild-to-moderate UC and Crohn’s disease
Mild-to-moderate disease is most often satisfactorily managed with oral and/or rectal therapies, such as 5-aminosalicylic acid preparations, to induce and maintain remission. Induction of remission can also be achieved with pulsed corticosteroids (CSs), such as oral prednisone or budesonide, which are tapered over a number of weeks. Immunomodulators, such as thiopurines or methotrexate, may be required for remission maintenance in certain individuals.12
Moderate-to-severe UC and Crohn’s disease
More severe disease phenotypes will often require frequent courses of CSs (steroid-dependent) or may fail to respond appropriately to steroid therapy (steroid refractory), requiring an escalation to biological therapies (with or without immunomodulators) or progression to surgery.12,13 Although there has been an observed global decrease in the surgical rate for individuals with IBD that may be attributed to increased utilization of immunosuppressant and biologic therapies, and access to specialized IBD care,6,14,15 surgery will still be required for approximately 23%–45% of individuals with UC and up to 75% of individuals with CD.16
Biological therapies
Tumor necrosis factor alpha inhibition
The dysregulated immune response of IBD triggers activation of the innate immune system which in turn produces cytokines such as TNFα, and activation of the adaptive immune system with subsequent recruitment of pro-inflammatory T-cells and B-cells to the colon or small bowel. This consequently leads to a further amplification of pro-inflammatory cytokine release that bind to cognate receptors and activate intercellular pathways, such as JAK and STAT, to mediate tissue inflammation. Biological therapy directed toward the inhibition of anti-TNFα has been studied as a treatment modality for IBD since the early 1990s17 with the introduction of infliximab (Remicade®; Janssen Pharmaceutical, Beerse, Belgium).18 Since then, adalimumab (Humira®; AbbVie Corporation, North Chicago, IL, USA),19 golimumab (Simponi®; Janssen Pharmaceutical),20 and certolizumab pegol (Cimzia; UCB Inc.; Brussels, Belgium)21 have been approved for use in UC and/or CD treatment. These immunoglobulin G1 (IgG1) monoclonal antibodies (mAbs) or Fragment AB, F(ab′)2 bind to and neutralize soluble and transmembrane TNFα, thereby impeding TNFα-receptor binding, disrupting the TNFα inflammatory pathway, and modifying the cellular immune response.
In CD, infliximab (ACCENT 1;22 SONIC23) and adalimumab (CLASSIC I,24 CLASSIC II,25 CHARM26) were shown to be efficacious in inducing and maintaining sustained clinical response and clinical remission in moderately-to-severely active disease in adults who have not achieved an adequate response with conventional therapy, as well as pediatric patients (infliximab-REACH;27 adalimumab-Study M06-806 IMAgINE28). Additionally, infliximab demonstrated effectiveness in inducing mucosal healing and reducing corticosteroid use (ACCENT 1;22 SONIC23), and was shown to be a successful maintenance therapy for fistulizing CD (ACCENT 229) in this adult population. Table 1 summarizes the pivotal anti-TNFα studies conducted in CD.
Similarly, in UC, efficacious induction and sustained maintenance of clinical response and clinical remission in adults with moderately-to-severely active disease can be achieved with infliximab (ACT 1 and ACT 236) and adalimumab (ULTRA 1;37 ULTRA 238), and less robustly with golimumab (PURSUIT-SC;39 PURSUIT-M40). Infliximab was also shown to be effective in inducing mucosal healing and reducing corticosteroid use (ACT 1 and ACT 236). Infliximab is the only TNFα inhibitor to be approved for pediatric patients with moderately-to-severely active UC, due to its effectiveness in inducing and maintaining sustained clinical response, clinical remission, and mucosal healing (Study Peds UC41). Table 2 summarizes the pivotal anti-TNFα studies conducted in UC.
Complications of TNFα inhibitors
Safety profile and adverse events
Due to the nonselective and total body mechanism of action of TNFα inhibitors, they are associated with important safety issues. Medically significant adverse events that have been reported for the TNFα-blocking drug class in clinical trials, post-marketing registries, and pharmacovigilance systems include: serious infections (bacterial, mycobacterial, viral, parasitic, invasive fungal, and other opportunistic infections), tuberculosis (new infection or reactivation of latent infection), lymphoma (including hepatosplenic T-cell lymphoma) and other malignancies, pediatric malignancies, new or worsening congestive heart failure, hematologic abnormalities and blood dyscrasias, hepatobiliary events, hepatitis B virus reactivation, hypersensitivity reactions (including allergic reactions, anaphylactic reactions, acute infusion reactions, and serum sickness-like reactions), autoimmunity (including autoantibodies and lupus-like syndrome), and central nervous system and peripheral demyelinating disorders.18–21 Live vaccine administration is not recommended in patients receiving TNFα inhibitors because of the risk of clinical and disseminated infections.18–21
Primary and secondary loss of response
Despite the high effectiveness of anti-TNFα therapies in patients with IBD, more than one-third of patients are primary nonresponders, and another one-third develop secondary loss of response. Optimal clinical response requires the maintenance of clinically effective drug concentrations which is highly variable among IBD patients and can be influenced by numerous factors such as sex, body weight, associated treatments (immunomodulatory drugs are known to increase anti-TNFα trough levels), route of administration, serum albumin concentration, and systemic inflammation with a markedly decreased half-life in patients with severe disease.43 However, the main factor that impacts anti-TNFα pharmacokinetics and efficacy over time is immunogenicity, whereby anti-drug antibodies accelerate anti-TNFα mAb clearance and shorten the drug’s half-life.43 Although humanized (ie, certolizumab) and fully human mAbs (ie, adalimumab and golimumab) are logically less immunogenic when compared with chimeric ones (ie, infliximab), they can all induce anti-drug antibodies that target the murine and/or variable domains of the drug molecule. Other factors may promote immunogenicity such as genotype in a minority of patients, and drug agitation or freeze–thaw cycles that can induce immunogenic protein aggregates. Concomitant immunomodulator use has been shown to reduce the risk of anti drug antibodies.44
Targeting of leukocyte trafficking in IBD
Until recently, targeting the pro-inflammatory cytokines TNFα and lL-12/23 (Ustekinumab)45,46 have been the only treatments for individuals with moderately-to-severely active CD or UC (unresponsive or inadequately responsive to CS and immunomodulators), yet a significant proportion of such patients will be refractory to these biological drug classes or eventually lose response to therapy. This has resulted in intense investigations to identify other cytokines and pathways that become activated in the dysregulated immune response of IBD. The most advanced alternative to TNFα antagonists has been therapy that targets integrins which mediate trafficking of lymphocytes to the gut. In order to understand how these novel inhibitors of lymphocyte recruitment function, it is crucial to have a basic overview of the molecules involved in gut-specific immune cell recruitment.47
Biological basis of leukocyte trafficking in IBD
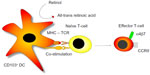
Dendritic cells (DCs), strategically positioned in the lamina propria in close proximity to small bowel enterocytes, continuously monitor the luminal microenvironment for antigens. When a potential antigen is encountered, these CD103+ DCs48 preferentially migrate to secondary lymphoid tissues, such as mesenteric lymph nodes and Peyer’s patches, and interact with naïve T- and B-cells to imprint knowledge of the gut-derived antigen.49 The appropriate immune response, either pro-inflammatory (for a potentially harmful antigen) or tolerance (for an auto-antigen), is thereby generated. Additionally, this interaction induces gut specificity, an important feature of regional immunity, as these newly programmed lymphocytes will be directed back to the gut where they are most likely to encounter the antigen.50 Figure 1 demonstrates the key interactions between DCs and naïve T-cells.
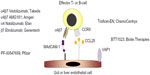
Gut-derived DCs that express CD103 (integrin alpha-E)48 have the capacity to convert retinol to all-trans retinoic acid, driving the process of gut specificity. The production of all-trans retinoic acid during lymphocyte priming induces the expression of the chemokine receptor CCR9 and the integrin combination of α4β7 on the surface of T- and B-lymphocytes, providing a molecular gut-specific postal code.51,52 Once these newly generated effector T-cells and IgA-producing B-cells exit the mesenteric lymphoid tissues, they enter the systemic circulatory system and are selectively recruited to gut. Circulating T- and B-lymphocytes interact with selectins (P- and E-selectins) expressed on the surface of endothelial cells that line the small venule beds within the intestinal tract. This tethering process slows down the velocity of the lymphocytes and allows them to roll along the endothelium to sample the microenvironment for potential chemokines that could trigger recruitment. CCL25, a chemokine whose expression is restricted to the gut, binds with CCR9 on the surface of the gut-homing lymphocytes, thereby activating the integrin α4β7 to bind with MAdCAM-1. These tight adhesions cause arrest of the lymphocyte on the endothelium with subsequent migration and extravasation into target tissue.53 Figure 2 summarizes these key molecules and drugs currently under development to target them in IBD.
Although CCR9, α4β7, CCL25, and MAdCAM-1 represent the major elements of gut specificity, other integrins and chemokines are also expressed in the gut during inflammation, including α4β1 which binds to VCAM-1 and the CXCR3 ligands, CXCR9, and CXCR10. These molecules do not contribute to tissue specificity, but rather are a feature of generalized inflammation that can be found at other sites in the body.50
Pre clinical targeting of leukocyte trafficking in IBD
In IBD, inflammation and resulting tissue injury depends on the recruitment of inflammatory cells – leukocytes such as neutrophils, lymphocytes, and monocytes – to the involved intestinal mucosa from the vascular system, in response to signaling mechanisms and mediators.54 The result is sustained inflammation and ongoing tissue damage. Early pre-clinical experiments focused on interfering with lymphocyte homing to the gut by targeting α4 integrins. Using cotton-top tamarins (CTTs) as an ideal study model of colitis given the symptomatic, histologic, and treatment response similarities to UC in humans, Podolsky et al55 reported a statistically significant reduction in acute colonic inflammation in histological samples following the administration of anti-α4 integrin mAb. This disrupted adhesion mediated by both α4β1 and α4β7 integrins on the surface of gut-homing leukocytes. In this placebo-controlled blinded trial, HP1/2 mAb 2 mg/kg (n=12 CTT) or placebo (n=12 CTT) was administered intramuscularly on days 0–7, with blinded evaluation of acute colonic activity of day 0 and day 10 colonic mucosal pinch biopsies. The mean histologic activity grade, used to quantify colonic inflammation, was significantly reduced with the HP1/2 treatment group when comparing pre-treatment and post-treatment scores (1.6±0.3 pre-treatment vs 0.2±0.1 post-treatment; P=0.005), and when the HP1/2 treatment group was compared with placebo control group for mean Δ in pre-treatment/post-treatment scores for each arm (1.4 vs 0.6; P<0.01). This study demonstrated the potential therapeutic impact of interfering with the recruitment (“trafficking”) of circulating inflammatory cells to areas of intestinal inflammation in response to signaling mechanisms.55
Natalizumab–first adhesion molecule antagonist in IBD
The first biological therapy to specifically target gut-specific leukocyte trafficking and recruitment in IBD was natalizumab (Tysabri®; Biogen Canada, Mississauga, ON, Canada).56 Acting as an antagonist against the cell adhesion molecule α4-integrin, this humanized mAb has the ability to block both α4β1 and α4β7 integrins (which bind to VCAM-1 and MAdCAM-1, respectively), making it selective for inflammation and infection, but nonselective for the GI tract. As VCAM-1 also has a role in cell survival, its mode of action may also impact integrin-dependent survival of cells, thereby promoting cell apoptosis of leukocytes already recruited to affected tissue.57 In a double-blind placebo-controlled study of 248 patients with moderate-to-severe CD, this drug proved to be efficacious and demonstrated significantly higher clinical response rates at weeks 4, 6, 8, and 12 in patients that received two infusions of either natalizumab 3 mg/kg or natalizumab 6 mg/kg, compared with those who received placebo.58 The onset of treatment was rapid and sustained through 8 weeks. Clinical efficacy of this drug was demonstrated in a number of subsequent clinical trials for CD, as summarized in Table 3.
Because natalizumab not only targets gut-specific inflammation through α4β7/MAdCAM-1 interactions, but also impacts nonspecific inflammation through α4β1/VCAM-1 binding, the drug gained an indication for the treatment of multiple sclerosis as α4β1/VCAM-1 represents an important inflammatory pathway in the central nervous system.59 Unfortunately, the ability of this drug to interfere with the α4β1/VCAM-1-mediated inflammatory pathway also became its downfall, as it was capable of suppressing the body’s natural immunologic control of the John Cummingham virus in individuals who were infected. Reactivation of the John Cummingham virus can lead to the development of PML, a rare and fatal demyelinating disease of the brain, as was observed in a small number of individuals treated with natalizumab.60,61 Given the significant risk for PML and the poor risk–benefit ratio of this drug, natalizumab is no longer used for the treatment of CD.
Vedolizumab–gut-specific targeting of leukocyte trafficking in IBD
Efforts have been refined toward specifically targeting the gut-specific α4β7 integrin in order to minimize, and ideally prevent, unwanted systemic immunosuppression associated with α4β1/VCAM-1 interactions (Figure 2). Potential emerging therapies in development include selective targeting of the β7 subunit (etrolizumab; Genentech) and selective inhibition of the MAdCAM-1 (PF-00547659; Pfizer).47 VDZ (Entyvio®; Takeda Pharmaceutical Company Limited) is, however, the lead compound in this class and it has recently become commercially available for the treatment of IBD. Administered as an intravenous infusion, VDZ is a humanized anti-α4β7 integrin IgG1 mAb that binds the activated heterodimer of α4β7.62 Whereby natalizumab only recognized the α4 subunit of the α4β1 heterodimer with no specificity for the β subunit, VDZ solely recognizes the activated α4β7 heterodimeric integrin and not the individual α4 or β7 subunits. This binding specificity results in gut-selective blockade of lymphocyte trafficking, thereby making it an attractive treatment option for IBD.
Vedolizumab in UC
The role of VDZ as an effective induction and maintenance therapy for UC was established in the GEMINI 1 study.66 This Phase III integrated induction and maintenance randomized, double-blind, placebo-controlled trial enrolled patients with active UC (defined as a Mayo score of 6–12 at screening) who had failed or were intolerant to glucocorticosteroids, immunomodulators, and/or anti-TNFα therapy. The induction therapy trial included a total of 895 patients in two treatment cohorts. Cohort 1 comprised 374 patients – stratified by corticosteroid use/nonuse, concomitant immunomodulator use/nonuse, and prior use of TNFα antagonist – who were randomized in a 3:2 ratio to receive VDZ 300 mg or placebo at weeks 0 and 2. A total of 521 patients in cohort 2 received open-label VDZ 300 mg at weeks 0 and 2. Both cohorts underwent endoscopic disease evaluation at week 6 to assess for clinical response, defined as a decrease in an individual’s Mayo score of at least 3 points (and at least 30% from baseline), as well as a decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1. The 121 patients from cohort 1 and 252 patients from cohort 2 who had a clinical response to VDZ at induction continued in the maintenance therapy trial for up to 52 weeks and were randomly assigned (1:1:1) to receive VDZ 300 mg q8weeks, VDZ 300 mg q4weeks, or placebo. Patients (n=373) who did not show response at week 6 to VDZ induction therapy received VDZ 300 mg q4weeks as maintenance; patients (n=149) who received placebo as induction therapy continued to received placebo for the duration of the maintenance trial.
A significantly significant 47.1% of patients from cohort 1 treated with VDZ showed a clinical response at week 6 vs 25.5% for the placebo group (P<0.001); 44.3% of patients from cohort 2 treated with open-label VDZ also showed a clinical response. Clinical remission at week 52, defined as a Mayo score ≤2 with no subscore greater than 1, was achieved in 41.8% of patients treated with VDZ 300 mg q8weeks and 44.8% of patients treated with VDZ 300 mg q4weeks compared with 15.9% of individuals switched to placebo for maintenance (P<0.001 against placebo for both VDZ regimens). The efficacy between VDZ 300 mg dosed q4weeks compared with q8weeks was not appreciably different. Commonly reported adverse events, serious infections, opportunistic infections, and enteric infections occurred in patients treated with VDZ at a frequency similar to those treated with placebo.66 Table 4 summarizes the pivotal VDZ studies conducted in UC.
Vedolizumab in CD
An identical study design framework was utilized in the GEMINI 267 study to evaluate the effectiveness of VDZ as induction and maintenance therapy in active CD. The study population was patients with moderate-to-severe CD, defined as a Clinical Disease Activity Index (CDAI) score between 220 and 450 points at screening, who had failed or were intolerant to glucocorticosteroids, immunomodulators, and/or anti-TNFα therapy. Three hundred and sixty-eight patients, stratified by corticosteroid use/nonuse, concomitant immunomodulator use/nonuse, and prior use of TNFα antagonist, were entered into cohort 1 and randomized to receive VDZ 300 mg or placebo at weeks 0 and 2 (3:2 ratio). Cohort 2 consisted of 747 patients who were infused with open-label VDZ 300 mg at weeks 0 and 2. Endoscopic evaluation was performed at week 6 to assess for clinical remission (CDAI score of ≤150 points) and CDAI-100 response (decrease in CDAI score of 100 or more points from baseline). Patients (n=461) who achieved at least a 70 point decrease in the CDAI score at week 6 (96 patients from cohort 1; 365 patients from cohort 2) were randomly assigned (1:1:1) to receive VDZ 300 mg q8weeks, VDZ 300 mg q4weeks, or placebo for up to 52 weeks within the maintenance therapy trial. Participants (n=506) who did not show response at week 6 to VDZ induction therapy received VDZ 300 mg q4weeks as maintenance and those individuals (n=168) who received placebo as induction therapy continued to received placebo for the duration of the maintenance trial.
At week 6 endoscopic evaluation, 14.5% of patients from cohort 1 induced with VDZ were in clinical remission at week 6 compared with 6.8% for the placebo group (P=0.02); although not found to be statistically different (P=0.23), 31.4% of cohort 1 patients treated with VDZ and 25.7% of patients treated with placebo achieved a CDAI-100 response at this same time point. Clinical remission was observed in 17.7% of patients from cohort 2 treated with open-label VDZ, with 34.4% having a CDAI-100 clinical response. For patients from both cohorts who responded to VDZ induction therapy, clinical remission at week 52 was confirmed in 39.0% of patients treated with VDZ 300 mg q8weeks and 36.4% of patients treated with VDZ 300 mg q4weeks compared with 21.6% of individuals switched to placebo for maintenance (P<0.001 for VDZ q8weeks vs placebo; P=0.004 for VDZ q4weeks vs placebo). The frequency of adverse events (discussed next) was higher in the VDZ treatment group compared with individuals receiving placebo.67
The GEMINI 3 study68 was carried out to assess the efficacy and safety of VDZ as an induction agent for moderately-to-severely active CD patients (CDAI score between 220 and 400 at baseline) with previous anti-TNFα therapy failure, defined as primary nonresponse, secondary nonresponse (loss of response), or intolerance to 1 or more TNFα antagonists. This Phase III, double-blind, placebo-controlled trial lasting 10 weeks enrolled 416 patients of which 315 (75.7%) were individuals with previous TNFα antagonist failure and 101 (24.3%) were anti-TNFα naïve. Patients were randomized in a 1:1 fashion to receive VDZ 300 mg or placebo at weeks 0, 2, and 6. In patients with prior TNFα antagonist failure, clinical remission (CDAI score ≤150 points) was not statistically significant at week 6 (15.2% of VDZ-treated patients vs 12.1% of placebo-treated patients; P=0.433), but was statistically significant at week 10 (26.6% of VDZ-treated patients vs 12.1% of placebo-treated patients; nominal P=0.001). As CDAI-100 response (≥100 point decrease from baseline CDAI score) was observed in 39.2% of patients receiving VDZ at week 6 compared with 22.3% of patients treated with placebo (P=0.001), the authors of the study concluded the onset of clinical remission is likely more gradual in this patient population, thereby encouraging clinicians to consider a time point beyond week 6 to evaluate patient response before manipulating concomitant medical therapies or abandoning VDZ as a therapeutic agent. Similar to safety data reported in the GEMINI 1 study,66 the frequency of adverse events in this study was comparable between VDZ-treated individuals and those treated with placebo. Table 4 summarizes the pivotal VDZ studies conducted in CD.
In order to evaluate VDZ as a maintenance therapy in moderately-to-severely active CD patients with previous anti-TNFα therapy failure, an open-label VDZ extension study is presently underway with results expected in 2016. This long-term study rolls over CD patients with no unacceptable adverse events and no need for CD-related surgery during their participation in the GEMINI 3 study.68
Clinical trials data confirm VDZ to be an effective agent for the induction and maintenance of clinical remission in IBD. Entyvio® (VDZ; Takeda Pharmaceutical Company Limited, Osaka, Japan) was approved for the treatment of moderately-to-severely active UC and moderately-to-severely active CD by the US Food and Drug Administration on May 20, 2014 and the European Commission on May 27, 2014. Health Canada approved its use in moderately-to-severely active UC on January 29, 2015. VDZ can be considered for patient’s naïve to anti-TNFα agents that have failed or are intolerant to conventional therapy with glucocorticosteroids and/or immunomodulators, or that have failed TNFα antagonists. Previous exposure to a TNFα antagonist did not substantially affect the efficacy of VDZ for inducing and maintaining clinical response and remission in patients with UC,66 and statistically significant clinical response and remission was achievable in patients with CD who had previously failed or been intolerant to up to three anti-TNFα agents.68 This is an important clinical consideration when there is a significant proportion of individuals who are refractory or who ultimately lose response to anti-TNFα therapy.68
The Toronto Consensus Statement released in May 2015 positions VDZ as a therapy to consider in the moderately-to-severely active ambulatory UC patient when symptoms persist after 2 weeks of oral corticosteroid therapy or if symptomatic response has not been achieved with anti-TNFα as monotherapy or in combination with an immunomodulator.12 It is anticipated that a formal consensus statement for the use of VDZ in CD will be forthcoming as clinicians integrate this drug into clinical care.
Vedolizumab versus anti-TNFα therapy in Crohn’s disease
As there are no direct comparative trials, it is not clear if anti-TNFα therapy as monotherapy or in combination with an immunosuppressant is equivalent or superior to VDZ for both the induction of remission and the maintenance of remission in CD. A recent network meta-analysis73 found VDZ to be superior only to placebo (odds ratio [OR], 2.0; 95% Credible Interval [CrI], 1.2–3.3), azathioprine/6-mercaptopurine (OR, 1.6; 95% CrI, 0.78–3.2), methotrexate (OR, 1.3; 95% CrI, 0.53–3.2), and certolizumab (OR, 1.4; 95% CrI, 0.77–2.7) for the induction of remission. When considering the maintenance of remission, VDZ was found to be superior only to placebo (OR, 2.2; 95% CrI, 1.3–3.7), azathioprine/6-mercaptopurine (OR, 1.3; 95% CrI, 0.65–2.3), and certolizumab (OR, 1.1; 95% CrI, 0.57–2.1). These data are fraught with potential biases, as the individual drugs might not necessarily be targeting the same responder who may have different biology and disease distribution. As such, proper head-to-head studies are required.
Vedolizumab and acute, severe IBD
Given the observed differences in the efficacy of VDZ in UC compared with CD, one must consider this gradual onset of clinical response and remission as a characteristic of lymphocyte trafficking modulators, as this delayed onset was also observed with natalizumab.61 We speculate there may be a necessity for a “co-inducer” such as bridging CSs when VDZ treatment is initiated, as exploratory subgroup analysis reported statistically significant increased rates of clinical remission (CDAI score ≤150 points) at week 10 both in the TNFα antagonist failure population on concomitant CSs receiving VDZ compared with the TNFα antagonist failure population on concomitant CS receiving placebo (30.2% versus 10.6%; P=0.002), and in the overall population on concomitant CSs receiving VDZ compared with the overall population on concomitant CS receiving placebo (34.5% versus 12.0%; P=0.0001).68 Given this gradual onset of therapeutic benefit observed in clinical trials, it is unlikely that VDZ will have a significant role in the urgent management of extremely severe or fulminant IBD.
Vedolizumab safety
With respect to the safety of VDZ, no cases of PML occurred across the three Phase III studies.66–68 In GEMINI 1, the frequency of commonly reported adverse events, serious infections, opportunistic infections, and enteric infections in UC patients treated with VDZ was similar to placebo. In comparison, the incidence of infections, serious infections, serious adverse events, and nasopharyngitis was more frequently observed in CD patients treated with VDZ compared with placebo. Nasopharyngitis, headache, arthralgia, upper respiratory tract infection, cough, abdominal pain, fatigue, and influenza are listed as the most common side effects of the drug in the Patient Medication Information leaflet (part III of product monograph).74
Clinically significant infusion reactions were uncommon, only requiring discontinuation of VDZ therapy for three individuals in GEMINI 1 and one individual in GEMINI 2 (no data reporting infusion reactions available for GEMINI 3). Immunogenicity is uncommon, with only 3.7% of the 620 patients with blood samples suitable for drug antibody testing in GEMINI 1 having VDZ antibodies at any given time through the induction and maintenance periods, and 4.1% of patients in GEMINI 2 having detectable VDZ antibodies. Pre-medications (antihistamine, hydrocortisone, corticosteroid, and/or acetaminophen) are not standardly required or recommended, unless the patient has experienced a mild-to-moderate infusion-related reaction to VDZ. Concomitant treatment with an immunomodulator was associated with a decrease in immunogenicity and may be considered for individual patients.
Vedolizumab and enteric pathogens
Clostridium difficile and other enteric infections did not occur more frequently than placebo in clinical trials,66–68 a valid concern when considering the gut-specific mechanism of action of VDZ. Wyant et al75 conducted a Phase I, randomized, double-blind, placebo-controlled, parallel-group single center non-inferiority trial, whereby 127 healthy volunteers were randomized 1:1 to receive a single dose of VDZ 750 mg or placebo at day 0, followed by an accelerated immunization dosing schedule of intramuscular hepatitis B vaccine (HBV) on days 4/32/60 and oral cholera vaccine (OCV; Dukoral®) on days 4/18. HBV seroconversion (defined as hepatitis B surface antibody titer ≥10 IU/L) and OCV seroconversion (defined as >fourfold increase in serum cholera toxin antibodies from baseline) was tested at day 74. HBV seroconversion to the parenteral hepatitis B vaccine was observed in 90.3% of individuals initially treated with placebo and 88.5% of individuals treated with VDZ, whereas OCV seroconversion to the enteral OCV was observed in 96.8% of individuals initially treated with placebo and 82.5% of individuals treated with VDZ. Further, the humoral response to OCV was markedly reduced in individuals treated with VDZ who demonstrated an OCV seroconversion, compared with individuals treated with placebo who seroconverted in response to OCV, providing further evidence of the gut selectivity of this molecule. The study authors speculated that T-cell-dependent immune defenses are attenuated, but not completely blocked, in response to OCV with concurrent administration of VDZ. From this study and the safety outcomes reported in clinical trials, it may be concluded that VDZ does exert an effect on the lymphocyte trafficking to the gut, but does not obliterate an individual’s own immune response to enteric infections. This is an important observation as IBD patients are more prone to infections of the gut and require appropriate immune responses to control enteric microorganisms encountered in the luminal environment while limiting the inappropriate immune response characteristic of IBD.
Potential extended indications for vedolizumab
There is the potential for extended therapeutic use of VDZ beyond IBD. Primary sclerosing cholangitis (PSC) is a chronic immune-mediated cholestatic liver disease of unknown etiology that results in progressive fibrostenotic strictures of the entire biliary tree eventually leading to liver cirrhosis and end-stage liver disease.76 PSC stands out among other forms of liver disease owing to its close association with IBD. Between 2.5% and 7.5% of individuals with IBD will eventually develop PSC and, conversely, between 60% and 70% of patients with PSC will develop IBD.77 This association is discontinuous as PSC can arise many years after the initial diagnosis of IBD or, in some instances, after a curative colectomy for UC has been performed. It is also well recognized that IBD can arise de novo after a successful liver transplant for PSC, thus suggesting a very close relationship and shared pathogenesis between PSC and IBD.
In PSC, there is aberrant expression of gut-specific chemokine CCL25 on hepatic sinusoidal endothelium which binds to CCR9 on gut tropic T-cells, activating α4β7 to recruit pro-inflammatory gut T-cells from the intestinal tract to the PSC liver. MAdCAM-1 and CCL25 expression, usually confined to the gut, has also been observed on liver endothelium in association with PSC, leading to increased trafficking of mucosal T-cells and chronic IBD related-liver inflammation.78–80 Therefore, VDZ may be a potential effective therapy for the treatment of PSC. To further investigate this hypothesized mechanism of chronic liver inflammation and injury, a Phase III randomized control study of VDZ in PSC is planned to commence in 2016.
Individuals with IBD often develop seronegative arthritis as an extra-intestinal manifestation of their IBD. Joint arthropathies may be the consequence of flawed mucosal leukocyte trafficking to the synovium of affected joints, as has been demonstrated in experimental models where gut derived T-cells were observed to bind to synovial tissue.81 The exact mechanism by which gut-homing lymphocytes bind to inflamed synovium is not well understood; earlier attempts hypothesized that α4β7 lymphocytes found in inflamed synovium must partially use integrins and adhesion molecules other than MAdCAM-1 such as VAP-1 to transmigrate across the endothelium into synovial tissue, as MAdCAM-1 is lacking on the endothelium of inflamed synovial blood vessels.82 This necessitates further investigations, but aberrant expression of gut-adhesion molecules may be implicated in seronegative arthritis given the association of this extra-intestinal manifestation with IBD. Long-term clinical use of VDZ in the IBD population combined with controlled clinical trials specifically designed to investigate the impact of this drug on seronegative arthritis will be required to further delineate the exact molecular mechanism of joint inflammation and chronic injury.
Conclusion
In summary, VDZ has emerged as a viable and efficacious alternative to TNFα antagonist treatment in both UC and CD without the serious complication of PML associated with its predecessor, natalizumab. The mechanism of action appears to be restricted to the gut without complete abrogation of the host immune system, preserving mucosal immunity that is relatively intact and capable of coping with enteric infections.
The future of anti-adhesion therapy in IBD will be focused on delineating the integrated, and often redundant, inflammatory pathways that contribute to chronic inflammation of the gut and associated extra-intestinal manifestations. Etrolizumab, PF-00547659 and CCR9 antagonists such as GSK-1605786 (formerly CCX-282; Traficet-EN, ChemoCentryx; New York, NY, USA) have been or continue to be investigated, but have not yet received regulatory approval for clinical use. The next logical progression in drug development is to consider new targets, such as the αE subunit of the gut-specific αEβ7 integrin, or multi-target therapy such as the collaboration between ChemoCentryx and GSK (Brenford, Middlesex, UK) to develop a next-generation CCR9 inhibitor (CCX507) that works in combination with an α4β7 integrin antagonist to provide an amplified treatment effect that surpasses monotherapy with either agent.
Future drug development in IBD is not solely restricted to gut-specific targets, but also include intracellular messengers such as tyrosine kinases. JAK1 plays important role in signal transduction of the interleukins. Tofacitinib is a JAK inhibitor that can block this signaling pathway and is under investigation in IBD.83 Other approaches include anti-interleukin 12/23 antibody treatments84 and non-gut-selective chemokine antagonists such as anti-CXCL10 antibodies.85 It is likely that combinations of these therapies will emerge and companion diagnostics to personalize treatments to target in individuals with IBD.
Acknowledgment
This research was supported by the Canadian Institutes for Health Research (CIHR).
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
The impact of inflammatory bowel disease in Canada: 2012 final report and recommendations. 2012. Available from: http://www.isupportibd.ca/pdf/ccfc-ibd-impact-report-2012.pdf.). Accessed July 4, 2015. | |
Fast facts: the impact of IBD in Canada 2012. 2012. Available from: http://isupportibd.ca/pdf/ccfc.ca-impact-report-fast-facts.pdf. Accessed July 4, 2015. | |
Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42; quiz e30. | |
Talley NJ, Abreu MT, Achkar JP, et al. An evidence-based systematic review on medical therapies for inflammatory bowel disease. American College of Gastroenterology IBD Task Force. Am J Gastroenterol. 2011; 106:S2–S25. | |
Loftus EV Jr, Shivashankar R, Tremaine W, Harmsen WS, Zinsmeister AR. Updated incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota (1970–2011). ACG 2014 Annual Scientific Meeting; October 2014. | |
Rocchi A, Benchimol EI, Bernstein CN, et al. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–817. | |
Bernklev T, Jahnsen J, Schulz T, et al. Course of disease, drug treatment and health-related quality of life in patients with inflammatory bowel disease 5 years after initial diagnosis. Eur J Gastroenterol Hepatol. 2005;17:1037–1045. | |
Theede K, Kiszka-Kanowitz M, Nordgaard-Lassen I, Mertz Nielsen A. The impact of endoscopic inflammation and mucosal healing on health-related quality of life in ulcerative colitis patients. J Crohn’s Colitis. 2015;9:625–632. | |
Wright EK, Kamm MA. Impact of drug therapy and surgery on quality of life in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2015;21:1187–1194. | |
McLean LP, Shea-Donohue T, Cross RK. Vedolizumab for the treatment of ulcerative colitis and Crohn’s disease. Immunotherapy. 2012;4:883–898. | |
Cheng FK, McLean LP, Cross RK. What is the role of vedolizumab in the era of anti-TNF agents? Ann Transl Med. 2014;2:4. | |
Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148:1035–1058. e3. | |
Nguyen GC, Devlin SM, Afif W, et al. Defining quality indicators for best-practice management of inflammatory bowel disease in Canada. Can J Gastroenterol Hepatol. 2014;28:275–285. | |
Kaplan GG, Seow CH, Ghosh S, et al. Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am J Gastroenterol. 2012;107:1879–1887. | |
Bernstein CN, Loftus EV Jr, Ng SC, et al. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622–629. | |
Singh S, Al-Darmaki A, Frolkis AD, et al. Post operative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2015;149:928–937. | |
van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995;109:129–135. | |
Janssen. Remicade® (infliximab) Product monograph. 2014. Available from: http://www.janssen.ca/subcategory_docdownload?id=2263. Accessed July 15, 2015. | |
Abbvie. Humira® (adalimumab) Product monograph. 2015. Available from: http://www.abbvie.ca/content/dam/abbviecorp/ca/english/docs/HUMIRA_PM_EN.pdf. Accessed July 15, 2015. | |
Janssen. Simponi® (golimumab) Product monograph. 2014. Available from: http://www.janssen.ca/subcategory_docdownload?id=2278. Accessed July 15, 2015. | |
UCB Canada. Cimzia® (certolizumab pegol) Product monograph. 2014. Available from: http://www.ucb-canada.ca/_up/ucbpharma_ca_en/documents/cimzia_pm_en_15jan2014.pdf. Accessed July 15, 2015. | |
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. | |
Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. | |
Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. | |
Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. | |
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. | |
Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863–873; quiz 1165–1166. | |
Hyams JS, Griffiths A, Markowitz J, et al. Safety and efficacy of adalimumab for moderate to severe Crohn’s disease in children. Gastroenterology. 2012;143:365–374. e2. | |
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350: 876–885. | |
Targan SR, Hanauer SB, van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosing factor α for Crohn’s disease. N Engl J Med. 1997;337(15):1029–1035. | |
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab. Ann Intern Med. 2007;146(12):829–838. | |
Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129(3):807–818. | |
Sandborn WJ, Schreiber S, Feagan BG, et al. Certolizumab pegol for active Crohn’s disease: A placebo-controlled randomized trial. Clin Gastroenterol Hepatol. 2011;9(8):670–678. | |
Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab for Crohn’s disease. N Engl J Med. 2007;357: 239–250. | |
Sandborn WJ, Schreiber S, Feagan BG, et al. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2011;9:670–678. | |
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353: 2462–2476. | |
Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60: 780–787. | |
Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–2565. e1–e3. | |
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95; quiz e14–e5. | |
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96–109. e1. | |
Hyams J, Damaraju L, Blank M, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:391–399. e1. | |
Probert CSJ, Hearing SD, Schreiber S, et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut. 2003;52:998–1002. | |
Khanna R, Sattin BD, Afif W, et al. Review article: a clinician’s guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:447–459. | |
Khanna R, Feagan BG. Current and future status of therapeutic drug monitoring in the treatment of IBD. Curr Treat Options Gastroenterol. 2014;12:76–89. | |
Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. | |
Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. | |
Lobaton T, Vermeire S, Van Assche G, Rutgeerts P. Review article: anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:579–594. | |
Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. | |
Agace W. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Lett. 2010;128:21–23. | |
Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nat Rev Immunol. 2006;6:682–692. | |
Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. | |
Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314: 1157–1160. | |
Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29(11):541–522. | |
Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. | |
Podolsky DK, Lobb R, King N, et al. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest. 1993;92:372–380. | |
Biogen Canada. Tysabri® (natalizumab) Product Monograph 2015. Available from: http://www.biogen.ca/Admin/Public/DWSDownload.aspx?File=Files%2fFiler%2fCanada%2fProduct_Information% 2fTYSABRI%2f2015_04_07-TYSABRI-PM-E.pdf. Accessed July 15, 2015. | |
Postigo AA, Teixido J, Sanchez-Madrid F. The alpha 4 beta 1/VCAM-1 adhesion pathway in physiology and disease. Res Immunol. 1993;144:723–735; discussion 54–62. | |
Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348:24–32. | |
Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha4beta1 integrin. Nature. 1992;356:63–66. | |
Monaco MC, Major EO. The link between VLA-4 and JC virus reactivation. Expert Rev Clin Immunol. 2012;8:63–72. | |
Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353: 1912–1925. | |
Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-{alpha}4{beta}7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330(3):864–875. | |
Gordon FH, Lai CWY, Hamilton MI, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to α4 integrin in active Crohn’s disease. Gastroenterology. 2001;121(2):268–274. | |
Targan SR, Feagan BG, Fedorak RN, et at. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE trial. Gastroenterology. 2007;132(5):1672–1683. | |
Sands BE, Korazek R, Spainhour J, et al. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm Bowel Dis. 2007;13(1): 2–11. | |
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. | |
Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369: 711–721. | |
Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147: 618–627. e3. | |
Feagan BG, McDonald J, Greenberg G, et al. An ascending dose trial of a humanized alpha4 beta7 antibody in ulcerative colitis (UC). Gastroenterology. 2000;118(4):AB74. | |
Feagan BG, Greenberg G, Wild G, et al. 2005. Treatment of ulcerative colitis with a humanized antibody to the α4β7 integrin. N Engl J Med. 2005;352(24):2499–2507. | |
Feagan BG, Greenberg GR, Wild G, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the α4β7 integrin. Clin Gastroenterol Hepatol. 2008;6(12):1370–1377. | |
Parikh A, Leach T, Wyant T, et al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18(8):1470–1479. | |
Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology. 2015;148:344–354. e5; quiz e14–e5. | |
Takeda. Entyvio® (vedolizumab) Product monograph. 2015. Available from: http://www.takedacanada.com/entyviopm/~/media/countries/ca/files/product%20pdfs/entyviopm_eng_2015jan25.pdf. Accessed July 15, 2015. | |
Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64:77–83. | |
Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382:1587–1599. | |
Eksteen B. Advances and controversies in the pathogenesis and management of primary sclerosing cholangitis. Br Med Bull. 2014;110:89–98. | |
Eksteen B, Miles AE, Grant AJ, Adams DH. Lymphocyte homing in the pathogenesis of extra-intestinal manifestations of inflammatory bowel disease. Clin Med. 2004;4:173–180. | |
Eksteen B, Grant AJ, Miles A, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511–1517. | |
Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244–251. | |
Salmi M, Andrew DP, Butcher EC, Jalkanen S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med. 1995;181:137–149. | |
Salmi M, Jalkanen S. Endothelial ligands and homing of mucosal leukocytes in extraintestinal manifestations of IBD. Inflamm Bowel Dis. 1998;4:149–156. | |
Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367: 616–624. | |
Bravata I, Fiorino G, Allocca M, Repici A, Danese S. New targeted therapies such as anti-adhesion molecules, anti-IL-12/23 and anti-Janus kinases are looking toward a more effective treatment of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:113–120. | |
Rivera-Nieves J. Strategies that target leukocyte traffic in inflammatory bowel diseases: recent developments. Curr Opin Gastroenterol. 2015;31:441–448. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.