Back to Journals » Journal of Pain Research » Volume 11
Second edition of SIMPAR’s “Feed Your Destiny” workshop: the role of lifestyle in improving pain management
Authors De Gregori M, Belfer I, De Giorgio R , Marchesini M, Muscoli C, Rondanelli M, Martini D, Mena P , Arranz LI, Lorente-Cebrián S, Perna S, Villarini A , Salamone M, Allegri M , Schatman ME
Received 22 December 2017
Accepted for publication 22 May 2018
Published 27 August 2018 Volume 2018:11 Pages 1627—1636
DOI https://doi.org/10.2147/JPR.S160660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Manuela De Gregori,1–3 Inna Belfer,2,4 Roberto De Giorgio,5 Maurizio Marchesini,2,3,6 Carolina Muscoli,7 Mariangela Rondanelli,2,8 Daniela Martini,9 Pedro Mena,9 Laura Isabel Arranz,2,10 Silvia Lorente-Cebrián,2,11 Simone Perna,8 Anna Villarini,12 Maurizio Salamone,2,13,14 Massimo Allegri,2,15 Michael
E Schatman2,16,17
1 Pain Therapy Service, Fondazione IRCCS Polclinico San Matteo, Pavia, Italy; 2Study in Multidisciplinary Pain Research Group, Parma, Italy; 3Young Against Pain Group, Parma, Italy; 4Faculty of Dentistry, McGill University, Montreal, QC, Canada; 5Department of Clinical Sciences, Nuovo Arcispedale S. Anna, University of Ferrara, Ferrara, Italy; 6Anesthesia, Intensive Care and Pain Therapy Service, Azienda Ospedaliero, Universitaria of Parma, Parma, Italy; 7Department of Health Sciences, Institute of Research for Food Safety and Health, University “Magna Graecia” of Catanzaro, Parma, Italy; 8Department of Public Health, Section of Human Nutrition and Dietetics, Azienda di Servizi alla Persona di Pavia, University of Pavia, Pavia, Italy; 9Human Nutrition Unit, Department of Food & Drugs, University of Parma, Parma, Italy; 10Department of Nutrition, Food Sciences and Gastronomy, University of Barcelona, Barcelona, Spain; 11Department of Nutrition, Food Science and Physiology, Faculty of Pharmacy, Center for Nutrition Research, University of Navarra, Pamplona, Spain; 12Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; 13Science department, Metagenics Italia srl, Milano, Italy; 14Società internazionale di Neuropsicocardiologia, Trapani, Italy; 15Anesthesia and Intensive Care Service – IRCCS MultiMedica Hospital, Sesto San Giovanni, Milano, Italy; 16Research and Network Development, Boston Pain Care, Waltham, MA, USA; 17Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, MA, USA
Abstract: This review is aimed to summarize the latest data regarding pain and nutrition, which have emerged during the second edition of Feed Your Destiny (FYD). Theme presentations and interactive discussions were held at a workshop on March 30, 2017, in Florence, Italy, during the 9th Annual Meeting of Study in Multidisciplinary Pain Research, where an international faculty, including recognized experts in nutrition and pain, reported the scientific evidence on this topic from various perspectives. Presentations were divided into two sections. In the initial sessions, we analyzed the outcome variables and methods of measurement for health claims pertaining to pain proposed under Regulation EC No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Moreover, we evaluated how the Mediterranean diet can have a potential impact on pain, gastrointestinal disorders, obesity, cancer, and aging. Second, we discussed the evidence regarding vitamin D as a nutraceutical that may contribute to pain control, evaluating the interindividual variability of pain nature and nurture, and the role of micro-RNAs (miRNAs), polyunsaturated omega 3 fatty acids, and phenolic compounds, with a final revision of the clinical role of nutrition in tailoring pain therapy. The key take-home message provided by the FYD workshop was that a balanced, personalized nutritional regimen might play a role as a synergic strategy that can improve management of chronic pain through a precision medicine approach.
Keywords: chronic pain, multidisciplinary pain management, personalized nutrition, nutritional supplements
Introduction
During the 9th Annual Meeting of Study in Multidisciplinary Pain Research (SIMPAR) in Florence, Italy, in March of 2017, the Feed Your Destiny (FYD) group convened for the second consecutive year to present recent data on the complex relationships between nutrition and pain.
Figure 1 provides an overview of the topics covered during the workshop.Following the presentations, interactive discussions on the topics of the lectures took place. The international faculty included numerous disciplines, with a goal of understanding the complex relationships from a variety of different perspectives. Many of the faculty members were present at the previous year’s FYD workshop, the proceedings of which were published in this journal in 2016.1 Given that we consider the role of nutrition in the development and maintenance of pain to be a topic of considerable importance, with the body of empirical literature on the topic growing copiously, our expectation is that we will meet annually and publish our proceedings in order to provide pain researchers and clinicians with crucial updates that will ideally inform both empirical investigation and pain management practice.
  | Figure 1 Description of FYD2 workshop. Abbreviations: FYD, Feed Your Destiny; SIMPAR, Study in Multidisciplinary Pain Research. |
Description of the main topics of FYD2
Based on Regulation (EC) No 1924/2006 of the European Parliament and of the Council, health claims state that food has beneficial properties on health, such as lowering cholesterol or helping maintain a suitable body weight.2,3 Concerning pain management, there are many opportunities for applying for a health claim, yet, at present, no successful applications have been submitted and more research is needed to reach this goal.
The Mediterranean diet
Chronic pain, including that of neuropathic origin, is generally related to inflammation.4,5 Hence the Mediterranean diet can be considered a viable strategy to lower systemic inflammation directly related to pain;6 indeed, it promotes the development of intestinal microbiota that are able to metabolize fiber, releasing short-chain fatty acids (SCFAs).7 SCFAs exert multiple roles in metabolic homeostasis.8 Several studies indicate that increased consumption of whole grains is associated with protection against various chronic degenerative disorders (e.g., cardiovascular diseases, diabetes, cancer, and metabolic syndromes), and helps maintain digestive health and body weight through fiber content and phytochemicals.9–12
The Mediterranean diet, with its balanced intake of energy and macronutrients,13 also improves physical and functional fitness, bodyweight, and patient quality of life.14
Moreover, in order to have a synergistic effect, it is advisable to maintain an active lifestyle, regulate one’s sleep cycle, develop a healthy bowel regimen, work to improve one’s mood, and minimize stress levels.15,16
The human gut microbiota
A Mediterranean diet is considered an ally for human gut microbiota (constituted of viruses, phages, bacteria, archea, fungi, protists, and nematodes).1,17 Consistent evidence indicates that gut microbiota play many key roles, including structural (e.g., epithelial barrier stabilization, immunoglobulin A induction), protective (pathogen displacement), and metabolic (contributing to energy homeostasis, epithelial cell maintenance, and food metabolism) functions. Sensory information is conveyed to the central nervous system via the enteric nervous system. The entero-endocrine cells, widely distributed throughout the gastrointestinal (GI) tract, have direct links with neurons and regulate GI secretion and motility, food intake, postprandial glucose levels, and metabolism.18 Intestinal gas (or other noxae) can evoke stretching of mechanoreceptors in the gut wall or activation of nociceptors, i.e., vascular and mesenteric afferents, which generate abdominal distension, bloating, and visceral pain. Notably, the occurrence of mechanisms leading to inflammation (or ischemia) at the tissue level is known to recruit “silent nociceptors” which they modulate.19,20
Key phyla of human gut microbiota change across different stages of life,21 and they are influenced by dietary habits.22 Irritable bowel syndrome (IBS) is a gut–brain axis disorder present in 10–20% of the population (with a female to male prevalence ratio of 3:1), characterized by visceral pain, bloating, and bowel habit changes.23,24
Recently, the role of gut microbiota in stress-related conditions and in the pathophysiology of functional gastrointestinal disorders has emerged; nevertheless, further clinical research is needed to confirm these preliminary results.25–27
Non-steroidal anti-inflammatory drugs (NSAIDs) are frequently prescribed to manage pain, and have the potential to produce GI toxicity, especially among patients older than 75 years of age.28
Consequences of excess of body weight and obesity
Most patients with chronic pain conditions have excesses of body weight and gastrointestinal disorders such as IBS, dyspepsia, food intolerances, or other GI disturbances such as bloating, constipation, abdominal pain, or altered bowel habits (i.e., diarrhea, constipation, or mixed).29 An effective correction of lifestyle, including proper water intake (mild hypohydration via fluid restriction increases pain sensitivity30) along with dietary changes, may improve pain relief.
Indeed, an increase of adipose tissue leads to a chronic, low-grade inflammatory state (at the systemic level), which has been associated with the development of obesity-related diseases such as hyperlipidemia and insulin resistance and, in the long run, atherosclerosis and type 2 diabetes.31
Obesity represents a major health issue more common than world hunger,32 as 39% of adults worldwide are overweight and 13% are obese (World Health Organization [WHO], 2015). Growing evidence suggests that the relationship between obesity and chronic pain is not random.33 Obesity and chronic pain coexist in a multifactorial disease state and adversely impact each other.
The presence of metabolic disorders leads to the development of a metabolic syndrome, characterized by an inflammatory state triggered at the adipose level through increased production of inflammatory cytokines, such as tumour necrosis factor alpha (TNF-α).34 This process can stimulate pre-adipocytes and other adipocytes to produce monocyte chemoattractant protein-1 (MCP-1).35 At the same time, a decrease in the secretion of anti-inflammatory factors occurs, due to adiponectin and secreted frizzled-related protein 1 (SFRP1).36 SFRP1 proteins act as modulators of Wnt signaling; they have a role in regulating cell growth and differentiation in specific cell types.
MCP-1 activates macrophage recruitment to adipose tissue and polarization toward more pernicious macrophage phenotypes (M1). In white adipose tissue, both M1 macrophages and adipocytes produce inflammatory factors/signals that perpetuate this vicious circle of inflammatory factor secretion, macrophage recruitment, impairment of adipocyte function, and tissue inflammation. This condition causes early aging (described as “inflammaging”), which is linked to neurodegenerative diseases and pain disorders.37,38
Breast cancer and body weight
In patients who have undergone breast cancer (BC) surgery, there are indications linking a better long-term outcome to healthy body weight39,40 and physical activity,41 a balanced diet heavy in fiber and soy, and lower intakes of total fat and saturated fat.42 Obesity and overweight are associated with poorer overall survival in pre- and post-menopausal BC, with both related to increased estrogen and insulin levels. A meta-analysis that included 12,108 women with invasive BC concluded that physical activity performed pre-diagnosis significantly reduced all-cause mortality by 18%, and post-diagnosis physical activity reduced BC mortality by 30% and decreased all-cause mortality by 41%.43
In estrogenic receptor (ER)-positive tumors, physical activity was demonstrated to reduce all-cause mortality (64%) and BC mortality (50%) (potentially attributed to the beneficial effect of exercise on estrogenic levels). No benefit was demonstrated among women with ER-negative disease. Observational data suggest that high soy intake (such as that found in a traditional Japanese diet) may be protective against the development of BC disease, although future clinical studies are needed to confirm this relationship.44 Until there are more data supporting safety, caution is warranted regarding high doses of isoflavones.
Nutrition and the elderly
In Italy, more than 20% of the population is older than 65 years, and life expectancy ranges have increased dramatically from 1961 to the present (67.2 years for men and 72.3 years for women in 1961; 78.8 years for men and 84.1 years for women currently). The above-mentioned data in 2015 has been provided by the Italian public research organization dealing with social and economic censuses and surveys, Istituto nazionale di statistica (ISTAT). Malnutrition and frailty sarcopenia are common in the elderly, and are due to different age-related causes such as endocrine and neurodegenerative diseases, suboptimal nutrition, malabsorption, disuse, and cachexia.45,46 Sarcopenia diagnosis is present with low muscle mass, low muscle strength, and low physical performance. The skeletal muscle index is calculated as the ratio between free fat mass of arms and legs (kg)/height (m2),47 and the cut-off is 7.26 kg/m2 for men and 5.5 kg/m2 for women.46 Strength is measured by the handgrip test, with the cut-off assigned as less than 30 kg for men and less than 20 kg for women. In the elderly, dual-energy X-ray absorptiometry analysis is utilized to measure osteoporosis, which is directly linked to visceral adipose tissue, waist/hip ratio, trunk to leg fat mass ratio, and fat mass index.
The current recommendations for diets of older people include the use of proteins to protect muscles; they should aim for a daily average intake of 1.0–1.2 g/kg body weight (BW)/d. There is also a recommended target of 25–30 g of protein per meal, with ~2.5–2.8 g of leucine, the most potent stimulator of muscle protein synthesis.48
More research is needed to define the exact protein needs in this frail and complex population, who often also experience vitamin D deficiency (25-hydroxyvitamin D [25OHD] lower than 10 ng/mL). This is due to several causes, including unbalanced diet, malabsorption, lack of exposure to sunlight, chronic liver disease, chronic kidney disease, and the use of drugs.
Vitamin D
Eighty percent of vitamin D content derives from ultraviolet (UV) radiation, and only 20% from food and dietary supplements.49 For example, 5 mL of cod liver oil contains ~400 International Units (IU) of vitamin D,50 while exposing the body to sunlight may result in the production of 10,000–25,000 IU.51
On the other hand, diet plays an important role in optimizing vitamin D concentration both in the mid-winter season and in the regions with moderate and high latitudes, characterized by low solar exposure and cold temperatures.52
Vitamin D levels, as reflected by serum 25OHD levels, are genetically highly regulated. Depending on the type of study, between 25% and 80% of the variation is genetically mediated and influenced by the vitamin D transport binding protein. Ethnic and racial differences in vitamin D levels are largely due to environmental factors such as latitude and exposure to sunlight.53 Vitamin D has pleiotropic activity on more than 2,100 genes with skeletal and extra-skeletal activities and acts as regulator of the immune response.
Low serum calcidiol has been related to an increased risk of cardiovascular events and mortality,54 and physiological serum 25OHD concentrations have been associated with improved thyroid function.55
There exists evidence that both exercise training and vitamin D supplementation may benefit musculoskeletal health in older adults, and it is plausible that in combination their effects may be additive.56
It is extremely challenging to assess worldwide variance in vitamin D levels, although empirical data have identified groups who are at risk of deficiency, such as newborns and institutionalized elderly.57
In 2011, the National Academy of Medicine, after an extensive review of the literature, concluded that for maximum bone health, a minimal blood level of 20 ng/mL of 25OHD is adequate.58
Vitamin D deficiency and insufficiency is a global health issue that afflicts more than one billion children and adults worldwide.59 Associations between vitamin D deficiency and myriad acute and chronic illnesses have been identified, including pre-eclampsia, childhood dental caries, periodontitis, autoimmune disorders, infectious diseases, cardiovascular disease, deadly cancers, type 2 diabetes, and neurological disorders.59
Vitamin D can be supplemented as cholecalciferol, a safe precursor of the active form of the vitamin/hormone D, and the European Food Safety Authority established a ceiling safe intake level of 4,000 IU/day for adults and pregnant women and 2,000 IU/day for children.60 Treatment of deficiencies is not identical to supplementation. International and Italian guidelines suggest that when a condition of deficiency has been established with 25(OH) cholecalciferol and parathyroid hormone blood tests, it is appropriate to restore normal values with a cumulative dose of 300,000–1,000,000 IU over 3–4 weeks followed by a maintenance dose of 800–2,000 IU/day.61
There are data focused on the role of vitamin D on chronic painful conditions such as osteoarthritis, with a general agreement that further research is required to elucidate its role in the development and progression of these conditions.62,63 Calcitriol, the hormonally active form of vitamin D, has anti-inflammatory effects, including suppression of prostaglandin action, inhibition of p38 stress kinase signaling, and pro-inflammatory cytokines, with the inhibition of nuclear factor kappa B (NFκB) signaling.64
Nutraceuticals
Nutraceuticals can deliver bioactive agent(s) at dosages able to affect human health,65 although these compounds require absorption in order to exert any bioactivity and, hence, formulation plays a major role in the enhancement of their absorption and physiological effect. On the other hand, Phase II metabolites of colonic origin may also exert bioactivity and be potential candidates for the development of nutraceuticals that exert analgesic properties.66,67
Citrus bergamia Risso et Poiteau is a fruit, commonly called bergamot, that is grown primarily in Calabria, Italy, where different cultivars are grown: Femminello, Fantastico, and Castagnaro. The (poly)phenolic profile of bergamot juice is due primarily to flavanones, in particular neoeriocitrin, naringin, and neohesperidin,68 which are antioxidant compounds endowed with biological activity.69 Free radicals play a crucial role in enhanced pain sensitivity experienced during inflammatory diseases, with Lauro et al recently demonstrating that thermal hyperalgesia induced by intraplantar injection of carrageenan is reversed by employment of bergamot juice in a dose-dependent manner.70 The development of thermal hyperalgesia causes carbonylation of mitochondrial proteins, included sirtuin 3 (SIRT3), an important epigenetic factor linked to inflammation, human metabolism, aging, and cancer. Defects in SIRT3 acetylation machinery lead to a mitochondrial hyperacetylation, with an imbalance in crucial enzyme control such as the forkhead box protein (FOXO) or mitochondrial antioxidant manganese superoxide dismutase (MnSOD) and histone acetylation that leads, in turn, to changes in chromatin structure and transcriptional deregulation of genes involved in the control of cell cycle progression, differentiation, and apoptosis.71 Knowing the putative benefits of the addition of nutraceuticals therapy to a healthy diet, it is important to emphasize that, while they may have similar general effects, food and drugs are distinct entities. Drugs generally involve single molecules, while nutraceuticals or food bioactives usually act as pools of compounds (both in terms of food composition and circulating metabolites). Moreover, most nutraceuticals and food bioactives are multi-target bio-activators; their final effect depends on interactions with several mediators and on their effectiveness at regulating specific targets.66,67
Nature and nurture of pain
Pain is variable and hereditable, and is shaped by interactions between nature (e.g., internal processes that predispose pain development according to a genetic code) and nurture (e.g., external processes and environmental effects).72
Several genes influence pain phenotypes (such as intensity, duration, impact on function, and response to analgesia), ranging from metabolic genes and those encoding enzymes and transcription regulators, to genes encoding receptors, cytokines, and ion channels. A significant number of genotype–phenotype correlations characterized in human gene association studies on pain have been addressed in order to characterize all genotype–phenotype correlations;73 however, the value of some of these studies is limited due to replication challenges.74,75
Nevertheless, recent advances in genetic pathway analysis, availability of unbiased genome-wide genetic data in large pain cohorts for meta-analysis, and novel bioinformatics strategies for function validation of discovered genetic factors may provide new insight on genetic mechanisms of pain and potential targets for future analgesic drugs.76 When nurture factors are considered, they may work as pain triggers and modifiers that influence pain behavior and pain perception.
Factors that predict pain development and perception include demographic characteristics (e.g., sex/gender, race/ethnicity, age), personal features (e.g., previous pain experiences, emotions, cognition), cultural setting (e.g., customs, beliefs), and socioeconomic factors (e.g., social support, acceptance, incentives, education, occupation, and quality of life).
Pain modifiers involve clinical features such as disease-related variables, treatment outcomes, operative procedures, degree of tissue trauma, lifestyle (e.g., diet and habits such as exercise, alcohol consumption, smoking, stress), and psychosocial factors including sleep, catastrophizing, fatigue, anxiety, fear, anticipation of more pain, emotional distress, and somatic awareness.
Nature and nurture contribute to pain and analgesic response both independently and through interplay between each other. George et al reported an example of such interplay in which different combinations of genotypes and psychological factors predicted pain phenotypes and disability outcomes following arthroscopic shoulder surgery: an interaction between the adrenoceptor beta 2 (ADRB2) gene and depression predicted worse shoulder pain and decreased function, while an interaction between arginine vasopressin receptor 1A (AVPR1A) alleles and kinesophobia predicted postoperative pain intensity, and an interaction between interleukin 6 (IL6) gene polymorphism and catastrophizing explained 8.1% of explained interindividual variability in postoperative pain and function.77 Similarly, high pain catastrophizing and low COMT enzyme activity (as determined by COMT haplotype) have been found to increase experimental shoulder pain.78
Nature and nurture may have a combined effect not only on pain but also on response to analgesia, in a sex-specific manner. For example, a single nucleotide polymorphism in the vasopressin-1A receptor gene (AVPR1A) interacted with stress levels in males only, influencing desmopressin inhibition of capsaicin pain.72 These findings support the importance of multi-dimensional pain phenotyping as well as comprehensive genetic studies that may improve our ability to predict and prevent painful conditions, and may lead to personalized and integrative pain management.
Epigenetics, inflammation, and pain: the role of miRNAs and specialized pro-resolving mediators (SPMs)
To develop personalized pain management, epigenetics might also play a key role in the near future. Micro-RNAs (miRNAs) are short noncoding RNA molecules that regulate 30–80% of genes by influencing gene transcription. They are short double-stranded RNA molecules that act by direct (physical) interaction with target messenger RNA (mRNA) in cytoplasm. miRNAs have been proposed as a novel mechanism regulating the development and progression of cancer and many other diseases characterized by an inflammatory component.
Resolvin D1 (RvD1) is a specialized lipid mediator derived from docosahexaenoic acid (DHA) that regulates resolution of inflammation, although the intimate mechanisms are not fully understood and might involve regulation of miRNAs. Recchiuti et al analyzed specific miRNAs involved in the resolution of acute inflammation following RvD1 injection.79 Their bioinformatics analysis revealed that RvD1-regulated miRNAs target genes with important roles in inflammation and resolution, including cytokines and interleukins, intermediates in TNF-α signaling, and NFκB pathway. The relationship between pain and miRNAs has already been described, suggesting an emergent topic in pain research and possible roles as biomarkers.80,81
Omega 3 fatty acids, inflammation, and pain
The omega-3 family comprises α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and DHA. EPA and DHA can be found in fish and fish oils, and are considered to be marine sources of fatty acids. Both EPA and DHA must be provided externally through diet and/or oral supplementation.82,83
EPA and DHA are precursors of eicosanoids (prostaglandins, leukotrienes, and thromboxanes) and other lipid-derived mediators, such as resolvins, protectins, and maresins, which contribute to resolution of inflammation.82 SPMs are generated during inflammation resolution and may control early events in acute inflammation.84 Specifically, these SPMs have important properties/actions in resolving inflammation by limiting neutrophil infiltration into damaged tissue, by counter-regulating cytokine production, and therefore reducing pain and edema, while promoting tissue repair and wound healing.79,84
It can be hypothesized that the development of a chronic inflammatory response is a consequence of unsolved inflammation, probably due to a defect in SPM synthesis. Another feasible hypothesis is that impaired synthesis of SPMs (likely via reduced oral intake of omega-3s) leads to the unresolved inflammation underlying chronic pathological conditions. Moreover, the production of SPMs may also be decreased due to comorbidities such as obesity.85
Final remarks
Finally, we analyzed the published data looking specifically at how nutrition may improve pain therapy. The topic is not well recognized in the clinical setting; indeed, a 2007 study indicated that only 45.7% of overweight patients with osteoarthritis were advised that weight loss might help their conditions.86
Some active substances introduced through diet or as supplemental factors can interact with numerous molecular targets, as DNA polymerase, leukotrienes, prostaglandins, and cyclooxygenase 2 (COX-2).87 Table 1 summarizes the key points discussed.
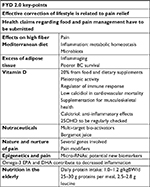  | Table 1 Key points discussed in FYD2 Abbreviations: FYD, Feed Your Destiny; BC, breast cancer; 25OHD, 25-hydroxyvitamin D; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; BW, body weight. |
Discussion
This workshop was intended to clarify the extent to which nutrition demonstrates efficacy and safety for pain therapy. A Mediterranean diet should be part of chronic pain management, as it normalizes proinflammatory states related to obese and overweight body mass index, which ideally ranges between 18.5 and 24.9 kg/m2, although is unfortunately increasing rapidly across developed nations. Furthermore, a Mediterranean diet has been established as decreasing chronic pain and gastrointestinal disorders. Additional randomized clinical trials are needed to assess the efficacy of dietary interventions for weight loss and survival in breast cancer patients.88 In the absence of more definitive empirical data, it is recommended that patients with breast cancer follow the guidelines provided by the American Cancer Society, which suggest at least 30 minutes of moderate physical activity, at a minimum of 5 days a week to maintain a healthy lifestyle.89
Diet is particularly important for seniors, particularly regarding protein consumption. Protein intake along with the type of protein consumed should be properly established, as well as when patients should consume them.90,91 Vitamin D should be regularly checked by measuring plasma 25OHD levels to avoid its insufficiency or deficiency.92
Safety is imperative for nutraceuticals or food supplements, which are ideally regulated. If a food chain compound demonstrates evidence for a beneficial effect with a wide safety window, it should receive approval as a New Dietary Ingredient (NDI) by the US Food and Drug Administration. NDIs must be safe in the recommended dose as established by appropriate safety data, and their efficacies should be evidenced by mechanistic plausibility and clinical data. In Europe, food supplements are also regulated. The objective of the harmonized rules regarding those products in Directive 2002/46/EC is to protect consumers against potential health risks from those products and to ensure that they are not provided with misleading information.
When it comes to supplements rich in phenolic compounds to ease pain, more evidence is required. At present, information is very limited, and most of the science behind supplements for pain relief is not supported by robust physiological investigation. Approaches taking into account the extensive metabolism that phenolic compounds generally undergo following consumption are essential if we are to better understand the prospects of these widespread plant bioactives for pain relief.
Among the future prospective of pain therapy, new studies will hopefully contribute to better recognition of specific molecular mechanisms and intracellular signaling pathways related to pain development and relief. Currently, therapeutic prospects of miRNAs in the pain field are largely unexplored. Manipulation of miRNAs offers the possibility to control multiple targets including neuro-immune interactions, nociceptive processing, and cognitive pathways. In the near future, we anticipate that it will be possible to determine whether miRNAs can be used as potential biomarkers of neuropathic pain (persistent stimulation of nociceptors, constant pain following resolution of tissue damage, or even in the absence of causative factors) and neurogenic pain syndromes.
New research will ideally address questions regarding the role and mechanisms of action of miRNA in decreasing chronic inflammation. We anticipate that it will be possible to determine whether there are miRNA differences between nociceptive (on-going tissue damage such as cancer, osteoarthritis) and neuropathic pain, and whether their analysis could help predict exactly what occurs in patients, thereby suggesting that miRNAs can be used as potential biomarkers for diagnosis, prognosis, or treatment of painful conditions. Given the anti-inflammatory component of EPA and DHA, it is evident that they might be beneficial in treating pain in which an inflammatory component is present. However, it is also feasible that they might have a role as co-adjuvants in other types of pain. Nevertheless, our body of knowledge is still limited and additional studies are needed to provide clarification.
In summary, current management of pain should include, in addition to aspects of traditional interdisciplinary care, education regarding a balanced diet, correction of maladaptive eating patterns, and, if necessary, use of supplements in order to provide the broadest, most effective approach to treating pain.
Disclosure
The authors report no conflicts of interest in this work.
References
De Gregori M, Muscoli C, Schatman ME, et al. Combining pain therapy with lifestyle: the role of personalized nutrition and nutritional supplements according to the SIMPAR Feed Your Destiny approach. J Pain Res. 2016;9:1179–1189. | ||
Martini D, Rossi S, Biasini B, et al. Claimed effects, outcome variables and methods of measurement for health claims proposed under European Community Regulation 1924/2006 in the framework of protection against oxidative damage and cardiovascular health. Nutr Metab Cardiovasc Dis. 2017;27(6):473–503. | ||
Martini D, Biasini B, Rossi S, et al. Claimed effects, outcome variables and methods of measurement for health claims on foods proposed under European Community Regulation 1924/2006 in the area of appetite ratings and weight management. Int J Food Sci Nutr. 2018;69(4):389–409. | ||
Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111(1):26–37. | ||
Bäckryd E, Lind AL, Thulin M, Larsson A, Gerdle B, Gordh T. High levels of cerebrospinal fluid chemokines point to the presence of neuroinflammation in peripheral neuropathic pain – a cross-sectional study of two cohorts of patients compared to healthy controls. Pain. 2017;158(12):2487–2495 . | ||
Bédard A, Lamarche B, Corneau L, Dodin S, Lemieux S. Sex differences in the impact of the Mediterranean diet on systemic inflammation. Nutr J. 2015;14:46. | ||
De Angelis M, Garruti G, Minervini F, Bonfrate L, Portincasa P, Gobbetti M. The food-gut human axis: the effects of diet on gut microbiota and metabolome. Curr Med Chem. Epub 2017 Apr 27. | ||
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. | ||
Kelly SA, Hartley L, Loveman E. Whole grain cereals for the primary or secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017; 8:CD005051. | ||
Chanson-Rolle A, Meynier A, Aubin F. Systematic review and meta-analysis of human studies to support a quantitative recommendation for whole grain intake in relation to type 2 diabetes. PLoS One. 2015;10(6):e0131377. | ||
Theodoratou E, Timofeeva M, Li X, Meng X, Ioannidis JPA. Nature, nurture, and cancer risks: genetic and nutritional contributions to cancer. Annu Rev Nutr. 2017;37:293–320. | ||
Vetrani C, Costabile G, Luongo D. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition. 2016;32(2):217–221. | ||
Vitiello V, Germani A, Capuzzo Dolcetta E, Donini LM, Del Balzo V. The New Modern Mediterranean Diet Italian Pyramid. Ann Ig. 2016;28(3):179–186. | ||
Landaeta-Díaz L, Fernández JM, Da Silva-Grigoletto M, et al. Mediterranean diet, moderate-to-high intensity training, and health-related quality of life in adults with metabolic syndrome. Eur J Prev Cardiol. 2013;20(4):555–564. | ||
Tremblay MS, Esliger DW, Tremblay A, Colley R. Incidental movement, lifestyle-embedded activity and sleep: new frontiers in physical activity assessment. Can J Public Health. 2007;98(Suppl 2):S208–217. | ||
Johnson BT, Acabchuk RL. What are the keys to a longer, happier life? Answers from five decades of health psychology research. Soc Sci Med. 2018;196:218–226. | ||
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. | ||
Latorre R, Sternini C, De Giorgio, Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil. 2016;28(5):620–630. | ||
Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol. 2004;61(1):45–54. | ||
McGuire C, Boundouki G, Hockley JRF, et al. Ex vivo study of human visceral nociceptors. Gut. 2018;67(1):86–96. | ||
Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. 2012;2:104. | ||
Franco-de-Moraes AC, de Almeida-Pititto B, da Rocha Fernandes G, Gomes EP, da Costa Pereira A, Ferreira SRG. Worse inflammatory profile in omnivores than in vegetarians associates with the gut microbiota composition. Diabetol Metab Syndr. 2017;9:62. | ||
Rajilic´-Stojanovic´ M, Biagi E, Heilig HG. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1792–1801. | ||
Jeffery IB, O’Toole PW, Öhman L. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61(7):997–1006. | ||
Holtmann G, Shah A, Morrison M. Pathophysiology of functional gastrointestinal disorders: a holistic overview. Dig Dis. 2017;35(Suppl 1):5–13. | ||
Moloney RD, Johnson AC, O’Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF. Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther. 2016;22(2):102–117. | ||
Foster JA, Rinaman L, Cryan JF. Stress and the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. | ||
Adams RJ, Appleton SL, Gill TK, Taylor AW, Wilson DH, Hill CL. Cause for concern in the use of non-steroidal anti-inflammatory medications in the community – a population-based study. BMC Fam Pract. 2011;12:70. | ||
Ho W, Spiegel BM. The relationship between obesity and functional gastrointestinal disorders: causation, association, or neither? Gastroenterol Hepatol (N Y). 2008;4(8):572–578. | ||
Bear T, Philipp M, Hill S, Mündel T. A preliminary study on how hypohydration affects pain perception. Psychophysiology. 2016;53(5):605–610. | ||
Stafeev IS, Vorotnikov AV, Ratner EI, Menshikov MY, Parfyonova YV. Latent inflammation and insulin resistance in adipose tissue. Int J Endocrinol. 2017;2017:5076732. | ||
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. | ||
Narouze S, Souzdalnitski D. Obesity and chronic pain: opportunities for better patient care. Pain Manag. 2015;5(4):217–219. | ||
Swaroop JJ, Rajarajeswari D, Naidu JN. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2012;135:127–130. | ||
Panee J. Monocyte Chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60(1):1–12. | ||
Lagathu C, Christodoulides C, Tan CY, et al. Secreted frizzled-related protein 1 regulates adipose tissue expansion and is dysregulated in severe obesity. Int J Obes (Lond). 2010;34(12):1695–1705. | ||
Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Healthspan. 2013;2(1):8. | ||
Szarc vel Szic K, Declerck K, Vidakovic´ M, Vanden Berghe W. From inflammaging to healthy aging by dietary lifestyle choices: is epigenetics the key to personalized nutrition? Clin Epigenetics. 2015;7(1):33. | ||
Braakhuis A, Campion P, Bishop K. The effects of dietary nutrition education on weight and health biomarkers in breast cancer survivors. Med Sci (Basel). 2017;5(2). pii: E12. | ||
Anderson C, Harrigan M, George SM. Changes in diet quality in a randomized weight loss trial in breast cancer survivors: the lifestyle, exercise, and nutrition (LEAN) study. NPJ Breast Cancer. 2016;2:16026. | ||
Ogunleye AA, Holmes MD. Physical activity and breast cancer survival. Breast Cancer Res. 2009;11(5):106. | ||
Holmes MD, Chen WY, Hankinson SE, Willett WC. Physical activity’s impact on the association of fat and fiber intake with survival after breast cancer. Am J Epidemiol. 2009;170(10):1250–1256. | ||
Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753–765. | ||
Mourouti N, Kontogianni MD, Papavagelis C, Panagiotakos DB. Diet and breast cancer: a systematic review. Int J Food Sci Nutr. 2015;66(1):1–42. | ||
Abellan van Kan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13(8):708–712. | ||
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Aging. 2010;39(4):412–423. | ||
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Epidemiol. 2004;159(4):413–421. | ||
Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–559. | ||
Holick MF. Sunlight and vitamin D. Both good for cardiovascular health. J Gen Intern Med. 2002;17(9): 733–735. | ||
Cortese M, Riise T, Bjørnevik K, et al. Timing of use of cod liver oil, a vitamin D source, and multiple sclerosis risk: the EnvIMS study. Mult Scler. 2015;21(14):1856–1864. | ||
Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3(2):118–126. | ||
Engelsen O. The relationship between ultraviolet radiation exposure and vitamin d status. Nutrients. 2010;2(5):482–495. | ||
Buillon R. Genetic and racial differences in the vitamin D endocrine system. Endocrinol Metab Clin N Am. 2017;46(4):1119–1135. | ||
Dror Y, Giveon SM, Hoshen M, Feldhamer I, Balicer RD, Feldman BS. Vitamin D levels for preventing acute coronary syndrome and mortality: evidence of a nonlinear association. J Clin Endocrinol Metab. 2013;98(5):2160–2167. | ||
Mirhosseini N, Brunel L, Muscogiuri G, Kimball S. Physiological serum 25-hydroxyvitamin D concentrations are associated with improved thyroid function-observations from a community-based program. Endocrine. 2017;58(3):563–573. | ||
Antoniak AE1, Greig C. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: a systematic review and meta-analysis. BMJ Open. 2017;7(7):e014619. | ||
Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr;111(1):23–45. | ||
IOM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D. Committee to Review Dietary Reference Intakes for Calcium and Vitamin D Washington, DC: The National Academies Press Institute of Medicine; 2011. | ||
Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153–165. | ||
Efsa Panel on dietetic products, nutrition and allergies (NDA). Scientific opinion on the tolerable upper intake level of vitamin D. EFSA J. 2012;10(7):2813. | ||
Adami S, Romagnoli E, Carnevale V, et al. Guidelines on prevention and treatment of vitamin D deficiency. Reumatismo. 2011(63)3 129–147. | ||
Straube S, Derry S, Straube C, Moore RA. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;5:CD007771. | ||
Mabey T, Honsawek S. Role of vitamin D in osteoarthritis: molecular, cellular, and clinical perspectives. Int J Endocrinol. 2015;2015:383918. | ||
Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. | ||
Bresciani L, Martini D, Mena P, et al. Absorption profile of (poly)phenolic compounds after consumption of three food supplements containing 36 different fruits, vegetables, and berries. Nutrients. 2017;9(3). pii: E194. | ||
Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18(14):1818–1892. | ||
Rodriguez-Mateos A, Vauzour D, Krueger CG, et al. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol. 2014;88(10):1803–1853. | ||
Gattuso G, Caristi C, Gargiulli C, Bellocco E, Toscano G, Leuzzi U. Flavonoid glycosides in bergamot juice (Citrus bergamia Risso). J Agric Food Chem. 2006;54(11):3929–3935. | ||
Khan MK, Zill EH, Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J Food Compost Anal. 2014;33(1):85–104. | ||
Lauro F, Giancotti LA, Ilari S, et al. Inhibition of spinal oxidative stress by bergamot polyphenolic fraction attenuates the development of morphine induced tolerance and hyperalgesia in mice. PLoS One. 2016;11(5):e0156039. | ||
Tao R, Coleman MC, Pennington JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893–904. | ||
Mogil JS, Sorge RE, LaCroix-Fralish ML, et al. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat Neurosci. 2011;14(12):1569–1573. | ||
Mogil JS. Pain genetics: past, present and future. Trends Genet. 2012;28(6):258–266. | ||
NCI-NHGRI Working Group on Replication in Association Studies, Chanock SJ, Manolio T, et al. Replicating genotype-phenotype associations. Nature. 2007;447(7145):655–660. | ||
Ioannidis JP. Why most published research findings are false: author’s reply to Goodman and Greenland. PLoS Med. 2007;4(6):e215. | ||
Renthal W. Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease. 5th ed. San Diego, CA: Academic Press; 2015. | ||
George SZ, Wu SS, Wallace MR, et al. Biopsychosocial influence on shoulder pain: influence of genetic and psychological combinations on twelve-month postoperative pain and disability outcomes. Arthritis Care Res (Hoboken). 2016;68(11):1671–1680. | ||
George SZ, Dover GC, Wallace MR, et el. Biopsychosocial influence on exercise-induced delayed onset muscle soreness at the shoulder: pain catastrophizing and catechol-o-methyltransferase (COMT) diplotype predict pain ratings. Clin J Pain. 2008;24(9):793–801. | ||
Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-MiRNA circuits. FASEB J. 2011;25(2):544–560. | ||
Kress M, Hüttenhofer A, Landry M, et al. microRNAs in nociceptive circuits as predictors of future clinical applications. Front Mol Neurosci. 2013;6:33. | ||
Sommer C. Exploring pain pathophysiology in patients. Science. 2016;354(6312):588–592. | ||
Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem. 2013;69(3):633–651. | ||
Lorente-Cebrián S, Costa AG, Navas-Carretero S, et al. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem. 2015;71(2):341–349. | ||
Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31(4):1273–1288. | ||
Neuhofer A, Zeyda M, Mascher D, et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 2013;62(6):1945–1956. | ||
Fontaine KR, Haaz S, Bartlett SJ. Are overweight and obese adults with arthritis being advised to lose weight? J Clin Rheumatol. 2007;13(1):12–15. | ||
Lakhan SE, Ford CT, Tepper D. Zingiberaceae extracts for pain: a systematic review and meta-analysis. Nutr J. 2015;14:50. | ||
Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. | ||
Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. | ||
Rondanelli M, Perna S, Faliva MA, Peroni G, Infantino V, Pozzi R. Novel insights on intake of meat and prevention of sarcopenia: all reasons for an adequate consumption. Nutr Hosp. 2015;32(5):2136–2143. | ||
Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12(1):86–90. | ||
Mosekilde L. Vitamin D and the elderly. Clin Endocrinol (Oxf). 2005;62(3):265–281. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
