Back to Journals » Pragmatic and Observational Research » Volume 8
Seasonal patterns of oral antihistamine and intranasal corticosteroid purchases from Australian community pharmacies: a retrospective observational study
Authors Carney AS, Price DB , Smith PK , Harvey R, Kritikos V , Bosnic-Anticevich SZ , Christian L, Skinner DA , Carter V, Durieux AMS
Received 8 February 2017
Accepted for publication 17 May 2017
Published 30 August 2017 Volume 2017:8 Pages 157—165
DOI https://doi.org/10.2147/POR.S134266
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Stefan Glück
A Simon Carney,1 David B Price,2,3 Pete K Smith,4 Richard Harvey,5,6 Vicky Kritikos,7 Sinthia Z Bosnic-Anticevich,7,8 Louise Christian,9 Derek A Skinner,10 Victoria Carter,10 Alice MS Durieux3
1Department of Otolaryngology – Head and Neck Surgery, Flinders University, Adelaide, SA, Australia; 2Centre of Academic Primary Care, University of Aberdeen, Aberdeen, UK; 3Observational and Pragmatic Research Institute, Singapore; 4Clinical Medicine, Griffith University, Southport, QLD, 5Applied Medical Research Centre, University of New South Wales, 6Faculty of Medicine and Health Sciences, Macquarie University, 7Woolcock Institute of Medical Research, University of Sydney, 8Central Sydney Area Health Service, Sydney, NSW, 9NostraData, Kew, VIC, Australia; 10Optimum Patient Care, Cambridge, UK
Purpose: To explore patterns in the purchase of prescription and over-the-counter (OTC) oral antihistamines (OAHs) and intranasal corticosteroids (INCSs) by patients, from pharmacies in different geographical regions of Australia.
Patients and methods: Retrospective observational study using a database containing anonymous pharmacy transaction data from 20.0% of the pharmacies in Australia that link doctor prescriptions and OTC information. Pharmacy purchases of at least one prescription or OTC rhinitis treatment during 2013 and 2014 were assessed.
Results: In total, 4,247,193 prescription and OTC rhinitis treatments were purchased from 909 pharmacies over 12 months. Of treatments purchased, 75.9% were OAHs and 16.6% were INCSs. OTC purchases of both treatments exceeded purchases through prescription. OTC OAHs purchasing patterns were seasonal and almost identical in the Australian Capital Territory, Victoria, Western Australia, South Australia, and New South Wales, and similar seasonal patterns for OTC INCSs were noted in most regions except for South Australia and Tasmania. Prescription purchasing patterns of both OAHs and INCSs remained unchanged throughout the year in most regions.
Conclusion: This large-scale retrospective observational study identified seasonal purchasing patterns of OTC and prescription OAHs and INCSs in a real-world setting. It highlighted that seasonality only affects OTC purchasing patterns of OAHs and INCSs across Australia and that practitioner prescribing remains unchanged, suggesting that it is only for persistent disease.
Keywords: allergic rhinitis, medication, over-the-counter, prescription, therapy, treatment
Introduction
Rhinitis is an umbrella term that encompasses a collection of disorders of the nose resulting from inflammation and/or dysfunction of the nasal mucosa. It is characterized by one or more of the following nasal symptoms: anterior or posterior rhinorrhea, sneezing, nasal congestion, and/or itching.1 Rhinitis is classified as allergic rhinitis (AR) or non-allergic rhinitis (NAR), but some types of rhinitis can have both AR and NAR components (known as “mixed rhinitis” [MR]).2 Regardless of the type of rhinitis, untreated or uncontrolled symptoms can have a significant negative impact on patient’s quality of life, including impairments in work productivity, school performance, social interactions, and sleep.3,4 Rhinitis is a significant cause of morbidity and poses a substantial economic and health burden to individuals and society.5,6
AR is the most prevalent form of rhinitis affecting 10%–40% of the global population and its prevalence is increasing in children and adults.3,5 In Australia, AR affects 17% of the population and is predicted to increase in prevalence by 70% in the next 35 years.7 In 2007–2008, AR rates were the highest in Australian Capital Territory (ACT), Western Australia (WA), Victoria (VIC), and South Australia (SA). New South Wales (NSW) and Queensland (QLD) had rates significantly lower than the overall rate for Australia.8 In contrast, estimates of the prevalence of NAR and MR in Australia have yet to be determined. International studies have reported that the ratio of AR to pure NAR is 3:1,9 and data suggest that 44%–87% of patients with AR may have MR.10,11
Traditionally, AR was classified based on the timing of allergen exposure into either seasonal (relating to AR caused by outdoor allergens, such as pollens and molds) or perennial (relating to AR caused by indoor allergens, such as house dust mites, molds, and animal dander). A revised system, proposed in the 2001 Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines, classifies AR into either intermittent or persistent according to the duration of symptoms, and either mild or moderate-to-severe based on the severity of symptoms and their impact on quality of life.12 The ARIA system is appropriate for use in Australia, as in certain regions of Australia: 1) pollens and molds may be perennial allergens; 2) even patients allergic to “seasonal” allergens may have symptoms for most of the year as the pollen season can be difficult to define, particularly in QLD; and 3) most patients are sensitized to multiple allergens and thus symptoms may be present throughout the year.13
In Australia, the most troublesome pollens tend to be airborne pollens produced by Northern Hemisphere grasses, trees, and flowering weed species. Grass pollens from temperate grasses, such as perennial ryegrass, timothy, and subtropical grasses including Bermuda (couch) and Bahia are clinically important seasonal allergens that can trigger AR in some individuals.14,15 Small wind-distributed pollens from trees (including cypress, silver birch, English oak, and Murray pine) and weeds (including ragweed, parthenium weed, pellitory weed, and Paterson’s curse) are more likely to trigger AR. Owing to geographical variability in climate, subtropical grasses have different pollination seasons across Australia. Moreover, VIC has higher peak pollen counts than many other regions of Australia because of the impact of springtime northerly winds that carry pollen from grasslands north of its border.16
The goal of AR management is to achieve optimal symptom control so as to prevent complications and disease progression.6,17 Achieving this goal depends on identifying the triggers and assessing the nature and severity of symptoms. For AR, management strategies include reducing allergen exposure, pharmacotherapy, and immunotherapy.17 ARIA guidelines recommend that pharmacological treatment should consider the severity and duration of the disease, the patient’s preference, and the efficacy, safety, availability, and costs of medications.17 Recommended therapies include intranasal corticosteroids (INCSs), second-generation oral antihistamines (OAHs), oral leukotriene receptor antagonists (LTRAs), and intranasal antihistamines.17,18
All guidelines agree that INCSs are the most effective monotherapy for AR.6,17–19 INCSs are key to the management of moderate-to-severe AR. They have some efficacy on ocular symptoms20 and have been shown to be superior to either OAHs or LTRAs.21,22 The newer INCSs have similar efficacy with excellent safety and tolerability profiles;23 however, several days’ use may be needed to reach maximum response.24 Second-generation OAHs are recommended for mild intermittent AR and have minimal adverse effects.25 Until recently, INCSs were regarded as the most effective treatment option for AR. A new intranasal formulation of a corticosteroid and an antihistamine combined in a single intranasal device has been shown to provide added efficacy and for some patients, that is substantial.26 Of more importance is the convenience factor for patients who use both intranasal antihistamines and INCSs. Although the addition of an OAH to an INCS has been investigated, no additional efficacy above that achieved by INCS monotherapy was demonstrated,27 with most published data confirming no clinical benefits gained by adding oral therapy to intranasal therapy.17
The Allergic Rhinitis in Australia report published in 2011,8 which used pharmaceutical wholesale supply information, found that the wholesale cost to community pharmacies of INCSs and OAHs had almost doubled in the previous 10 years. In 2001, the total wholesale cost of these medications was $107.8 million and in 2010 it was $226.8 million. It was reported that this trend had strengthened over the 10-year period owing largely to increasing availability of over-the-counter (OTC) OAHs. The majority of OAH products available in Australia in 2010 (125 out of 147) had OTC status, making 9 out of 10 OAH products obtainable without consulting a pharmacist or medical practitioner. Of the 12 INCS products available in 2010, 5 had OTC status and 7 had either pharmacist-only (Schedule 3) or prescription-only (Schedule 4) status. Each year monthly wholesale supplies of OAHs started to increase around July and peaked around October to November and monthly wholesale supplies of INCSs started to increase in August, peaking around November.
AR is one of the most underestimated respiratory conditions, by physicians and patients. Its management is frequently complicated by delayed diagnosis and proper treatment because of attempts by patients to self-medicate with a wide range of OTC medications available from pharmacies without the need for doctors’ prescription.13 In Australia, apart from reported patterns and costs of wholesale supplies of rhinitis treatments to pharmacies,8 little is known about the nature or extent of rhinitis treatment purchases from pharmacies, in light of the increasing size of the OTC medicine market and number of people with rhinitis who choose to self-medicate. This study aimed to explore patterns in the purchases of prescription and OTC OAHs and INCSs from pharmacies in different geographical regions of Australia.
Materials and methods
This was a retrospective observational study of a historical cohort conducted with data from a database collected during 2013 and 2014. The study was registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (registration number ENCEPP/SDPP/8507) and approved by the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee (approval reference number ADEPT0215).
Data source
NostraData (https://www.nostradata.com.au/Public/Home/About) provided a suitable dataset of anonymous pharmacy transaction data available from 20% of pharmacies in Australia that links doctor prescriptions and OTC information. Data within this dataset describe the details of valid transactions completed at the pharmacy including name(s) of product(s) purchased, prescription or OTC status, postcode of purchase, and price paid. As the dataset does not contain patient demographic information or longitudinal data, it is, therefore, not possible to track individual patient purchases at different NostraData pharmacies or on different occasions.
From the pharmacy claims dataset, we assessed pharmacy purchases of at least one prescription or OTC rhinitis treatment (used as a proxy for a diagnosis of rhinitis) during 2013 and 2014. Categories of rhinitis treatments included OAHs, INCSs, intranasal antihistamine and corticosteroid combinations, non-steroidal nasal sprays, LTRAs, eye drops for allergic conjunctivitis, oral corticosteroids, and injectable corticosteroids. A list of drugs included in each therapeutic class and the most representative in terms of prescription and OTC purchases is presented in Table 1. As LTRAs alone are likely to be purchased for asthma treatment rather than rhinitis, and individual patients could not be tracked in this dataset, LTRAs were only included as rhinitis therapy for pharmacy transactions without additional asthma/chronic obstructive pulmonary disease treatment. Categories of asthma/chronic obstructive pulmonary disease treatments included short-acting beta2 agonists, inhaled corticosteroids, long-acting beta2 agonists, inhaled corticosteroid/long-acting beta2 agonist combination therapy, short-acting muscarinic antagonists, long-acting muscarinic antagonists, chromones, and theophylline. Patient purchases of oral and systemic corticosteroids (without other rhinitis therapy) were excluded.
Data analysis
Data were analyzed using MySQL and Microsoft Excel 2011 software. Descriptive statistics were used to summarize sample characteristics and pharmacy transactions involving purchases of prescription and OTC OAHs and/or INCSs specifically. All pharmacy purchases during the study period were analyzed together and reported as average numbers and percentages per month for each of the following geographical regions: NSW, ACT, QLD, VIC, SA, WA, Tasmania (TAS), and Northern Territory (NT).
Results
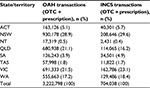
Pharmacy transaction data collected from 909 pharmacies during 2013 and 2014 were assessed. Sample characteristics of pharmacy transactions in different geographical regions over 12 months are shown in Table 2. Of the 8,334,472 pharmacy transactions assessed, 4,247,193 (51.0%) included rhinitis therapy. Prescription and OTC purchases of OAHs and INCSs in different geographic regions over 12 months are shown in Table 3. Of the 4,247,193 prescription and OTC rhinitis treatments purchased over 12 months, 3,222,798 (75.9%) were OAHs and 704,038 (16.6%) were INCSs (Table 2). The highest rates of prescription and OTC OAH purchases (in descending order) were in NSW, VIC, and QLD, whereas the highest rates of prescription and OTC INCS purchases were in NSW, VIC, and WA over the 12 months.
Seasonality in purchasing patterns in different geographical regions
OTC OAHs
Of the 3,222,798 OAHs purchased over 12 months, 2,995,056 (92.9%) were OTC purchases. Seasonal patterns in pharmacy purchases of OTC OAHs in each geographical region are shown in Figure 1A. Almost identical seasonal patterns in purchases were noted in ACT, VIC, WA, SA, and NSW, remaining high from September to November and peaking in October. The highest peak in purchases occurred in the ACT, followed by VIC and WA (Figure 1A).
OTC INCSs
Of the 704,038 INCSs purchased over 12 months, 456,639 (64.9%) were OTC purchases. Seasonal patterns in pharmacy purchases of OTC INCSs in each geographical region are shown in Figure 1B. Seasonal patterns in purchases were similar in most regions remaining high from September to November and peaking in October. However, in SA and TAS, purchasing patterns differed from other regions, with purchases dipping in September and peaking in October in SA, and peaking in December in TAS (Figure 1B).
Prescription OAHs
Of the 3,222,798 OAHs purchased over 12 months, 227,742 (7.1%) were prescription purchases. Seasonal patterns in pharmacy purchases of prescription OAHs in each geographical region are shown in Figure 2A. With the exception of SA, rates of pharmacy purchases of prescription OAHs were low and stable in most regions throughout the 12 months, with a higher rate of purchases in QLD.
Prescription INCSs
Of the 704,038 INCSs purchased over 12 months, 247,399 (35.1%) were prescription purchases. Seasonal patterns in pharmacy purchases of prescription INCSs in each geographical region are shown in Figure 2B. In most regions, patterns in purchases of prescription INCSs, generally, remained stable throughout the 12 months.
Seasonality of purchasing patterns across Australia
Seasonal patterns in pharmacy purchases of prescription and OTC OAHs and INCSs across Australia are shown in Figure 3. Seasonal purchasing patterns across Australia of prescription INCS and OAHs were similar and remained stable throughout the 12 months, but differed from those of OTC INCSs and OAHs, remaining high from September to December, and peaking in October (Figure 3).
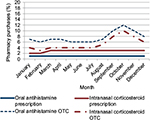  | Figure 3 Seasonal purchasing patterns of oral antihistamines (N=3,222,798) and intranasal corticosteroids (N=704,038) across Australia. Abbreviation: OTC, over-the-counter. |
Discussion
This study is the first to provide a unique “snapshot” of seasonal patterns in purchases of OAHs and INCSs from community pharmacies in different geographical regions of Australia, using prescription and OTC information. It provides valid pharmacy transaction data on rhinitis treatment purchases for patients in a real-world setting. Of the OTC and prescription treatments purchased, 75.9% were OAHs, and 16.6% were INCSs. The highest rates of prescription and OTC OAH purchases (in descending order) were in NSW, VIC, and QLD, whereas the highest rates of prescription and OTC INCS purchases were in NSW, VIC, and WA over the 12 months. OTC purchases of both treatments exceeded purchases through prescription. Similarities and differences were identified in seasonal purchasing patterns of prescription and OTC treatments across various regions. The seasonality of purchasing patterns across Australia of both OTC OAHs and INCSs indicates that most instances of seasonal disease are managed by OTC treatments while the stable purchasing patterns of prescription OAHs and INCSs represent unchanged prescribing behavior for those with persistent disease.
Few published studies have explored patterns of rhinitis treatment purchases from community pharmacies using prescription and OTC information. Research in this area has primarily focused on examining wholesaler supply patterns of rhinitis therapy,8 investigating demographics and medication use, and evaluating clinical and humanistic outcomes of individuals suffering with symptoms of rhinitis who visit the pharmacy, using self-report questionnaire-based surveys without validation of the accuracy of the recording of symptoms and treatments.28–31 Therefore, exploring patterns in pharmacy purchases of OAHs and INCSs using valid prescription and OTC information was considered important, given the increasing size of the OTC medicine market, the number of people with rhinitis who choose to self-medicate, the quality use of medicines in achieving optimal patient outcomes, and the paucity of data about OTC and prescription treatment purchases for rhinitis symptoms in the primary care setting.
This study showed that 75.9% of purchased treatments were OAHs and 16.6% were INCSs. Our results are consistent with those from a 2001 longitudinal community pharmacy-based study in the UK, which found that of the patient reported treatments obtained for AR symptoms, over 70% were OAHs and 14% were INCSs.31 Williams and Scadding reported similar findings upon survey completion of a random sample of French, German, and UK population.32 The Allergic Rhinitis in Australia report also found that three times as many OAHs than INCSs were supplied to pharmacies in 2010.8
This study also showed that 92.9% of OAHs purchases were OTC purchases. OAHs are recommended for mild intermittent AR symptoms, and are effective in relieving sneezing, itching, and rhinorrhea, yet often fail to relieve nasal congestion, which is often the most troublesome symptom.6,17,19 In NAR, second-generation OAHs are of no benefit as histamine release has not been seen in NAR.2 Furthermore, AR can sometimes copresent in association with other types of rhinitis (MR) leading to increased severity requiring INCS treatment.6,19 It is, therefore, likely that a proportion of OTC OAH purchases may not be appropriate first-line treatment according to ARIA guidelines. This not only puts the person with rhinitis at risk of future adverse outcomes due to sub-optimal disease management, but also places the pharmacist at a risk of failure to identify sub optimal treatment and intervene appropriately.
In Australia, although the majority of second-generation OAHs are available OTC (Schedule 2), first-generation sedating OAHs have Schedule 3 status (Pharmacist Only Medicine), requiring pharmacist counseling and advice. Although it is not possible to determine whether patients self-selected OAHs with or without seeking pharmacist advice prior to purchasing them in this study, these findings have wide implications for pharmacy. Over 60% of rhinitis patients are known to self-medicate, often with inappropriate medication, leading to poorly controlled symptoms and sub optimal management.30,31 Moreover, many people who initially present during the pollen season can still have symptoms 6 months later, suggesting that much AR that appears initially to be intermittent is in fact persistent in nature.31 Our study results support the need for new tools and strategies for primary health care providers, which take into account patients’ perception of symptoms and the effect of ongoing treatment. These tools and strategies should aim at facilitating the application of clinical guidelines in practice and quality use of medicines so that rhinitis can be better managed in the primary care setting.
In our study, a greater number of prescription and OTC OAHs were purchased in NSW, VIC, and QLD compared with other regions over 12 months. A possible explanation for this finding is that a greater number of NostraData pharmacies were located in these regions, and hence were able to capture a greater proportion of purchases of OAHs, which represent the most commonly used treatment for AR.17,19 Furthermore, OTC OAH and INCS purchases were found to exceed those of prescription OAHs and INCSs. This is not surprising, since most OAHs and INCSs on the market for the management of rhinitis are now available from Australian pharmacies, without the need for doctor’s prescription.
This study also explored seasonal patterns in purchases of OAHs and INCSs in different geographical areas of Australia. Seasonal patterns in prescription purchases of both treatments were similar and stable in most regions, indicating that prescribing patterns were unaffected by geographical variability in climate and pollination seasons, but were influenced by treatment requirements for persistent symptoms throughout the year. In contrast, the similar and fluctuating seasonal purchasing patterns of OTC OAHs in the ACT, VIC, WA, SA, and NSW indicate these regions share common clinically relevant seasonal aeroallergens despite geographical variability in climate and temperature. Seasonal purchasing patterns of OTC INCSs were similar in most regions, with patterns differing in the southern states of SA and TAS from August to December. This finding could be explained by the fact that, during the time of the study, these states may have experienced a longer winter resulting in a delayed pollination season and/or that they may have undergone weather changes in temperature and humidity, thus triggering nasal symptoms in patients with NAR or MR. As global temperatures increase, it is anticipated that allergen seasons may become longer and subtropical grasses and weeds will become more important allergens in regions with temperate climates.15
A key contribution of our study has been the identification of seasonal pharmacy purchasing patterns across Australia of both prescription and OTC OAHs and INCSs. The seasonality in pharmacy purchasing pattern of OTC OAHs appears to be consistent with the seasonality in pharmaceutical wholesale supply pattern of OAHs8 and with the ARIA guidelines for the treatment of mild intermittent (seasonal) AR.18 The overall seasonal pattern and peak in pharmacy purchases of OTC INCSs seems to mimic that of OTC OAHs, suggesting that INCSs are being appropriately used for the treatment of moderate-to-severe intermittent (seasonal) AR based on the latest AR guidelines.18 It is well recognized that AR is often regarded by patients as a background noise, a nuisance, and a trivial disease as it is not life-threatening. For those reasons, AR is frequently self-managed by patients with OTC treatments from community pharmacies, and the pharmacist, therefore, represents the first-point of contact for advice on appropriate medication.33–36 A population survey in 2010 found that 63% of Australian patients with AR visited a pharmacist for advice on AR treatment.37 The purchasing pattern of prescription INCSs, however, appears to be unaffected by seasonal changes and represents ongoing treatment requirements for those with moderate-to-severe persistent rhinitis.17 Studies have shown that most patients who visit a doctor for their AR have moderate-severe persistent disease,38 with most patients first visiting their doctor when AR symptoms become “intolerable”.34 Similarly, the stable purchasing pattern of prescription OAHs is unaffected by seasonal changes and represents unchanged prescribing behavior for those with persistent disease.
Strengths and limitations of this study
This large dataset included pharmacy transaction data from 909 Australian community pharmacies and information on 4,247,193 rhinitis treatments for “real-life” patients with or without additional respiratory disease in 2013 and 2014. Data related to rhinitis therapy underwent rigorous quality assurance procedures prior to statistical analyses. As the dataset used prescription and OTC information, rather than patient-reported outcomes, it provided a unique insight into prescribing and self-medication behavior, and the significant burden posed on community pharmacy to address the needs of people with rhinitis symptoms. The sample of pharmacy transactions involving OAH and INCS treatments is representative of the Australian population as a whole, based on the latest published data of pharmaceutical wholesale supply of OAHs and INCSs to community pharmacies.8 Finally, another strength of the study is its observational nature, which allowed a “snapshot” of the current state of rhinitis treatment purchases via prescription and OTC supply in Australia. This approach provides insight into prescriber behavior and patient purchasing behavior that would have be difficult to obtain through other approaches, such as surveys, which can misrepresent patient and prescriber behavior.
The limitations of the study were associated with the cross-sectional design, variation in the number of pharmacies in each geographical region and lack of patient demographic data. In addition, we used prescription and OTC purchases of rhinitis therapy as a proxy for a rhinitis diagnosis. Although there is a possibility that prescription and OTC purchases of treatment classified as rhinitis therapy could be used for another indication, the marked seasonality in pharmacy purchases of OTC INCS and OAH treatments observed in our study provides evidence to support the use of rhinitis therapy as a proxy for a diagnosis of intermittent (seasonal) AR. Furthermore, in this study it was not possible to check whether therapies purchased together in the dataset were all destined to the same patient nor was it possible to document purchases or rhinitis therapy from pharmacies outside NostraData coverage.
Conclusion
This large-scale retrospective observational study identified seasonal purchasing patterns of OTC and prescription OAHs and INCSs in a real-world setting across Australia. It highlighted that seasonality only affects OTC purchasing patterns of OAHs and INCSs, confirming that spring is the worst time for nasal allergies and that practitioner prescribing remains unchanged, suggesting that it is only for those with persistent (perennial) disease.
Acknowledgments
The abstract of this paper was presented at the Respiratory Effectiveness Group 2016 Annual Summit as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in The Journal of Thoracic Disease (Vol. 8, Supplement 5, 5 July 2016). http://jtd.amegroups.com/article/view/8504.
Disclosure
A Simon Carney is a consultant for Olympus and Smith and Nephew and has received honoraria from MEDA Pharmaceuticals. David B Price has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from UK National Health Service, British Lung Foundation, Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals, Zentiva, and Theravance; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Novartis and Mundipharma; payment for travel/accommodation/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, Teva Pharmaceuticals, and AstraZeneca; funding for patient enrolment or completion of research from Chiesi, Teva Pharmaceuticals, Zentiva, and Novartis; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd, UK, and 74% of Observational and Pragmatic Research Institute Pte Ltd, Singapore; and is peer reviewer for grant committees of the Medical Research Council, Efficacy and Mechanism Evaluation programme, and Health Technology Assessment. Pete Smith has received honoraria from AstraZeneca, GlaxoSmithKline, MEDA Pharmaceuticals, and Mundipharma. Richard Harvey is a consultant for Medtronic, Neilmed, and Olympus, he has received honoraria from Sequiris and grant support from MEDA Pharmaceuticals, Neilmed and Stallergenes.
Vicky Kritikos has received honoraria from AstraZeneca, GlaxoSmithKline, and Pfizer.
Sinthia Bosnic-Anticevich has received honoraria from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mundipharma, and TEVA Pharmaceuticals for her contribution to advisory boards/key international expert forum. Alice Durieux was an employee of Observational and Pragmatic Research Institute, which has conducted paid research in respiratory disease on behalf of UK National Health Service, British Lung Foundation, Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, Chiesi, MEDA Pharmaceuticals, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Takeda, TEVA Pharmaceuticals, Theravance, and Zentiva. The other authors report no conflicts of interest in this work.
References
Settipane RA, Kaliner MA. Nonallergic rhinitis. Am J Rhinol Allergy. 2013;27(Suppl 1):48–51. | ||
Scarupa MD, Kaliner MA. Nonallergic rhinitis, with a focus on vasomotor rhinitis. World Allergy Organ J. 2009;2(3):20–25. | ||
Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28(1):3–9. | ||
Melzer EO, Farrar JR, Sennett C. Findings from an online survey assessing the burden and management of seasonal allergic rhinoconjunctivitis in US patients. J Allergy Clin Immunol Pract. 2017;5(3):779–789.e6. | ||
Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(Supp 2):12–16. | ||
Seidman MD, Schwartz SR, Bonner JR, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(1S): S1–S43. | ||
Australian Institute of Health and Welfare. Australia’s health 2014; 2014. Available from: http://www.aihw.gov.au/publication-detail/?id=60129547205. Accessed November, 2016. | ||
Australian Institute of Health and Welfare. Allergic rhinitis (‘hay fever’) in Australia; 2011. Available from: http://www.aihw.gov.au/publication-detail/?id=10737420595. Accessed November, 2016. | ||
Settipane RA. Rhinitis: a dose of epidemiological reality. Allergy Asthma Proc. 2003;24(3):147–154. | ||
Settipane RA, Charnock DR. Epidemiology of rhinitis: allergic and nonallergic. Clin Allergy Immunol. 2007;19:23–34. | ||
Settipane RA. Demographics and epidemiology of allergic and nonallergic rhinitis. Allergy Asthma Proc. 2001;22(4):185–189. | ||
Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl): S147–S334. | ||
Walls RS, Heddle RJ, Tang MLK, Basger BJ, Solley GO, Yeo GT. Optimising the management of allergic rhinitis: an Australian perspective. Med J Aust. 2005;182(1):28–33. | ||
Davies JM. Grass pollen allergens globally: the contribution of subtropical grasses to burden of allergic respiratory diseases. Clin Exp Allergy. 2014;44(6):790–801. | ||
Beggs PJ. Adaptation to impacts of climate change on aeroallergens and allergic respiratory diseases. Int J Environ Res Public Health. 2010;7(8):3006–3021. | ||
Australian Society of Clinical Immunology and Allergy: Pollen allergy; 2015. Available from: http://www.allergy.org.au. Accessed November, 2016. | ||
Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63 (Supp 86):8–160. | ||
Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–476. | ||
Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38(1):19–42. | ||
Bielory L, Chun Y, Bielory BP, Canonica GW. Impact of mometasone furoate nasal spray on individual ocular symptoms of allergic rhinitis: a meta-analysis. Allergy. 2011;66(5):686–695. | ||
Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317(7173):1624–1629. | ||
Ratner PH, Howland WC III, Arastu R, et al. Fluticasone propionate aqueous nasal spray provided significantly greater improvement in daytime and nightime nasal symptoms of seasonal allergic rhinitis compared with montelukast. Ann Allergy Asthma Immunol. 2003;90(5):536–542. | ||
Neilsen LP, Mygind N, Dahl R. Intranasal corticosteroids for allergic rhinitis: superior relief? Drugs. 2001;61(11):1563–1579. | ||
Klimek L, Mullol J, Hellings P, Gevaert P, Mösges R, Fokkens W. Recent pharmacological developments in the treatment of perennial and persistent allergic rhinitis. Exp Opin Pharmacother. 2016;17(5):657–669. | ||
Simons FE, Simons KJ. Histamine and H1-antihistamines:celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139–1150. | ||
Meltzer EO, Wallace D, Dykewicz M, Shneyer L. Minimal clinically important difference (MCID) in allergic rhinitis: agency for healthcare research and quality or anchor-based thresholds? J Allergy Clin Immunol Pract. 2016;4(4):682–688. | ||
Benninger M, Farrar JR, Blaiss M, et al. Evaluating approved medications to treat allergic rhinitis in the United States: an evidence-based review of efficacy for nasal symptom class. Ann Allergy Asthma Immunol. 2010;104(1):13–29. | ||
Lombardi C, Musicco E, Rastrelli F, Bettoncelli G, Passalacqua G, Canonica GW. The patient with rhinitis in the pharmacy. A cross-sectional study in real life. Asthma Res Pract. 2015;1:1–6. | ||
Lourenco O, Calado S, Sa-Sousa A, Fonseca J. Evaluation of allergic rhinitis and asthma control in a Portuguese community pharmacy setting. J Mang Care Pharm. 2014;20(5):513–522. | ||
Mehuys E, Gevaert P, Brusselle G, et al. Self-medication in persistent rhinitis: overuse of decongestants in half of the patients. J Allergy Clin Immunol Pract. 2014;2:313–319. | ||
Sinclair H, Bond C, Largue G, Price D, Hannaford P. Community pharmacy provision of allergic rhinitis treatments: a longitudinal study of patient reported outcome. Int J Pharm Pract. 2005;13:249–256. | ||
Williams A, Scadding G. Is reliance on self-medication and pharmacy care adequate for rhinitis patients? Int J Clin Pract. 2009;63(1):98–104. | ||
Demoly P, Allaert FA, Lecasble M. ERASM, a pharmacoepidemiologic survey on management of intermittent allergic rhinitis in every day general practice in France. Allergy. 2002;57:546–554. | ||
Maurer M, ZuberbierT. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007;62(9):1057–1063. | ||
Fromer LM, Ortiz G, Ryan SF, Stoloff SW. Insights on allergic rhinitis from the patient perspective. J Fam Pract. 2012;61(2 Suppl):S16–S22. | ||
Storms W, Meltzer EO, Nathan RA, Selner JC. Allergic rhinitis: the patient’s perspective. J Allergy Clin Immunol. 1997;99(6):S825–S828. | ||
Katelaris CH, Lai CK, Rhee C, et al. Nasal allergies in the Asia-Pacific population: results from the Allergies in Asia-Pacific survey. Am J Rhinol Allergy. 2011;25(Suppl 1):S3–S15. | ||
Demoly P, Bousquet PJ, Mesbah K, Bousquet J, Devillier P. Visual analogue scale in patients treated for allergic rhinitis: an observational prospective study in primary care: asthma and rhinitis. Clin Exp Allergy. 2013;43(8):881–888. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.